High frequency of false-positive results of aPTT-based lupus anticoagulant tests in patients receiving argatroban
Argatroban is a selective direct thrombin inhibitor (DTI),1 that has been shown to improve clinical outcomes, particularly new thrombosis and death due to thrombosis, in patients who have heparin-induced thrombocytopenia (HIT).2 Argatroban and other DTIs are being increasingly used as alternative anticoagulants for prophylaxis and treatment of thrombosis in known or suspected HIT,1 and their use has been recommended by the American College of Chest Physicians in these clinical situations.3 The clinical coagulation laboratory frequently receives specimens containing argatroban along with lupus anticoagulant (LA) testing ordered; therefore, it is important to know whether argatroban interferes with the tests.
We have previously shown that argatroban interferes with the activated protein C resistance ratio, potentially causing missed diagnosis of factor V Leiden.4 However, to our knowledge, the effect of argatroban on LA testing has not been assessed in prospective studies nor longitudinally in patients before and after receiving argatroban. Thus, it is essential to determine which coagulation tests may be erroneous due to the administration of this drug to ensure proper diagnosis and management in patients receiving it. Therefore, this study was designed to determine the effect of argatroban on aPTT-based LA testing, by determining prospectively the false-positive and false-negative rates in patients being treated with it at a large hospital with a high-volume coagulation laboratory.
Patients receiving argatroban anticoagulation therapy at Massachusetts General Hospital (MGH) were enrolled over a 2.5-year period. We prospectively identified patients receiving argatroban by daily examination of medical records from the total pool of 3.2% citrate blood specimens submitted for specialized coagulation testing to our laboratory. Patient clinical histories were reviewed through the MGH electronic medical record system. The decision of administration/discontinuation of argatroban was independent of this study.
Once a patient was determined to be receiving argatroban, an additional plasma specimen before starting argatroban or after discontinuation of argatroban was retrieved from the pool of existing clinical samples in the laboratory. For plasma specimens collected after argatroban was discontinued, a thrombin time was performed and was demonstrated to be normal to ensure that the drug was no longer present. Each patient was tested for LA while receiving argatroban as well as when argatroban was not present. LA testing for each patient included a lupus anticoagulant-sensitive activated partial thromboplastin time (PTT-LA) assay, 1:1 mixing of PTT-LA with normal pooled plasma, and the hexagonal phase lipid neutralization (Staclot-LA) assay. A Student's paired t test was used to determine the statistical significance. The Pearson correlation coefficient (r) and simple linear regression were used to assess for correlation.
A total of 15 526 plasma samples were submitted for specialized LA testing during the study period. We identified 44 patients receiving argatroban and included 37 patients who had specimens sent when on and off argatroban. The mean ± SD patient age was 57.7 ± 16.7 years (range, 15-83 years). Most patients were males (75%) and had a history of a thrombotic event (75%).
LA testing was confirmed true-positive in 38% (14/37) of samples from patients at baseline when not receiving argatroban therapy. All patients who were LA positive off argatroban were also positive on argatroban (14/14, no false negatives).
Argatroban significantly prolonged the baseline PTT-LA in all patients but one (off argatroban mean ± SD: 42 ± 12.3 seconds; on argatroban mean ± SD: 83.9 ± 30.3 seconds; P < .0001). Among patients with LA at baseline, the mean ± SD PTT-LA was 43.92 ± 12.4 seconds when not receiving argatroban and 84 ± 25.8 seconds when receiving argatroban (P < .0001) (Figure 1A). Among patients without LA at baseline, the mean ± SD PTT-LA was 41 ± 12.5 seconds when not receiving argatroban and 85.2 ± 33.9 seconds when receiving argatroban (P < .0001) (Figure 1B). Argatroban-induced PTT-LA prolongation was reversed by mixing with 1:1 normal plasma in only one patient without LA. Thus, the PTT-LA mix was false positive in 22/23 (96%) of LA-negative patients. The PTT-LA mix was prolonged in all LA-positive patients on argatroban (14/14, 100%, no false-negatives).
