Duvelisib, an oral dual PI3K-δ, γ inhibitor, shows clinical activity in indolent non-Hodgkin lymphoma in a phase 1 study
Funding information: Infinity Pharmaceuticals; Verastem Inc.
Abstract
Duvelisib (IPI-145) is an oral dual inhibitor of phosphoinositide-3-kinase (PI3K)-δ and -γ in clinical development for the treatment of hematologic malignancies, including indolent non-Hodgkin lymphoma (iNHL). In a Phase 1, open-label study to determine the maximum tolerated dose (MTD), pharmacokinetics, pharmacodynamics, clinical activity, and safety of duvelisib monotherapy in patients with advanced hematologic malignancies, duvelisib was administered at eight dose levels (8-100 mg BID) in a dose-escalation phase (n = 31 evaluable patients). Two dose-limiting toxicities (DLTs), Grade 3 transaminase elevations and Grade 3 rash, occurred at 100 mg BID, and the MTD was determined to be 75 mg BID. Across all doses, 58.1% of iNHL patients had a response (19.4% complete, 35.5% partial, and 3.2% minor); median time to response was 1.84 months and duration of response was 16.9 months. Median progression-free survival was 14.7 months, and the probability of overall survival at 24 months was 71.7%. Severe (Grade ≥ 3) adverse events included elevated liver enzymes (38.7%), diarrhea (25.8%), and neutropenia (29.0%). Three patients, all in the 75 mg BID cohort, experienced fatal AEs: E. coli sepsis, acute respiratory failure, and fungal pneumonia. No iNHL patients experienced Pneumocystis pneumonia. Duvelisib demonstrated favorable clinical activity and an acceptable safety profile in these high-risk, heavily pretreated, relapsed/refractory iNHL patients, with 25 mg BID selected for further clinical development.
1 INTRODUCTION
Non-Hodgkin lymphoma (NHL) is a heterogenous group of lymphoid malignancies with clinical behaviors ranging from indolent (iNHL) to aggressive. iNHL accounts for approximately 40% of all NHL cases, and includes follicular lymphoma (FL) and marginal zone lymphoma (MZL). Approximately 71 000 and 93 500 new cases of NHL are diagnosed each year in the U.S. and Europe, respectively, with almost 19 000 and 38 000 persons dying from these diseases annually.1, 2
Standard treatments for iNHL include monoclonal antibodies (eg, anti-CD20) often in combination with chemotherapy (chemoimmunotherapy, CIT), radioimmunotherapy (RIT), and external-beam radiotherapy. While frontline treatment of advanced iNHL patients with CIT-based regimens induces overall response rates (ORRs) of 90% or higher,3 eventual relapse is expected, with response rates and durations decreasing with each successive line of therapy. Although median survival with current therapies is estimated at greater than 10 years for newly diagnosed patients, iNHL remains incurable, necessitating the development of new effective oral treatments with novel mechanisms of action and acceptable toxicity profiles.
Phosphoinositide 3-kinase (PI3K) is a lipid kinase involved in intracellular signal transduction. PI3K's catalytic subunit (p110) exists in four isoforms: α, β, δ, γ. While α and β isoforms are virtually ubiquitous, δ and γ isoforms are preferentially expressed in leukocytes and play an important role in adaptive and innate immune responses in B-cell, T-cell, and myeloid cell function4-7 PI3K-δ inhibition includes multiple key effects on malignant B-cells, including blockade of tumor microenvironment-mediated malignant B-cell proliferation and migration, and inhibition of tumor cell-derived chemokine secretion.8-10 In addition, PI3K-γ inhibition is believed to block the formation of the tumor microenvironment through inhibition of T-cell and macrophage migration towards microenvironment-derived chemokines, and to block macrophage polarization to a tumor-supporting M2 phenotype.8, 11-13 In transformed FL xenograft models, combined inhibition of PI3K-δ and PI3K-γ enhanced tumor-growth suppression compared to inhibition of either isoform alone,14 suggesting complementary dual isoform contributions to tumor growth and survival. Accordingly, when compared with PI3K-δ inhibition alone, dual PI3K-δ,γ inhibition may enhance therapeutic benefit in patients with lymphoid malignancies.
