Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: Results of two phase 3, randomized, placebo-controlled trials
Abstract
Spleen tyrosine kinase (Syk) signaling is central to phagocytosis-based, antibody-mediated platelet destruction in adults with immune thrombocytopenia (ITP). Fostamatinib, an oral Syk inhibitor, produced sustained on-treatment responses in a phase 2 ITP study. In two parallel, phase 3, multicenter, randomized, double-blind, placebo-controlled trials (FIT1 and FIT2), patients with persistent/chronic ITP were randomized 2:1 to fostamatinib (n = 101) or placebo (n = 49) at 100 mg BID for 24 weeks with a dose increase in nonresponders to 150 mg BID after 4 weeks. The primary endpoint was stable response (platelets ≥50 000/μL at ≥4 of 6 biweekly visits, weeks 14-24, without rescue therapy). Baseline median platelet count was 16 000/μL; median duration of ITP was 8.5 years. Stable responses occurred in 18% of patients on fostamatinib vs. 2% on placebo (P = .0003). Overall responses (defined retrospectively as ≥1 platelet count ≥50 000/μL within the first 12 weeks on treatment) occurred in 43% of patients on fostamatinib vs. 14% on placebo (P = .0006). Median time to response was 15 days (on 100 mg bid), and 83% responded within 8 weeks. The most common adverse events were diarrhea (31% on fostamatinib vs. 15% on placebo), hypertension (28% vs. 13%), nausea (19% vs. 8%), dizziness (11% vs. 8%), and ALT increase (11% vs. 0%). Most events were mild or moderate and resolved spontaneously or with medical management (antihypertensive, anti-motility agents). Fostamatinib produced clinically-meaningful responses in ITP patients including those who failed splenectomy, thrombopoietic agents, and/or rituximab. Fostamatinib is a novel ITP treatment option that targets an important mechanism of ITP pathogenesis.
1 INTRODUCTION
Immune thrombocytopenia (ITP) is an autoantibody-mediated bleeding disorder, characterized primarily by accelerated platelet destruction and also by impairment of megakaryocyte function.1, 2 The constellation of symptoms varies between patients and can include severe thrombocytopenia, with bleeding ranging from none to skin and mucosal bleeding, to intracranial, gastrointestinal, and genitourinary bleeding.2-4 Mortality rates are very low but increase with age, certain comorbidities, and long-term severe disease.3, 5 Quality of life is often impaired in areas of physical functioning and mental health.6-8
Patients needing treatment usually receive steroids with or without intravenous immunoglobulin (IVIG) as first-line therapy, but the great majority of patients require additional treatment due to intolerability or relapse. Current guidelines for second-line and subsequent therapies are broad and nonprescriptive. There are many therapies available, but no comparative, randomized, controlled studies exist to facilitate treatment choice.9, 10 Splenectomy is the only potentially curative approach, which is effective in a majority of patients initially, but some patients subsequently relapse. This surgical option is declining in use due to the availability of noninvasive options.11 Medical therapies, such as rituximab, thrombopoietin receptor agonists, and immunosuppressive agents, have been effective in many patients and are chosen according to physician and patient preference. Some patients do not respond, or respond but relapse, when treated with second line treatments and develop persistent and then chronic disease. Those who do not improve often cycle endlessly through the various treatment options. Despite the multiple agents available, there is an unmet medical need for patients with ITP.6, 7, 12, 13
Fostamatinib, an orally bioavailable, small molecule spleen tyrosine kinase (Syk) inhibitor, was approved in April 2018 by the US Food and Drug Administration (FDA) for the treatment of chronic ITP in adults who have had an insufficient response to a prior therapy. All activating Fc receptors signal via Syk, which has roles in cellular proliferation, differentiation, survival, immune regulation, and cytoskeletal rearrangements during phagocytosis.14-16 Syk is also linked to signaling of the B cell receptor and has been implicated in autoantibody production.17 Inhibition of Syk using fostamatinib reduced platelet destruction in a mouse model of passive ITP, underscoring the role of Syk in phagocytosis of antibody-coated platelets.18 Clinical results with fostamatinib were first reported in patients with chronic ITP in a single center study in 2009. Of 16 patients, 8 (50%) achieved long-term responses, with manageable adverse events (AEs).18 Fostamatinib was also extensively studied in rheumatoid arthritis, demonstrating efficacy and establishing a safety profile in a total of more than 3000 patients.19-25 This report describes two identical, parallel, multicenter, randomized, double-blind, placebo-controlled, phase 3 studies comparing fostamatinib to placebo in 150 adults with persistent and primarily chronic ITP of very long-lasting duration, who had failed a number of prior treatments.
