Outcome of children with newly diagnosed acute lymphoblastic leukemia treated with CCLG-ALL 2008: The first nation-wide prospective multicenter study in China
Funding information: National Key Technology R&D Program of China (grant number 2007BAI04B03); the National Science and Technology Major Project of the Ministry of Science and Technology of China (grant number 2017ZX09304029); the National Natural Science Foundation of China (grant numbers 81200392, 81170504); the Beijing Natural Science Foundation (grant number 7152054); the Scientific Research Common Program of Beijing Municipal Commission of Education (KM201810025025); the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Grant (grant number ZY201404); the Beijing Municipal Administration of Hospitals DengFeng Program (No. DFL20151101); the Capital Health and Development of Special Grant (No. 2016-1-2091); the Tianjin Science and Technology Program (grant number 12ZCDZSY18100); the Children Cancer Foundation of Hong Kong and New Sunshine Charity Foundation of China
ClinicalTrials.gov identifier: NCT00707083
Abstract
Acute lymphoblastic leukemia (ALL) is the most common malignancy among children. The trial Chinese Children Leukemia Group (CCLG)-ALL 2008 was a prospective clinical trial designed to improve treatment outcome of childhood ALL through the first nation-wide collaborative study in China. Totally 2231 patients were recruited from ten tertiary hospitals in eight cities. The patients were stratified according to clinical-biological characteristics and early treatment response. Standard risk (SR) and intermediate risk (IR) groups were treated with a modified BFM based protocol, and there was 25%-50% dose reduction during intensification phases in the SR group. Patients in high risk (HR) group received a more intensive maintenance treatment. Minimal residual disease (MRD) monitoring with treatment adjustment was performed in two hospitals (the MRD group). Complete remission (CR) was achieved in 2100 patients (94.1%). At five years, the estimate for overall survival (OS) and event-free survival (EFS) of the whole group was 85.3% and 79.9%, respectively. The cumulative incidence of relapse (CIR) was 15.3% at five years. The OS, EFS and CIR for the SR group were 91.5%, 87.9%, and 9.7%, respectively. The outcome of the MRD group is better than the non-MRD group (5y-EFS: 82.4% vs 78.3%, P = .038; 5y-CIR: 10.7% vs 18.0%, P < .001). Our results demonstrated that the large-scale multicenter trial for pediatric ALL was feasible in China. Dose reduction in the SR group could achieve high EFS. MRD-based risk stratification might improve the treatment outcome for childhood ALL.
1 INTRODUCTION
Acute lymphoblastic leukemia (ALL) is the most common malignancy in children below age of 14 years.1 This is a heterogeneous disease as shown by variable gene expression in many studies.2-4 With advances in treatment through large-scale clinical trials, the long-term survival rates for childhood ALL were approaching 90% in many developed countries.5 However, treatment-related mortality was still high in the developing countries.6 With the improvement in socio-economic condition and the establishment of the national insurance system, more children with ALL can now receive appropriate treatment in China. The Chinese Children Leukemia Group (CCLG) was organized in 2008 with funding support from the National Key Technology R&D Program of China. The CCLG-ALL 2008 Study was designed to improve treatment outcome of childhood ALL through the first nation-wide collaborative study, involving ten tertiary hospitals from different parts of China. This study might establish a platform for future national based treatment strategy,7 and serve as a model for future similar studies for other types of childhood cancer and severe hematological disorders in the country.
The CCLG-ALL 2008 Study was based on BFM ALL treatment backbone with some modifications to reduce the treatment-related mortality. Here, we reported the treatment results of this trial involving 2231 children from China.
2 PATIENTS AND METHODS
2.1 Study design and participants
In this multicenter clinical trial, participation centers were hospitals with experience in treating children with ALL, equipped with dedicated facilities and personnel for diagnosis and treatment of ALL in China. The 10 centers from eight cities were either hematology departments of children's hospitals or hematology divisions of pediatric departments in university general hospitals. The participation centers followed the unified inclusion and exclusion criteria. All previously untreated patients presented to the ten centers were recruited. The five hospitals in Hong Kong were grouped as one institution because of small number of patients.