The expanding recognition of PI3K-δ and PI3K-γ's roles in hematologic malignancies has triggered the recent development of class-specific inhibitors of PI3K for use as anticancer agents. Idelalisib, an oral inhibitor of PI3K-δ only, is approved for treatment in iNHL and in relapsed chronic lymphocytic leukemia (CLL) in combination with rituximab. Duvelisib (also known as IPI-145)15 is an oral dual inhibitor of both PI3K-δ and PI3K-γ that has demonstrated selective antiproliferative activity against leukemia cells in vitro while sparing normal lymphoid cells.9, 16, 17
Here we report on the efficacy, safety, and pharmacodynamic activity of duvelisib in patients with relapsed/refractory iNHL based on results from the first clinical study of duvelisib in patients with advanced hematologic malignancies.
2 PATIENTS AND METHODS
2.1 Study design and treatment
Study IPI-145-02 was a Phase 1, open-label, dose-escalation study in patients with advanced hematologic malignancies. Duvelisib was administered as oral capsules twice daily (BID) continuously in 28-day cycles until disease progression or unacceptable toxicity. Dose interruptions and dose reductions (up to 2) were allowed in the event of duvelisib-related toxicities. Clinic visits occurred weekly during the first three treatment cycles, biweekly during Cycles 4 and 5, monthly during Cycles 6 through 19, and once every third cycle thereafter.
During the expansion phase, patients with certain hematologic malignancies, including iNHL, were treated with either the MTD (75 mg BID) or 25 mg BID. The rationale for selecting the latter dose for expansion was based on the review of preliminary data collected during the dose escalation phase (DEP), including safety, pharmacokinetics (PK), pharmacodynamics (PD), and clinical activity.18
All patients provided informed consent before undergoing any study procedure. The protocol was approved by site Institutional Review Boards, and the study was conducted according to local and federal regulations and the Declaration of Helsinki.
2.2 Patients and eligibility criteria
Patient enrollment criteria included age ≥ 18 years, life expectancy >3 months, and a diagnosis of advanced hematologic malignancy. Patients were also required to have progressed on, been refractory to, or intolerant of established therapy. For the iNHL expansion cohort, eligible subtypes included FL, MZL, or Waldenström's macroglobulinemia (WM)/lymphoplasmacytic lymphoma (LPL), and prior PI3K inhibitor therapy was permitted. Key exclusion criteria included a diagnosis of overt leptomeningeal leukemia or CNS lymphoma; ongoing high-dose immunosuppression for chronic conditions; inadequate hepatic function, chronic hepatitis, or other chronic liver disease; inadequate renal function; HIV infection; history of alcohol abuse; and pregnancy. Medications or foods that are strong CYP3A inhibitors or inducers were prohibited. During the course of the study, the protocol was amended to require concomitant Pneumocystis prophylaxis due to three cases of Pneumocystis jirovecii pneumonia: two fatal cases in patients with diffuse large B-cell lymphoma (DLBCL) and one case in a CLL patient.
2.3 Pharmacodynamic methods
Serum collected at Cycle 1, Day 1 (C1D1) (predose), C1D8, C2D1, and C3D1 was processed for pharmacodynamic biomarker analysis. Cytokines, chemokines, and matrix metalloproteinases (MMPs) (total of 74 analytes) were evaluated using Luminex xMAP technology (Luminex Corp., Austin, TX) (Supporting Information Table S1). The PD analysis set consisted of all iNHL patients for whom at least the C1D1 sample and one other sample at C1D8, C2D1, or C3D1 were available.