2 METHODS
2.1 Study design
FIT1 (NCT02076399) and FIT2 (NCT02076412) were identically-designed, parallel, phase 3, multicenter, randomized, double-blind, placebo-controlled trials of fostamatinib compared to placebo for the treatment of persistent/chronic ITP. FIT1 patients enrolled from July 2014 to April 2016 at 35 centers in North America, Australia, and Europe; FIT2 patients enrolled from January 2015 to August 2016 at 23 centers in Europe. Patients were randomized 2:1 to receive fostamatinib or placebo twice daily (BID) by mouth for 24 weeks. Randomization was stratified to balance patients with prior splenectomy and degree of thrombocytopenia (platelet count < or ≥15 000/μL). Both studies were approved by independent ethics committees at participating centers, performed in accordance with the Declaration of Helsinki, and designed in accordance with FDA Guidance. All patients provided written informed consent.
2.2 Patients
Eligible patients had persistent/chronic ITP, in accordance with the American Society of Hematology 2011 Practice Guidelines10 and 2014 European Medicines Agency guideline on clinical development of medicinal products for ITP.26 Eligible patients were ≥18 years of age with primary ITP for at least 3 months. The average platelet counts had to be <30 000/μL, based on ≥3 qualifying counts (2 during screening) within the 3 months preceding study entry (and no counts >35 000/μL unless from rescue therapy). Other inclusion criteria included ≥1 prior treatment of ITP and Karnofsky score ≥70. Exclusion criteria included: secondary ITP, a major cardiovascular event, coagulopathy (including prothrombotic conditions such as Factor V Leiden, APC resistance, AT-III deficiency and lupus anticoagulant, or arterial or deep venous thrombosis) within 6 months, ITP Bleeding Scale Grade 2 at the screening visit,27 poorly-controlled hypertension, or disorders that, in the Investigator's opinion, could affect the conduct of study.
2.3 Treatments
Prior to randomization, patients had a washout period, during which therapeutic agents, other than those allowed as concomitant ITP therapies, were discontinued for up to 8 weeks. Patients were allowed to continue one concomitant ITP medication (corticosteroids at <20 mg prednisone equivalent per day, azathioprine, or danazol) throughout the study without any changes if they were on a stable dose for 14 days prior to baseline. Rescue therapies (e.g., increased dosing of concomitant ITP therapy, IVIg, IV anti-D, steroids, platelet transfusion) were allowed.
Fostamatinib was administered at 100 mg BID and could be increased to 150 mg BID after 4 weeks or later, depending on platelet count. Doses could be reduced to fostamatinib 100 or 150 mg once daily if a dose-limiting AE occurred. The need for dose modifications was determined during biweekly visits.
Patients who completed the 24-week study treatment could enroll in the long-term, open-label extension study (Study 049; NCT02077192). Nonresponders who were treated for ≥12 weeks and received 150 mg BID of study drug for ≥4 weeks could also enter the extension study.
2.4 Efficacy and safety assessments
The primary efficacy endpoint was a stable response by week 24 (defined as platelet counts ≥50 000/μL on at least 4 of the 6 clinic visits occurring every 2 weeks during weeks 14–24 inclusive); patients receiving rescue medication after week 10 were considered nonresponders. A secondary efficacy platelet endpoint was measured in patients with baseline platelet count <15 000/μL: achievement of platelet counts ≥30 000/μL and at least 20 000/μL above baseline at weeks 12 and 24. Bleeding events were tabulated both for efficacy and safety. Data from FIT1and FIT2 were analyzed separately and pooled. Safety assessments and platelet counts were conducted during biweekly clinic visits 13 times over the 24-week treatment period.
2.5 Post hoc assessments
Post hoc assessments included overall response, which was defined as a platelet count ≥50 000/μL within the first 12 weeks without rescue medication in the preceding 4 weeks; the initial 12 weeks were selected since most nonresponding patients from both arms discontinued FIT1 and FIT2 and entered the open-label extension study at or very soon after week 12. Other post hoc analyses included the time to response and median platelet counts according to response (overall or stable response and none). Responders were categorized by age, sex, prior splenectomy, prior rituximab use, prior thrombopoietin receptor agonist (TPO-RA) use, ITP duration, and baseline platelet count.
2.6 Anti-platelet antibody assay
Plasma from 60 patients in the fostamatinib group was collected 2 weeks after the first dose of fostamatinib and analyzed for the presence of platelet autoantibodies (Lifecodes PakLx kit, Immucor GTI Diagnostics, Waukesha, WI, and indirect platelet immunofluorescence test [iPIFT]). Platelet suspensions from 2 healthy donors were used for autoantibody detection. Details are provided in the Supplemental Material.
2.7 Statistical analyses
Analysis populations included: (1) intent-to-treat (ITT): all randomized patients, primary population for efficacy analyses; and (2) safety: all randomized patients receiving a dose of study drug, the primary population for safety analyses. Sample size (75 patients per study: 50 fostamatinib, 25 placebo) was calculated to provide 90% power for the primary efficacy endpoint, using 2-sided, Fisher's Exact Test with alpha level of 0.05 and 2:1 fostamatinib:placebo allocation, assuming true proportions 0.40 for fostamatinib and 0.05 for placebo. One patient from FIT2, randomized to placebo, incorrectly received fostamatinib for 2 weeks; that patient's efficacy data were analyzed with the placebo group, but safety data with the fostamatinib group.