Between April 1, 2008 and December 31, 2012, a total of 2231 patients under age of 18 years with newly diagnosed ALL were recruited into the trial (Supporting Information Figure S1). The clinical study was approved by institutional ethical committees of all participating hospitals, and patients were only recruited when the parents or legal guardian provided written informed consent for the research study. Separate informed consent forms were obtained at the time of randomization of the maintenance arms. Standardized informed consent forms were applied in all participating centers. Assents from the patients were also obtained when the patients were old enough to understand the treatment and research study.
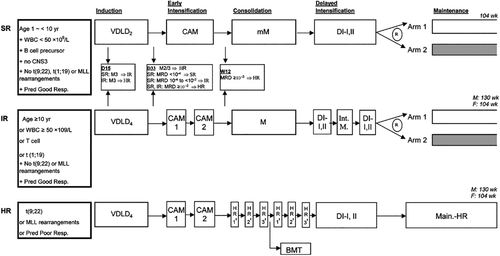
Treatment Outline in CCLG-ALL 2008 Study. Shaded boxes depict experimental arms of Maintenance for SR/IR. SR indicated standard risk; IR, intermediate risk; HR, high risk; CNS, central nervous system; Pred Good Resp., Prednisone Good Response; Pred Poor Resp., Prednisone Poor Response; VDLD2, Vincristine, Dexamethasone, L-Asparaginase and Daunorubicin (2 doses for SR); VDLD4, Vincristine, Dexamethasone, L-Asparaginase and Daunorubicin (4 doses for IR/HR); CAM, Cyclophosphamide, Cytarabine and 6-mercaptopurine; mM, Methotrexate (MTX) 2 g/m2 per dose for 4 doses; M, MTX 5 g/m2 per dose for 4 doses; DI, Delayed Intensification; Int. M., Interim maintenance; Main.-HR, Maintenance for HR
2.2 Procedures
The treatment strategy of CCLG-ALL 2008 study was shown in Figure 1, and the details of the treatment elements were provided in Supporting Information Table S1. ALL were diagnosed if at least 25% lymphoblasts were present in the bone marrow (BM). Immunophenotypic and karyotypic features of leukemic cells were determined according to standard techniques.8, 9 Cytogenetic study for TEL-AML1, BCR-ABL1, E2A-PBX1, and MLL rearrangement was tested by karyotyping, fluorescence in situ hybridization (FISH) and/or polymerase-chained reaction (PCR) methods.10 The central nervous system (CNS) status was defined as follow: CNS1, no detectable blast cells in Cerebrospinal fluid (CSF); CNS2, fewer than five leucocytes per μl with detectable blast cells in a cytospin preparation of CSF; and CNS3 or CNS involvement, five or more leucocytes per μl with identifiable blast cells, or the presence of cranial-nerve palsies, or intracerebral infiltrates on cranial computed tomography.