2.4 Efficacy assessments
Disease response—complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD)—according to the International Working Group (IWG) criteria19 was determined by the investigator on Day 1 of Cycles 3, 5, and 7, every third cycle from C10-19, and every sixth cycle thereafter using computed tomography (CT) scans and positron emission tomography (PET) where indicated. A bone marrow biopsy and/or aspiration was performed to confirm a radiographic CR. For WM patients, a WM-specific response assessment, which included a minor response (MR), was used.20
Efficacy endpoints included ORR (CR + PR + MR), time to response (TTR), lesion response (percent change from baseline in the sum of the product of diameters of baseline lesions), duration of response (DOR; months from first response to progressive disease or death), progression-free survival (PFS; months from first dose to either progressive disease or death), and OS (months from first dose to death). Summaries of ORR, PFS, and OS are presented for all iNHL patients who received at least one dose of duvelisib. The TTR and DOR analyses include only iNHL patients who achieved a CR, PR, or MR. Lesion response rate was calculated for patients for whom baseline and post-baseline CT scan results were available.
2.5 Safety assessments
Adverse event (AE) monitoring and laboratory assessments were performed at all clinic visits. AEs were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.03. Summaries include all iNHL patients who received at least one dose of duvelisib.
2.6 Statistical methods
Descriptive statistics, including median and range or mean and coefficient of variation for continuous endpoints and frequency and percentage for categorical endpoints, are presented. Analyses of pharmacodynamic data included two-sample t test comparisons of C1D1 values compared to values from samples from healthy donors (serum purchased from Bioreclamation IVT, Hicksville, NY); and paired t tests of the change from C1D1 to postdose time points. These analyses were evaluated at an alpha of .05 after Bonferroni correction for multiple comparisons. For the ORR, the Clopper-Pearson 95% confidence interval (CI) is provided. DOR, PFS, and OS are summarized using Kaplan-Meier methods.
Results are presented for all doses combined and for 25 and 75 mg BID doses individually. Statistical analyses comparing dose groups were not performed.
3 RESULTS
3.1 Patient demographic and baseline characteristics, disposition, and treatment
Thirty-one iNHL patients were treated with duvelisib BID at the following doses: 15 mg (n = 1), 25 mg (n = 14), 50 mg (n = 1), or 75 mg (n = 15). The characteristics of these 31 patients are summarized in Table 1. iNHL patients had a median age of 64 years (range 37-78), were mostly (58%) male, and predominately (90%) white. Most iNHL patients had FL (24; 77%), while four patients had WM/LPL, two had MZL, and one had an unspecified iNHL. At baseline, the majority of patients had an ECOG performance status of 0 (42%) or 1 (55%) and a Follicular Lymphoma International Prognosis Index (FLIPI) score of 2 (32%) or 3 (48%). The median number of prior systemic therapies was three, and 87% of patients had Stage 3 or higher disease. Nearly all patients had previously received rituximab (94%), with alkylating agents (excluding bendamustine; 42%), and bendamustine (39%) representing other common prior treatments. Two patients had prior idelalisib therapy and six (19%) had prior stem cell transplant (five autologous, one not specified).
| 25 mg BID | 75 mg BID | All doses | |
|---|---|---|---|
| (n = 14) | (n = 15) | (n = 31) | |
| Demographics | |||
| Age (years), median (range) | 61 (37-76) | 70 (42-78) | 64 (37-78) |
| Race, white, n (%) | 13 (93) | 13 (87) | 28 (90) |
| Sex, male, n (%) | 10 (71) | 6 (40) | 18 (58) |
| Baseline disease status | |||
| iNHL diagnosis, n (%) | |||
| Follicular lymphoma (FL) | 13 (93) | 9 (60) | 24 (77) |
| Waldenström's macroglobulinemia/lymphoplasmacytic lymphoma | 1 (7) | 3 (20) | 4 (13) |
| Marginal zone lymphoma | 0 | 2 (13) | 2 (7) |
| iNHL (NOS) | 0 | 1 (7) | 1 (3) |
| Years from initial diagnosis, median (range) | 6 (1-21) | 7 (1-14) | 7 (1-21) |
| Baseline disease ≥stage 3, n (%)a ,b | 12 (86) | 12 (80) | 26 (87) |
| Bulky disease (>5 cm lesion), n (%)c | 5 (36) | 5 (33) | 11 (36) |
| ECOG score, 0/1/2, % | 50/50/0 | 27/67/7 | 42/55/3 |
| Baseline FLIPI score, 0-1/2/3 factors (%) | 29/29/43 | 13/33/53 | 19/32/48 |
| Number of prior systemic therapies, median (range) | 3 (1-7) | 3 (1-8) | 3 (1-8) |
| Prior therapy regimen, n (%)d | |||
| Rituximab | 13 (93) | 14 (93) | 29 (94) |
| Alkylating agent (excl. Bendamustine) | 7 (50) | 5 (33) | 13 (42) |
| Bendamustine | 5 (36) | 6 (40) | 12 (39) |
| Purine analog | 3 (21) | 4 (27) | 7 (23) |
| Anthracycline | 3 (21) | 2 (13) | 6 (19) |
| RIT | 3 (21) | 2 (13) | 5 (16) |
| Prior stem cell transplantatione | 5 (36) | 0 | 6 (19) |
| Patients with <6 months from most recent systemic therapy, n (%) | 6 (43) | 5 (33) | 11 (36) |
- Abbreviations: ECOG, Eastern Cooperative Oncology Group; FL, follicular lymphoma; FLIPI, Follicular Lymphoma International Prognostic Index; NOS, not otherwise specified; RIT, radioimmunotherapy.