The primary efficacy endpoint was analyzed using the prespecified imputation method of last observation carried forward (LOCF). A sensitivity analysis was conducted such that missing data were imputed as <50 000/µL (nonresponse). One patient in FIT2 randomized to fostamatinib had 5 consecutive study counts > 50 000/μL culminating at week 16; the patient then moved and thus had to leave the study. This patient was considered a stable responder in the prespecified analysis using LOCF and was a nonresponder in the sensitivity analysis.
Efficacy endpoints were summarized using counts and percentages, and 95% exact (Clopper-Pearson) confidence intervals (CI). A 2-sided Fisher's Exact Test with significance level of 0.05 was used to evaluate efficacy. AEs were coded by the Medical Dictionary for Regulatory Activities (MedDRA) v18.1
3 RESULTS
3.1 Patient demographics, baseline characteristics, and disposition
Patient characteristics at baseline were well-balanced between the placebo and fostamatinib groups, including age (median 53 vs 54 years), sex (61% vs 60% female), and number of prior treatments (median 3 for both groups, range 1–13) except that FIT2 placebo patients had higher median platelet counts (Table 1). Patient characteristics were similar between FIT1 and FIT2 except that FIT1 and FIT2 patients were from different geographic areas. Compared to FIT1 patients, FIT2 patients had a longer median duration of ITP, lower rates of prior splenectomy, IVIg/anti-D, TPO-RA, and rituximab use, and higher rates of prior cyclophosphamide, vinca alkaloids, and danazol (Table 1).
| FIT1 | FIT2 | Pooled | ||||
|---|---|---|---|---|---|---|
| Placebo(n = 25) | Fostamatinib(n = 51) | Placebo(n = 24) | Fostamatinib(n = 50) | Placebo(n = 49) | Fostamatinib(n = 101) | |
| Age, median (range), years | 57 (26–77) | 57 (20–88) | 50 (20–78) | 50 (21–82) | 53 (20–78) | 54 (20–88) |
| Sex, n (%) | ||||||
| Female | 17 (68) | 30 (59) | 13 (54) | 31 (62) | 30 (61) | 61 (60) |
| Male | 8 (32) | 21 (41) | 11 (46) | 19 (38) | 19 (39) | 40 (40) |
| Race, n (%) | ||||||
| White | 21 (84) | 44 (86) | 24 (100) | 50 (100) | 45 (92) | 94 (93) |
| Asian | 2 (8) | 3 (6) | 0 | 0 | 2 (4) | 3 (3) |
| Black/African American | 2 (8) | 2 (4) | 0 | 0 | 2 (4) | 2 (2) |
| Other | 0 | 2 (4) | 0 | 0 | 0 | 2 (2) |
| Region, n (%) | ||||||
| North America | 8 (32) | 17 (33) | 0 | 0 | 8 (16) | 17 (17) |
| Europe | 13 (52) | 25 (49) | 24 (100) | 50 (100) | 37 (76) | 75 (74) |
| Australia | 4 (16) | 9 (18) | 0 | 0 | 4 (8) | 9 (9) |
| ITP Classification, n (%) | ||||||
| Persistent | 3 (12) | 3 (6) | 1 (4) | 3 (6) | 4 (8) | 6 (6) |
| Chronic | 22 (88) | 48 (94) | 23 (96) | 47 (94) | 45 (92) | 95 (94) |
| Duration of ITP, median (range), years | 5.5 (0.4–45.0) | 7.5 (0.6–53.0) | 10.8 (0.9–29.1) | 8.8 (0.3–50.2) | 7.8 (0.4–45) | 8.7 (0.3–53) |
| Duration of ITP ≥3 years, n (%) | 17 (68) | 38 (75) | 18 (75) | 38 (76) | 35 (71) | 76 (75) |
| Prior unique treatments for ITP, median (range) | 5.0 (1–10) | 3.0 (1–9) | 3.0 (1–10) | 3.0 (1–13) | 3.0 (1–10) | 3.0 (1–13) |
| Prior treatments, n (%) | ||||||
| Corticosteroids | 25 (100) | 46 (90) | 22 (92) | 48 (96) | 47 (96) | 94 (93) |
| IVIg or IV Anti-D | 17 (68) | 33 (65) | 10 (42) | 19 (38) | 27 (55) | 52 (51) |
| Thrombopoietic agents | 15 (60) | 27 (53) | 10 (42) | 20 (40) | 25 (51) | 47 (47) |
| Immunosuppressants | 12 (48) | 22 (43) | 10 (42) | 22 (44) | 22 (45) | 44 (44) |
| Splenectomy | 10 (40) | 20 (39) | 9 (38) | 14 (28) | 19 (39) | 34 (34) |
| Rituximab | 11 (44) | 26 (51) | 3 (13) | 8 (16) | 14 (29) | 34 (34) |
| Danazol | 4 (16) | 7 (14) | 5 (21) | 13 (26) | 9 (18) | 20 (20) |
| Chemotherapy | 2 (8) | 4 (8) | 4 (17) | 5 (10) | 6 (12) | 9 (9) |
| Other (Dapsone) | 3 (12) | 10 (20) | 0 | 0 | 3 (6) | 10 (10) |
| Baseline platelet count, mean, /μL (range) |
15 844 (1000-48 000) |
16 202(1000-51 000) | 23 958(1000-156 000) | 15 900(1000-33 000) | 19 818(1000-156 000) | 16 052(1000-51 000) |
| Baseline Platelet count of <15 000 /μL, n (%) | 13 (52) | 26 (51) | 15 (63) | 28 (57) | 28 (57) | 54 (54) |
The patient characteristics illustrate a difficult-to-treat population with long-standing ITP (median of 8.5 years and approximately 75% had had ITP for ≥3 years), many attempts at prior therapy for ITP (median of 3 unique prior therapies and as many as 13), and an average platelet count at baseline that is typically associated with bleeding episodes (more than half were below 15 000/μL).
