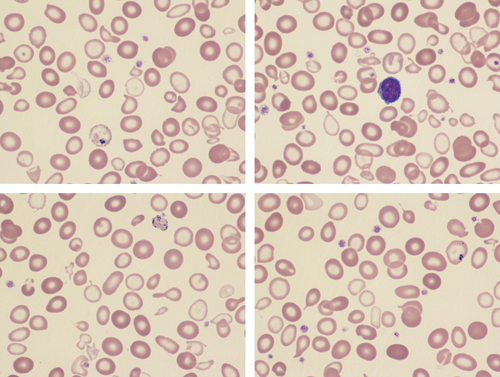
Congenital sideroblastic anemia in a female
A 20-year-old female of Pakistani descent was referred because of a microcytic hypochromic anemia refractory to oral iron that had been present since early childhood. A diagnosis of thalassemia intermedia had previously been suspected but high performance liquid chromatography showed normal hemoglobin A2 and F levels and no mutation of the α- or β-globin genes was detected by DNA sequencing or deletion analysis. Her parents were consanguineous. Neither parent had any hematological abnormality. On physical examination she was of short stature (<4th centile) but exhibited no dysmorphism or other somatic abnormalities. A blood count showed a hemoglobin concentration of 70 g/L with a normal red cell count, MCV of 66.6 fL and MCH of 18.3 pg. The reticulocyte count was 58.6 × 109/L and there were no biochemical markers of hemolysis. Transferrin saturation and serum ferritin were raised at 96% and 349 μg/L, respectively. A blood film was dimorphic with a large proportion of poorly hemoglobinized microcytes (Images); there was anisopoikilocytosis with dacrocytes, elliptocytes, and occasional target cells. Pappenheimer bodies were present, some being very large (bottom right image).The morphological features suggested sideroblastic anemia, most likely congenital in the context. To confirm this, next-generation sequencing of a comprehensive gene panel for inherited red cell disorders was undertaken. This identified a homozygous mutation of the SLC25A38 gene c.401G>A p.(Arg134His). This involves a highly conserved amino acid and has previously been described as a pathogenic variant in autosomal recessive pyridoxine-refractory sideroblastic anemia.1 Both parents were heterozygous for this variant.
Congenital sideroblastic anemias are a rare group of inherited disorders characterized by the pathological deposition of iron in mitochondria of erythroid precursors in the bone marrow leading to ineffective erythropoiesis with iron overload, and hypochromic red cells in the peripheral blood. They can be divided into syndromic and nonsyndromic forms and are genetically heterogeneous with nine monogenic forms and others due to mitochondrial DNA deletion. Nonsyndromic forms are either X-linked or autosomal recessive. Their morphological hallmark is the presence of ring sideroblasts in the bone marrow. These represent iron-laden mitochondria which form a ring around the nucleus of erythrocyte precursors. This feature points to a common pathophysiology due to genetic mutations that disrupt heme–iron biosynthesis in the mitochondria. SLC25A38 encodes an erythroid-specific mitochondrial carrier protein that transports glycine into mitochondria for the first step in heme synthesis. In this case molecular diagnosis prompted by characteristic morphological atypia obviated the need for bone marrow examination and enabled genetic counselling and screening to be provided to the patient and her family.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest with the contents of this article.