- a Assessed using Ann Arbor method (except for 4 patients).
- b Data missing for one 75 mg BID patient.
- c Data missing for one 25 mg BID patient and three 75 mg BID patients.
- d Patients are counted for each prior therapy they received; the sum of % >100% because many patients received multiple prior therapies.
- e One patient received 50 mg BID; autologous stem cell transplant (n = 5), unspecified bone marrow transplant (n = 1).
The median duration of duvelisib treatment was 18 weeks (range: 0.3-160) across all doses, 36 weeks (range 0.3-152) for those who received 25 mg BID, and 12 weeks (range: 3-102) for those who received 75 mg BID.
At the time of this analysis, two patients remained on duvelisib 25 mg BID and 29 had discontinued treatment.
3.2 Duvelisib pharmacodynamics in iNHL
In an exploratory analysis, a group of 74 serum factors, including chemokines, cytokines, matrix metalloproteinases, and other proteins were evaluated (Supporting Information Table S1). These factors were chosen because of their relevance to the tumor microenvironment in supporting malignant B cells, including cell-migration, cell-cell associations, cell-proliferative, and differentiation functions.21-30
Pharmacodynamic data were available from 9 to 24 iNHL patients, depending on the analyte and time point; 10 of the 74 serum analytes tested decreased significantly from baseline at C1D8: CCL1, CCL4, CCL17, CCL22, CXCL10, CXCL13, IL-10, IL-16, MMP-9, and TNFα. (Supporting Information Table S2 and Figure S1). Three of the 10 analytes (CCL17, CXCL13, and MMP-9) also exhibited a significant change at C2D2. No analyte increased significantly from baseline. With the exception of CXCL10, each of the analytes noted above was elevated at baseline in serum from iNHL patients when compared with serum from healthy donors (n = 33). After 8 days of duvelisib treatment, the levels of five of the analytes elevated at baseline were no longer significantly higher than the values from the healthy donor samples.
3.3 Efficacy
3.3.1 iNHL patients
Across doses, 18 of the 31 iNHL patients had a response (ORR = 58.1%), including 6 (19.4%) CRs, 11 (35.5%) PRs, and one (3.2%) MR (WM patient; Table 2). Of the two patients who had received prior treatment with idelalisib, one achieved a CR on duvelisib. Ten (32.3%) patients had stable disease and two (6.5%) patients had progressive disease as best response, and one patient had no post-baseline assessment. The ORR was 64.3% and 46.7% in the 25 mg BID (n = 14) and 75 mg BID (n = 15) groups, respectively.
| 25 mg BID | 75 mg BID | All doses | |
|---|---|---|---|
| (n = 14) | (n = 15) | (n = 31) | |
| Overall response ratea | 9 (64.3) | 7 (46.7) | 18 (58.1) |
| 95% CI | (35.1, 87.2) | (21.3, 73.4) | (39.1, 75.5) |
| Best overall response | |||
| Complete response | 4 (28.6) | 1 (6.7) | 6 (19.4) |
| Partial response | 4 (28.6) | 6 (40.0) | 11 (35.5) |
| Minor responseb | 1 (7.1) | 0 | 1 (3.2) |
| Stable disease | 3 (21.4) | 7 (46.7) | 10 (32.3) |
| Progressive disease | 1 (7.1) | 1 (6.7) | 2 (6.5) |
| Unknownc | 1 (7.1) | 0 | 1 (3.2) |
- Abbreviation: CI, confidence interval.