The 24 weeks of treatment were completed by more patients on fostamatinib compared with patients on placebo (Supporting Information Figure S1). Rates of and reasons for study discontinuation were similar between studies. The majority of nonresponders on fostamatinib and most patients on placebo discontinued study treatment at Week 12 to enter the open-label, long-term extension study: 88% of patients in FIT1 and 79% in FIT2 on placebo vs. 55% in FIT1 and 66% in FIT2 on fostamatinib.
3.2 Efficacy
More patients on fostamatinib achieved the primary endpoint of stable response (platelets > 50 000/µL without rescue at 4 of 6 visits, weeks 14–24) compared with placebo. Stable responses occurred in 9 of 51 (18%) patients on fostamatinib vs. 0 of 25 patients on placebo in FIT1 (P = .026), and in 9 of 50 (18%) on fostamatinib vs. 1 of 24 (4%) patients on placebo in FIT2 (P = .152; Figure 1A). In the pooled analysis, stable responses were seen in 18 of 101 (18%) on fostamatinib vs. 1 of 49 (2%) on placebo (P = .0003). Among stable responders, 15 of 18 (83%) responded at 5 of 6 clinic visits and 14 of 18 (77%) at all 6 clinic visits. The primary analysis was conducted using the prespecified imputation method of last observation carried forward. Using the most conservative sensitivity analysis where missing data are imputed as <50 000/µL, one patient in FIT2 is then considered a nonresponder. The FIT1 findings were unchanged. In FIT2, stable responses occurred in 8 of 50 (16%) on fostamatinib vs. 1 of 24 (4%) patients on placebo (P = .2559). In the pooled sensitivity analysis, stable responses were seen in 17 of 101 (17%) vs. 1 of 49 (2%) on fostamatinib and placebo respectively (P = .0071). These findings are not substantively changed between the two analyses.
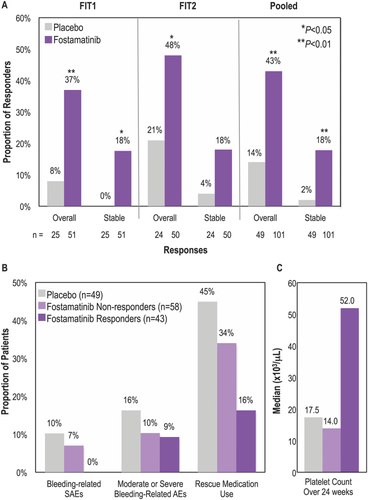
Platelet responses, median platelet counts, bleeding-related events and rescue medication use by response. (A) Proportion of patients on placebo or fostamatinib who achieved a stable response (platelet counts ≥50 000/μL on at least 4 of 6 biweekly clinic visits during weeks 14–24, using the prespecified imputation method of last observation carried forward) or an overall response (≥1 platelet count ≥50 000/μL during weeks 0–12) in the FIT1, FIT2, and pooled populations. (B) Proportion of overall responders, nonresponders and placebo patients in the pooled population with bleeding-related SAEs, moderate or severe bleeding-related AEs, or requiring rescue medication. Bleeding-related AEs included epistaxis, menorrhagia, contusion, gastrointestinal hemorrhage, ITP, petechiae, and vaginal hemorrhage. (C) Median platelet count over 24 weeks in overall responders, nonresponders and placebo patients in the pooled population
On fostamatinib, 43 of 101 (43%) patients achieved an overall response, defined as at least 1 platelet count > 50 000/μL within the first 12 weeks (including stable responders), compared with 7 of 49 (14%) on placebo (P = .0006; Figure 1A). Overall responses occurred in 37% of patients on fostamatinib vs. 8% on placebo in FIT1 (P = .007), and in 48% on fostamatinib vs. 21% on placebo in FIT2 (P = .025). Among patients with more severe thrombocytopenia at baseline (platelet counts <15 000/µL), an increase of ≥20 000/µL to a platelet count of ≥30 000/µL at weeks 12 and 24 was achieved by 10/47 (21%) and 7/47 (15%) on fostamatinib and by 1/21 (5%) and 0/21 (0%) on placebo in the pooled analysis.