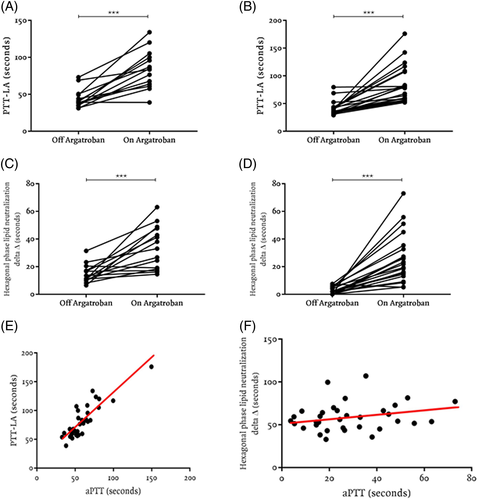
Argatroban also significantly increased the confirmatory hexagonal phase lipid neutralization delta both in patients with and without LA at baseline. Among patients with LA at baseline, the mean ± SD hexagonal phase lipid neutralization delta was 14.7 ± 6.5 seconds when not receiving argatroban and 33.6 ± 15.5 seconds when receiving argatroban (P < .0001) (Figure 1C). Among patients who were LA negative off argatroban, all but one tested LA positive when on argatroban, leading to a false-positive rate of 96% (22/23). Among that group of LA-negative patients, the mean ± SD phase delta was 2.7 ± 2.6 seconds when not receiving argatroban and 25.8 ± 17.8 seconds when receiving argatroban, a difference that was highly significant (P < .0001, positive is delta >8 seconds) (Figure 1D). The effects of argatroban on PTT-LA and confirmatory hexagonal phase lipid neutralization delta were resolved after drug discontinuation. We also obtained these findings in a small number of patients on other DTIs—bivalirudin and lepirudin (data not shown) and dabigatran5—false-positive PTT-LA mix and false-positive hexagonal phase with increased delta.
Although we found high false-positive rates for PTT-LA and hexagonal phase lipid neutralization assay, the dilute Russell viper venom time (dRVVT) LA test does not seem to be as affected by argatroban.6, 7 Genzen and Miller showed that argatroban significantly increased the dRVVT ratio, but it did not significantly alter the test/confirm dRVVT ratio and the percent correction of dRVVT ratio when spiked into samples from healthy donors and LA-positive samples at both low and high doses.6 Seheult et al did not find that argatroban induced any significant interference in the dRVVT ratio from patient specimens in a retrospective study with a limited number of clinical specimens, and testing was not performed on the same group of patients without anticoagulation for comparison.7 Gosselin et al also showed that argatroban did not cause false-positive dRVVT when spiked into pooled normal plasma at different concentrations.8 The clinical significance of the effect of argatroban on the dRVVT remains to be fully elucidated since none of these past studies were done in patients receiving vs not receiving argatroban.
Because aPTT is positively correlated with infusion dose and argatroban levels in plasma,9 we used it as an indication of the level of argatroban anticoagulation to evaluate the effect of the drug concentration in different coagulation-based tests. As the aPTT increased, the PTT-LA tended to increase, suggesting that increasing concentration of argatroban has a greater effect on the PTT-LA results. The coefficient of correlation (r = .9) and the coefficient of determination (r2 = .7) were very strong (Figure 1E). Not surprisingly, argatroban-related prolongations on PTT-LA appear to be dose-dependent. PTT-LA prolongations were observed at lower and higher aPTT prolongations in argatroban specimens, and thus presumably at lower and higher doses of argatroban, suggesting that these results would be observed throughout the therapeutic concentration range of argatroban in plasma (50-70 seconds in our laboratory). This phenomenon occurs presumably because PTT-LA is essentially an aPTT with a much lower concentration of phospholipid to enhance sensitivity to LA.
The hexagonal phase lipid neutralization delta also tended to increase as the aPTT increased. However, the coefficient of correlation (r = .2) and the coefficient of determination (r2 = .05) were very weak (Figure 1F), presumably because the result is a delta (change in seconds) rather than a clotting time.
In conclusion, the rate of false-positive results in aPTT-based LA testing is unacceptably high in patients receiving argatroban, and argatroban is likely to lead to an incorrect diagnosis of LA, because almost all patients without LA tested falsely positive for LA while receiving argatroban. Clinicians and coagulation laboratory directors should be advised that aPTT-based LA testing for patients receiving argatroban is highly inaccurate, causing false-positive results in almost all specimens, including specimens with low concentrations of argatroban. Argatroban did not cause false-negative results for LA.
ACKNOWLEDGMENTS
The authors acknowledge the personnel of the MGH Special Coagulation laboratory who performed the laboratory testing of the human samples.
CONFLICT OF INTEREST
The authors state that they have no conflict of interest.