- a Overall response rate = CR + PR + MR (Waldenström's macroglobulinemia[WM] patients only).
- b Assessed in WM patients only.
- c Includes one patient who did not have any response assessments after initiation of duvelisib.
Following treatment with duvelisib, a ≥ 50% reduction in adenopathy was observed in 61.5% (16/26) of patients across doses, including 66.7% and 50.0% of patients in the 25 mg and 75 mg BID groups, respectively (Supporting Information Figure S2).
Among all iNHL patients who had a response (n = 18), the median TTR was approximately 1.8 months across doses (range 1.5-4.1) and for the 25 mg and 75 mg BID dose groups. The median DOR was 16.9 months across doses, 12.9 months for the 25 mg BID group (n = 9), and 16.9 months for the 75 mg BID group (n = 7). The estimated probability of remaining in response at 6, 12, and 18 months was 76.6%, 67.0%, and 44.7%, respectively, across dose groups (Supporting Information Figure S3).
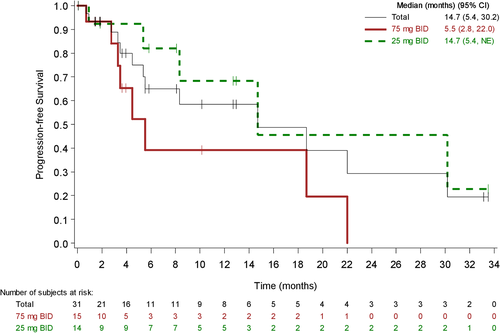
Across doses, 11 (35.5%) patients progressed and 2 (6.5%) died of other causes. Median PFS was 14.7 months for all doses, 14.7 months for the 25 mg BID group, and 5.5 months for the 75 mg BID group (Figure 1). The estimated probability of being progression free at 6, 12, and 18 months was 65.0%, 58.5%, and 48.8%, respectively, across doses (Figure 1).
For the overall survival analysis, 21 (67.7%) patients across doses were censored, which included patients who were either alive and in follow-up at the time of this analysis, alive after having completed the 2-year survival follow-up, or were lost to follow-up at the time of the data analysis. The median time of OS was not reached. The estimated probability of survival at 6, 12, and 18 months was 86.1%, 78.9%, and 75.3% (Supporting Information Figure S4).
As FL was the most common (77.4%) diagnosis in the iNHL cohort, an efficacy analysis was performed on this subpopulation. Among FL patients (n = 24), the overall response rate was 58.3% (25.0% CR, 33.3% PR), with a median TTR of 1.84 months, a DOR of 18.4 months, and a probability of remaining in response at 6, 12, and 18 months of 80.8%, 67.3%, and 50.5%, respectively. The median time of remaining progression-free for FL patients was 14.7 months, and the probability of remaining progression-free at 6, 12, and 18 months was 71.9%, 61.7%, and 46.2%, respectively. The estimated probability of survival at 6, 12, 18, and 24 months was 91.3%, 86.7%, 82.2%, and 82.2%, respectively.