The median duration of ITP at study entry was 8.7 years for stable responders, 6.5 years for overall responders and 10.3 years for nonresponders. The 8 patients between both studies with persistent ITP had 6 overall responses, 1 of which was a stable response. Overall responses were seen across a wide range of ITP durations, including those with a duration of <3 years (52%), 3 to <8 years (48%) and ≥8 years (36%); however, there was no correlation of response with duration of ITP at study entry.
Median platelet counts are shown in Figure 1B. Among stable and overall responders, median platelet counts increased by week 2 on fostamatinib and remained above 50 000/μL at nearly all time points (Figures 2A,B). By contrast, the median platelet counts for nonresponders and placebo patients remained well below 50 000/μL for the duration of the study with one exception. The median platelet count spiked at Week 18 in the placebo group based on 2 patients, the one placebo responder and one placebo nonresponder with fluctuating platelet counts. In the responder groups, fluctuations in median platelet counts were more apparent in the first 4 weeks due to the use of rescue medication, which caused the exclusion of Week 2 data from 5 and 3 patients in the overall and stable responder groups, respectively.
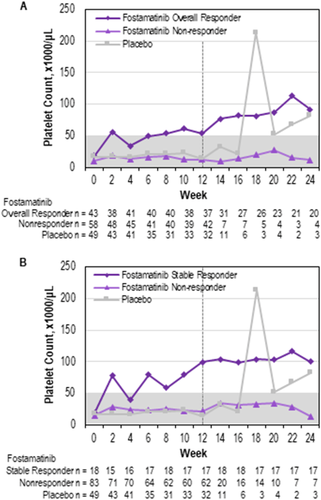
Platelet counts over time. (A) Median platelet count over time from 0 to 24 weeks in overall responders to fostamatinib, nonresponders, and placebo patients. (B) Median platelet count over time from 0 to 24 weeks in stable responders to fostamatinib, nonresponders, and placebo patients. Dotted line at week 12 represents cutoff time for response; nonresponders were allowed to enroll in the extension study at week 12
The median time to a platelet count ≥50 000/μL was 15 days for overall responders and 15.5 days for stable responders, at which time all patients were still receiving 100 mg BID dosing except one patient who had decreased the dose to 150 mg daily. A response was achieved by Week 4 in 22 (51%) overall responders and 11 (61%) stable responders. Of 101 patients on fostamatinib, 89 (88%) increased their dose to 150 mg BID at or after week 4, including 88% of stable responders. The median platelet count over the 24 weeks was 95 000/μL for the stable responders and 49 000/μL for overall responders.
Responses to fostamatinib were observed across all subgroups of age, sex, prior therapy (splenectomy, rituximab, or TPO-RA), baseline platelet count (≤ vs. >15 000/µL), or duration of ITP at study entry (Supporting Information Figure S2). Of the 47 patients with prior TPO-RA experience, 8 (17%) had a stable response, and all 8 had discontinued prior TPO-RA therapy due to a lack of effect. Numerically more of the younger patients (<65 years) responded to fostamatinib than older patients, and numerically more patients with platelet counts of 15–30 000/μL responded to fostamatinib compared to those with counts <15 000; however, sample sizes were small. Ten of 28 (36%) patients with detectable antiplatelet antibodies achieved stable responses to fostamatinib compared to 3 of 32 (9%) without detectable antibodies (see Supporting Information).
Patients were allowed to continue one concomitant ITP medication throughout the study without any changes if they were on a stable dose for 14 days prior to baseline, and 70 (46%) patients did so. Of those, 59 (39.3%) were on corticosteroids, 5 (3.3%) were on immunoglobulins, 7 (4.7%) were on azathioprine, and 2 (1.3%) were on danazol. Only 2 patients with a stable platelet response were on a concomitant medication, and both were on steroids (for 62 days and 14 years prior to first dose of fostamatinib). Of the 43 patients with an overall platelet response, 4 patients were on a concomitant medication, including 3 on steroids (for 62 days, 67 days and 14 years prior to first dose of fostamatinib) and 1 on azathioprine (for 197 days prior to the first dose of fostamatinib). The effect of these concomitant medications would very likely have been evident prior to study entry and thus are not considered likely to have influenced the results.
3.3 Bleeding-related events and rescue medication use
Moderate/severe bleeding-related AEs occurred in 4 of 43 (9%) overall responders to fostamatinib, (1/18 [6%] stable responders) vs. 8/49 (16%) patients on placebo (Figure 1B). Bleeding-related SAEs did not occur in any responders to fostamatinib compared with 5/49 (10%) patients on placebo.
There was a trend for patients on fostamatinib to receive rescue medication less often than those on placebo (30% vs. 45%, respectively; P = .07). Among overall responders to fostamatinib, 7/43 (16%) received rescue medication vs. 20/58 (34%) nonresponders and 22/49 (45%) patients on placebo. Among stable responders, 3/18 (17%) received rescue medication but only during the first week of treatment. In contrast, nonresponders received rescue medication throughout the study period (weeks 0–24).
3.4 Safety
In FIT1 and FIT2, 83% and 75% of patients experienced AEs in the fostamatinib and placebo groups, respectively. The most commonly reported AEs were diarrhea, nausea, hypertension, dizziness, and ALT and/or AST increases (Table 2). Overall infections were slightly more frequent in patients on fostamatinib than placebo (30% vs. 21%, respectively), but rates of moderate or severe infections were similar (8% vs. 6%, respectively). Most cases of AEs were either mild (39% on fostamatinib and 56% on placebo) or moderate (42% on fostamatinib and 25% on placebo).
| Adverse reaction | Fostamatinib (N = 102) | Placebo (N = 48) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mild % | Moderate % | Severe % | Total % | Mild % | Moderate % | Severe % | Total % | |
| Any AE | 32 | 35 | 16 | 83 | 42 | 19 | 15 | 75 |
| Diarrheaa | 21 | 10 | 1 | 31 | 13 | 2 | 0 | 15 |
| Hypertensionb | 17 | 9 | 2 | 28 | 10 | 0 | 2 | 13 |
| Nausea | 16 | 3 | 0 | 19 | 8 | 0 | 0 | 8 |
| Dizziness | 8 | 2 | 1 | 11 | 6 | 2 | 0 | 8 |
| ALT increased | 5 | 6 | 0 | 11 | 0 | 0 | 0 | 0 |
| AST increased | 5 | 4 | 0 | 9 | 0 | 0 | 0 | 0 |
| Respiratory infectionc | 7 | 4 | 0 | 11 | 6 | 0 | 0 | 6 |
| Rashd | 8 | 1 | 0 | 9 | 2 | 0 | 0 | 2 |
| Abdominal paine | 5 | 1 | 0 | 6 | 2 | 0 | 0 | 2 |
| Fatigue | 4 | 2 | 0 | 6 | 0 | 2 | 0 | 2 |
| Chest pain | 2 | 3 | 1 | 6 | 2 | 0 | 0 | 2 |
| Neutropeniaf | 3 | 2 | 1 | 6 | 0 | 0 | 0 | 0 |
- a Includes diarrhea and frequent bowel movement.
- b Includes hypertension, blood pressure (BP) increased, BP diastolic abnormal, and BP diastolic increased.
- c Includes upper respiratory tract infection, respiratory tract infection, lower respiratory tract infection, and viral upper respiratory tract infection.
- d Includes rash, rash erythematous and rash macular.
- e Includes abdominal pain, and abdominal pain upper.
- f Includes neutropenia and neutrophil count decreased.
- ALT, alanine aminotransferase; AST, aspartate aminotransferase.
- Note: Common adverse reactions defined as all adverse reactions occurring at a rate of ≥ 5% of patients in the fostamatinib group and greater than placebo rate.
Similar rates of AEs leading to treatment withdrawal were reported between treatment arms (10% fostamatinib vs. 8% placebo) but at different rates between studies (FIT1: 16% fostamatinib vs. 8% placebo; FIT2: 4% vs. 9%). Severe AEs leading to treatment withdrawal in the fostamatinib arm included 1 case each of nonserious chest pain and syncope, pneumonia, and thrombocytopenia. Three of 43 overall responders but none of the 18 stable responders discontinued treatment due to AEs. Patients on fostamatinib had higher rates of AEs leading to dose reductions (9% fostamatinib vs. 2% placebo) and temporary dose interruptions (18% vs. 10%). The most common AEs (2%) leading to fostamatinib dose reductions were diarrhea and hypertension. The most common AEs (≥2%) leading to fostamatinib dose interruptions were ALT increased, diarrhea, and influenza-like illness.
AEs known to occur with fostamatinib and assessed as groups of relevant preferred terms included: neutropenia (7% on fostamatinib vs 0% on placebo), gastrointestinal events (41% vs 21%), transaminase elevation to > 3 times normal (9% vs 0%; no drug-induced liver injury in either group), and hypertension-type events (28% vs 13%).