3.4 Safety
The most frequently reported (>15% patients) all-causality treatment-emergent AEs and investigation abnormalities in iNHL patients treated with duvelisib are presented in Table 3. Most events were grades 1-2 with a few exceptions. Transaminase elevations and diarrhea were the most frequent nonhematologic AEs (both 54.8%) and the most common grade 3 or 4 events (38.7% and 25.8%, respectively). Transaminase elevation AE incidences were similar across dose levels (57.1% for 25 mg BID; 66.7% for 75 mg BID). These events were observed early in treatment, with a median time to onset of 36 days (range 28-63 days). Most patients with transaminase elevations (11 of 17) were managed with dose modifications (interruption/reduction), and 4 patients (12.9%) discontinued treatment. The first diarrhea AEs typically occurred later in the treatment course, with the median time to onset of approximately 3 months (106 days, range 57-153). Diarrhea was more frequently reported in the 75 mg than the 25 mg BID cohort (66.7% vs. 42.9%) and was largely managed with supportive medications, with only three of 17 patients with diarrhea having a dose modification (interruption/ reduction), and one patient discontinuing treatment due to diarrhea. Toxicities believed to be immune related, colitis and pneumonitis, were observed in 4 patients (2 each, 6.5%), and did not appear to be dose dependent.
| AE | 25 mg BID | 75 mg BID | All doses | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (n = 14) | (n = 15) | (n = 31) | |||||||
| Gradea | Any | Grade 3 | Grade 4 | Any | Grade 3 | Grade 4 | Any | Grade 3 | Grade 4 |
| Hematologic | |||||||||
| Neutropenia | 3 (21.4) | 1 (7.1) | 2 (14.3) | 7 (46.7) | 2 (13.3) | 3 (20.0) | 12 (38.7) | 5 (16.1) | 5 (16.1) |
| Anemia | 2 (14.3) | 1 (7.1) | 0 | 3 (20.0) | 2 (13.3) | 0 | 6 (19.4) | 4 (12.9) | 0 |
| Thrombocytopenia | 3 (21.4) | 1 (7.1) | 1 (7.1) | 2 (13.3) | 0 | 0 | 6 (19.4) | 1 (3.2) | 1 (3.2) |
| Investigations | |||||||||
| ALT or AST increased | 8 (57.1) | 5 (35.7) | 1 (7.1) | 10 (66.7) | 4 (26.7) | 2 (13.3) | 18 (58.1) | 9 (29.0) | 3 (9.7) |
| Nonhematologic | |||||||||
| Diarrhea | 6 (42.9) | 3 (21.4) | 0 | 10 (66.7) | 5 (33.3) | 0 | 17 (54.8) | 8 (25.8) | 0 |
| Pyrexia | 5 (35.7) | 0 | 0 | 9 (60.0) | 1 (6.7) | 0 | 16 (51.6) | 1 (3.2) | 0 |
| Fatigue | 4 (28.6) | 0 | 0 | 7 (46.7) | 0 | 0 | 13 (41.9) | 0 | 0 |
| Cough | 4 (28.6) | 0 | 0 | 7 (46.7) | 0 | 0 | 12 (38.7) | 0 | 0 |
| Nausea | 6 (42.9) | 1 (7.1) | 0 | 5 (33.3) | 1 (6.7) | 0 | 12 (38.7) | 2 (6.5) | 0 |
| Headache | 4 (28.6) | 1 (7.1) | 0 | 4 (26.7) | 0 | 0 | 8 (25.8) | 1 (3.2) | 0 |
| Decreased appetite | 2 (14.3) | 0 | 0 | 4 (26.7) | 0 | 0 | 7 (22.6) | 0 | 0 |
| Dyspnoea | 1 (7.1) | 0 | 0 | 6 (40.0) | 0 | 0 | 7 (22.6) | 0 | 0 |
| Rash | 3 (21.4) | 0 | 0 | 4 (26.7) | 0 | 0 | 7 (22.6) | 0 | 0 |
| Rash maculo-papular | 0 | 0 | 0 | 6 (40.0) | 2 (13.3) | 0 | 6 (19.4) | 2 (6.5) | 0 |
| Upper respiratory tract infection | 3 (21.4) | 0 | 0 | 2 (13.3) | 0 | 0 | 6 (19.4) | 0 | 0 |
| Vomiting | 5 (35.7) | 1 (7.1) | 0 | 1 (6.7) | 0 | 0 | 6 (19.4) | 1 (3.2) | 0 |
| Chills | 2 (14.3) | 0 | 0 | 2 (13.3) | 0 | 0 | 5 (16.1) | 0 | 0 |
| Myalgia | 1 (7.1) | 0 | 0 | 4 (26.7) | 0 | 0 | 5 (16.1) | 0 | 0 |
| Edema peripheral | 1 (7.1) | 0 | 0 | 4 (26.7) | 0 | 0 | 5 (16.1) | 0 | 0 |
| Oropharyngeal pain | 2 (14.3) | 0 | 0 | 3 (20.0) | 0 | 0 | 5 (16.1) | 0 | 0 |
| Stomatitis | 1 (7.1) | 0 | 0 | 4 (26.7) | 0 | 0 | 5 (16.1) | 0 | 0 |
- a AEs were coded using the Medical Dictionary of Regulatory Activities (MedDRA) version 16.1, and were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.03.
Neutropenia AEs were common (10; 32.2%), with maximum Grade 4 events occurring in 4 patients (12.9%). Three of these patients were managed with dose interruption or reduction; one patient discontinued treatment due to neutropenia. As may be expected for this advanced iNHL patient population, infections were frequently observed (61.3% all grades; 25.8% ≥ Grade 3) with upper respiratory tract infection (URTI) being the most commonly reported (6 [19.4%] patients). Unspecified lung infection was the only severe infection event reported in two or more patients. None of the iNHL patients experienced Pneumocystis pneumonia.
Three patients, all in the 75 mg BID cohort, experienced fatal AEs while on treatment. E. coli sepsis and acute respiratory failure, occurred in one patient each. The third patient died due to fungal pneumonia.
4 DISCUSSION
Advanced-stage iNHL remains incurable, with emerging resistance and cumulative toxicity to available therapies limiting treatment options. For nearly two decades, chemoimmunotherapy has been recognized as the standard treatment approach for iNHL.31 However, disease refractoriness to any or all components of chemoimmunotherapy regimens presents a serious challenge to its continued use in the relapsed setting, thus necessitating the continued development of novel therapies targeting pathways critical to tumor growth and survival and tumor microenvironment maintenance.
In this Phase 1 dose escalation trial, pharmacodynamics, clinical safety, and efficacy of duvelisib were evaluated across multiple advanced hematologic malignancies.18 During the dose escalation phase of the study, the MTD was determined to be 75 mg BID; expansion cohorts in relapsed/refractory (RR) chronic lymphocytic leukemia (CLL), treatment-naïve (TN) CLL, T-cell lymphoma, and iNHL were enrolled to further characterize the clinical activity of duvelisib at both the MTD and a 25 mg BID dose. This report summarizes data on patients with RR iNHL who primarily received 25 mg or 75 mg BID. Dose assignments were not determined by randomization, and the study was not designed to provide a formal statistical comparison between dose levels.
The safety profile of duvelisib in iNHL was consistent with the entire Phase 1 patient population,18 with most treatment-emergent AEs presenting as mild (grades 1-2) events. Many common AEs, such as infections and cytopenias, were expected based on the advanced nature of the underlying disease and the heavily pretreated patient population. Transaminase elevations and diarrhea, the most frequently reported AEs, were generally manageable with dose modifications and rarely resulted in treatment discontinuation. While the incidence of diarrhea was similar across the disease expansion cohorts, the incidence of transaminase elevations in the CLL cohort (O'Brien et al., a companion to the present publication, also in The American Journal of Hematology32) was nearly half that reported in iNHL patients, suggesting toxicity modulation by disease-specific factors, or differences in prior treatments or immune cell populations.
A deeper understanding of the tumor microenvironment's contribution to the pathogenesis and persistence of iNHL continues to evolve along with evidence supporting its disruption as an effective and novel approach to treating lymphoid malignancies. PI3K-δ and PI3Kγ play key roles in B- and T-lymphocyte regulatory functions and accordingly represent rational targets for tumor cell and microenvironment disruption.33 In this study, the significant on-treatment reductions of several serum factors expressed by malignant B cells34 and tumor-supporting myeloid and T cells suggests a pharmacodynamic correlate to duvelisib's potential impact on the tumor microenvironment.8, 35-39 In particular, CXCL13 and IL-10, which exhibited a sustained decrease from baseline, are tumor microenvironment factors of interest. While the significance of circulating levels of many of these factors is not understood, further investigation in future clinical studies is warranted.