SAEs were experienced by 13% of patients on fostamatinib and 21% on placebo. Only 3 types of SAEs were reported in more than 1 patient in either treatment group: epistaxis (2% fostamatinib vs. 2% placebo), thrombocytopenia (1% vs. 4%), and menorrhagia (0% vs. 4%). SAEs were considered related to study drug in 4% of patients on fostamatinib and 2% on placebo. There were 2 fatalities. In FIT1, a patient on placebo died of probable sepsis 19 days after discontinuing the study due to epistaxis. In FIT2, plasma cell myeloma led to withdrawal of fostamatinib on Day 19 and death 71 days later. There were no thromboembolic events.
4 DISCUSSION
FIT1 and FIT2 were identical, parallel, double-blind, randomized phase 3 trials evaluating a Syk inhibitor, fostamatinib, vs. placebo in a total of 150 adult patients with persistent or (predominantly) chronic ITP. The studies were designed in accordance with FDA guidance, using standard methods to avoid bias, and the efficacy endpoints were based on an objective laboratory assessment of platelet count. These phase 3 studies were the first to evaluate second- or third-line treatment for ITP in the current era of widespread use of TPO-RA and rituximab. FIT1 and FIT2 were relatively large studies, with 75 patients each, of a “rare” disease (ITP). These 2 studies included patients with a very long duration of disease and a median of 3 prior treatments. In the pooled analysis, stable platelet response, achieved by 18 of 101 patients on fostamatinib, and overall platelet response, achieved by 43 of 101 patients on fostamatinib, were both significantly higher in fostamatinib-treated patients than in placebo-treated patients. Furthermore, the overall (including the stable) responses were associated with reductions in both bleeding and use of rescue medication. These findings demonstrate that almost half of patients had overall platelet responses to fostamatinib, despite some not reaching the primary endpoint, and that these responses were clinically meaningful, based on decreased bleeding events and a decreased need for rescue medication and not just numerical values. The efficacy findings were robust since the responses were similar between the two studies and consistent with the phase 2 ITP study results. These studies led to the FDA approval of fostamatinib for the treatment of chronic ITP in adults who have had an insufficient response to a prior therapy.
The primary endpoint and inclusion criteria used in this study were more stringent than those used in certain studies of avatrombopag and eltrombopag, in which eligibility platelet counts were assessed on a single day.28-31 The primary endpoint in FIT1 and FIT2 required patients to achieve high platelet counts (>50 000/μL) at multiple timepoints (4 of 6 over weeks 14–24), which is similar (although not identical) to the pivotal romiplostim studies. In the latter study, the durable response endpoint was met by 38% of splenectomized patients, comparable to what was seen here.32 To provide a more complete description of meaningful responses elicited by fostamatinib, an additional post hoc efficacy endpoint of overall platelet response (>50 000/μL) within the first 12 weeks of the study was assessed. Overall responders had elevated median platelet counts corresponding to more consistently increased platelet counts rather than just one increased count, supporting the appropriateness of this post hoc endpoint.
Most patients (75%) in the study had ITP lasting more than 3 years (median 8.5 years) at study entry. Consistent with the long duration of disease, this was a population with at least 2–3 prior therapies in almost all cases. These exposures included frequent prior use of TPO-RA-based therapy (48%), rituximab (34%), and splenectomy (35%). More than half the patients had baseline platelet counts below 15 000/μL. During the study 16% of patients on placebo had moderate or severe bleeding, and 45% required rescue medication. Among these difficult-to-treat patients, Syk inhibition with fostamatinib produced clinically meaningful platelet responses within each group categorized by duration of ITP, prior therapy or baseline platelet count. For example, the rate of achieving the primary endpoint was identical for those patients who had tried and not responded to TPO-RA (17%) as it was for the overall population (18%). Furthermore, fostamatinib potentially represented the only effective treatment for certain patients. These were patients who had failed to respond to all of splenectomy, rituximab and TPO-RA. This suggests that inhibition of Syk has a unique role in the treatment of at least a small subset of adults with persistent and chronic ITP.
Responses to fostamatinib occurred regardless of age, gender, prior therapies (splenectomy, rituximab, TPO-RA), duration of ITP, and baseline platelet count; however, the study was not powered for correlation of response with these factors. Given the not significantly better but somewhat more favorable results in the 8 patients with persistent ITP and in those with ITP of <3 years duration, further studies appear warranted to determine whether treatment of patients with shorter duration of ITP would be associated with higher response rates to fostamatinib. Other predictors of response tentatively identified in this study included age <65 years, baseline platelet count of 15–30 000/μL, and a potential association with the presence of anti-platelet autoantibodies. The latter exploratory analysis and the mouse studies18 support the hypothesis that fostamatinib prevents platelet loss through inhibition of autoantibody-directed platelet destruction in ITP. In turn this suggests that patients with predominantly autoantibody-mediated ITP may be more responsive to Syk inhibition, as opposed to patients with T-cell mediated disease or other mechanisms. Similar findings of the predictive value of antibody testing were seen in one study of rituximab.33 Further studies are needed to explore clinical and laboratory factors predictive of fostamatinib sensitivity.
Dose selection for FIT1 and FIT2 was based on results of the phase 2 ITP study where doses up to 175 mg BID were associated with better response rates but apparently more toxicity. Therefore, the phase 3 trials were designed to start patients at a dose of fostamatinib 100 mg BID, with a dose increase to 150 mg BID permitted at week 4. Almost all stable responders up-titrated to 150 mg BID to achieve and/or sustain their responses to fostamatinib. The degree of Syk blockade at these doses was not investigated in this study. The likelihood that higher doses of fostamatinib would yield higher response rates is uncertain, but there would likely be more adverse events.
The safety profile of fostamatinib was consistent with prior experience in the phase 2 ITP study and the large rheumatoid arthritis studies; no new AEs of note were seen.18-24 The most commonly reported AEs included diarrhea, hypertension, nausea, and transaminase elevation. An increased rate of hypertension has been previously reported with fostamatinib in the Phase 2 ITP study and studies of rheumatoid arthritis20, 21 and appears to be due to off-target effects on VEGFR2.34-36 Most AEs were mild or moderate. Gastrointestinal disorders and hypertension were medically managed as needed with antihypertensive and anti-motility agents.37 The number of overall responders discontinuing fostamatinib was small (3 of 43) and did not include any stable responders; however, other patients required dose interruption or dose reduction because of adverse events and also medication to manage their AEs.
Limitations to these paired studies included the very long duration of disease in enrolled patients, which exceeds that of other published ITP studies. Furthermore, the prior use of many other well-established treatments, e.g., immunosuppressives, rituximab, splenectomy, and especially TPO-RA agents, likely also biased the study against a good response to fostamatinib. Other studies have shown that responses to rituximab and anti-CD40 ligand were lower in ITP patients with very long disease duration.38, 39
Other effective therapies for ITP are thought to inhibit platelet destruction, e.g., IVIG, IV anti-D, vincristine, and danazol. IVIg and IV anti-D both inhibit platelet destruction but very likely impact different FcR receptors, which may explain their apparently additive effects.39, 40 There are multiple distinct pathways involved in immune-mediated platelet destruction, and treatments interfering with platelet destruction may have nonoverlapping effects.39 This could explain responses to fostamatinib in patients who were unresponsive to other therapies.
In summary, fostamatinib targets the Syk-mediated pathway of platelet-destruction, producing a stable response (>50 000/μL at 4 of 6 counts during weeks 14–24) in 18% of patients on fostamatinib compared with 2% on placebo and an overall response in 43% on fostamatinib compared to 14% in placebo. Fostamatinib has a unique mechanism of action, dissimilar to other approved ITP therapies, and produced responses in patients who had relapsed or not responded to TPO-RA agents, rituximab, and/or splenectomy. The ongoing open-label extension study will broaden the understanding of the long-term clinical efficacy and safety of fostamatinib in adults with ITP. Future studies will investigate whether higher response rates will be seen when fostamatinib is given earlier in the course of ITP, further study the mechanism of Syk inhibition, and explore which patients are most likely to respond to treatment.
ACKNOWLEDGMENTS
We thank the patients, study investigators, nurses, and coordinators for their participation in the study. The study was funded by Rigel Pharmaceuticals, Inc. Editorial support was provided by Ingrid Koo, PhD, and funded by Rigel Pharmaceuticals.
CONFLICT OF INTERESTS
JB has received research support from Rigel, Novartis, and Amgen, and has participated on advisory boards for Rigel, Novartis, Amgen, Momenta, and Protalex. DMA has received research support from Novartis, Amgen, and Bristol Meyers Squibb, and has been a consultant for Rigel, Amgen, Novartis, and UCB. EG has been an employee and consultant for Rigel and holds stock options from Rigel. JM has received research funding from Rigel. WH has received honoraria from Rigel and is a principal investigator for this study. JW has received grant/research/clinical trials support from Baxalta, Biogen Idec, Baxter Healthcare, Bayer, CSL Behring, Novo Nordisk, Octapharma, Roche, Sanofi, and Shire. FZ has been a consultant for Novartis, Celgene, Roche, Janssen, Gilead, Abbvie, and Sandoz, has participated on speaker bureaus for Novartis, Amgen, Celgene, Roche, Janssen, Gilead, Abbvie, and BMS, has spoken at educational presentations for Novartis, and has received travel fees for Roche, Celgene, Novartis, Amgen, and Takeda. NC has been a consultant and spoken at educational events for Novartis and Amgen. VM, HZ, and AMD are employees of Rigel Pharmaceuticals and hold stock options from Rigel. No other conflicts-of-interests from other authors have been declared.
AUTHOR CONTRIBUTIONS
All authors analyzed and interpreted the data, critically revised the manuscript, and approved the final draft. EG contributed to the design of the study. JB developed the initial draft of the manuscript and provided critical direction during the development of the manuscript; HZ performed the statistical analyses.