PI3K inhibition as a therapeutic strategy in iNHL is supported by data from studies with idelalisib, an oral inhibitor of PI3K-δ, and copanlisib, an intravenously administered inhibitor of PI3K-α and PI3K-δ. Both therapies received accelerated approval from the FDA for the treatment of relapsed FL patients who had received two prior systemic therapies based upon data from single-arm Phase 2 trials. The ORRs for iNHL patients treated with idelalisib or copanlisib in the respective pivotal studies were 57% and 59%.40, 41 Clinical benefit with duvelisib in RR iNHL patients was demonstrated across dose levels, with 58% of patients responding, including 19% with a complete response. The time to response was rapid, with most responses achieved by the first response assessment (2 months). Clinically meaningful activity was further supported by a median DOR and PFS of 17 and 15 months, respectively. Among the subgroup of FL patients, the efficacy was equally robust, with an overall response rate of 58%, including 25% of patients with a CR, and a median DOR of 18.4 months. At the time of the analysis, the median OS for the overall iNHL patient population was not reached, with an estimated probability of survival of 75% at 18 months.
In heavily pretreated patients with relapsed/refractory iNHL, oral duvelisib monotherapy demonstrated rapid, clinically meaningful activity and an acceptable safety profile, with 25 mg BID selected as the Phase 2/3 dose. Findings from this first clinical evaluation of duvelisib, an oral dual inhibitor of PI3K-δ and PI3K-γ, suggest that duvelisib may be considered a potential new component of the iNHL treatment paradigm. DYNAMO (NTC01882803), a Phase 2 clinical trial further assessing the therapeutic potential of duvelisib 25 mg BID in patients with treatment-refractory iNHL for whom additional treatment options are needed, has been completed and met its primary endpoint.
ACKNOWLEDGMENT
Infinity Pharmaceuticals and Verastem provided financial support. We would like to thank the study investigators, coordinators, nurses, and patients and their families for their contributions. Steven Mousterakis and Justin McLaughlin, formerly of Infinity Pharmaceuticals, and Paul Guttry of Acumen Medical Communications provided graphical and editorial support.
CONFLICT OF INTERESTS
Dr. Flinn has received funding/grant support from the following: Acerta Pharma, Agios Pharmaceuticals, BeiGene, Celgene, Constellation Pharmaceuticals, Curis Inc., Forma Therapeutics, Forty Seven Inc., Genentech, Gilead Sciences, Incyte Corporation, Infinity Pharmaceuticals, Janssen Pharmaceutical, Kite Pharma, Merck, Pharmacyclics, Portola Pharmaceuticals, Seattle Genetics, Takeda Pharmaceuticals, TG Therapeutics, Inc., Trillium Therapeutics, Inc., and Verastem Inc. Dr. Patel has no conflict of interest to declare. Dr. Oki declares having received honoraria from Bristol-Myers Squibb and Takeda, and research funding from Infinity Pharmaceuticals, Inc., Rhizen Pharmaceuticals S.A., and Curis Inc. Dr. Horwitz has received research funding/grant support from Celgene, Millennium Pharmaceuticals, Seattle Genetics, Spectrum Pharmaceuticals, and Infinity Pharmaceuticals, and consulting fees/honoraria from Celgene, Millennium Pharmaceuticals, Seattle Genetics, and Spectrum Pharmaceuticals. Dr. Foss has received consulting fees as well as funding for a clinical study from Infinity Pharmaceuticals. Authors Allen, Douglas, Stern, Sweeney, Kharidia, and P. Kelly are former employees of Infinity Pharmaceuticals. Dr. Kahl has received consulting fees from Infinity Pharmaceuticals, Gilead, Pharmacyclics, Roche, Millennium, Seattle Genetics, and Cell Therapeutics. Dr. V.M. Kelly is a former employee of Infinity Pharmaceuticals, Inc. and currently a consultant for Verastem, Inc.