| Total (N = 2231) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Na | % | 5y-EFS,%(SE) | P | 5y-OS,%(SE) | P | SR, % (n = 868) | IR, % (n = 893) | HR, % (n = 470) |
| All | 2231 | 100 | 79.9(0.9) | 85.3(0.8) | 100 | 100 | 100 | ||
| Sex | |||||||||
| Male | 1337 | 59.9 | 78.5(1.2) | .143 | 84.2(1.1) | .130 | 56.9 | 60.4 | 64.7 |
| Female | 894 | 40.1 | 81.9(1.4) | 86.8(1.2) | 43.1 | 39.6 | 35.3 | ||
| Age(years) | |||||||||
| < 1 | 15 | 0.7 | 77.8(11.4) | < .001 | 71.8(12.0) | .001 | 0 | 0.8 | 1.7 |
| 1 -< 6 | 1393 | 62.4 | 82.9(1.1) | 87.3(1.0) | 79.8 | 55.4 | 43.6 | ||
| 6 -< 10 | 492 | 22.1 | 75.2(2.1) | 82.1(1.8) | 20.2 | 19.8 | 29.8 | ||
| ≥ 10 | 331 | 14.8 | 74.1(2.6) | 81.3(2.2) | 0 | 24.0 | 24.9 | ||
| Initial WBC(×109/L) | |||||||||
| < 20 | 1425 | 63.9 | 85.3(1.0) | 90.5(0.8) | 85.4 | 59.4 | 32.8 | ||
| 20 -< 50 | 348 | 15.6 | 77.3(2.5) | 79.3(2.4) | 14.6 | 14.1 | 20.4 | ||
| 50 -< 100 | 211 | 9.5 | 66.4(3.6) | < .001 | 73.7(3.4) | < .001 | 0 | 14.2 | 17.7 |
| 100 -< 200 | 129 | 5.8 | 69.7(4.2) | 79.3(3.8) | 0 | 7.4 | 13.4 | ||
| ≥ 200 | 118 | 5.3 | 55.4(5.0) | 62.8(4.6) | 0 | 4.9 | 15.7 | ||
| CNS status | |||||||||
| CNS1 | 2145 | 96.1 | 80.8(0.9) | < .001 | 85.9(0.8) | < .001 | 97.5 | 95.4 | 95.1 |
| CNS2 | 55 | 2.5 | 63.8(6.9) | 72.9(6.2) | 2.5 | 2.5 | 2.3 | ||
| CNS3 | 31 | 1.4 | 47.5(9.9) | 57.7(8.9) | 0 | 2.1 | 2.6 | ||
| Immunophenotype | |||||||||
| Precursor B | 2041 | 91.6 | 81.2(0.9) | < .001 | 86.2(0.8) | < .001 | 100 | 88.7 | 81.4 |
| T | 188 | 8.4 | 66.0(3.7) | 73.3(3.4) | 0 | 11.3 | 18.6 | ||
| BCR-ABL1 | |||||||||
| Negative | 2078 | 95.5 | 81.3(0.9) | < .001 | 86.3(0.8) | < .001 | 100 | 100 | 78.6 |
| Positive | 99 | 4.5 | 48.0(5.5) | 53.7(5.8) | 0 | 0 | 21.4 | ||
| TEL-AML1 | |||||||||
| Negative | 1787 | 83.7 | 78.3(1.1) | < .001 | 83.5(0.9) | < .001 | 77.1 | 84.7 | 93.6 |
| Positive | 349 | 16.3 | 89.5(1.8) | 93.0(1.5) | 22.9 | 15.3 | 6.4 | ||
| E2A-PBX1 | |||||||||
| Negative | 2014 | 94.3 | 79.8(1.0) | .175 | 84.9(0.8) | .650 | 100 | 87.9 | 96.2 |
| Positive | 121 | 5.7 | 86.2(3.2) | 86.9(3.2) | 0 | 12.1 | 3.8 | ||
| MLL rearrangement | |||||||||
| Negative | 2071 | 97.0 | 80.6(0.9) | < .001 | 85.5(0.8) | < .001 | 100 | 100 | 86.0 |
| Positive | 64 | 3.0 | 64.6(6.4) | 68.2(6.2) | 0 | 0 | 14.0 | ||
| Prednisone response | |||||||||
| Good | 2000 | 90.5 | 82.0(0.9) | < .001 | 87.2(0.8) | < .001 | 100 | 100 | 54.5 |
| Poor | 210 | 9.5 | 61.0(3.7) | 68.6(3.4) | 0 | 0 | 45.5 | ||
| Day 15 BM | |||||||||
| M1 | 1647 | 75.5 | 84.3(1.0) | < .001 | 88.4(0.8) | < .001 | 90.4 | 78.2 | 42.2 |
| M2 | 294 | 13.5 | 76.7(2.8) | 84.0(2.3) | 9.6 | 14.7 | 18.5 | ||
| M3 | 241 | 11.0 | 57.1(3.6) | 70.2(3.2) | 0 | 7.1 | 39.3 | ||
| Day 33 nonremission | |||||||||
| No | 2155 | 96.6 | 81.6(0.9) | < .001 | 86.4(0.8) | < .001 | 100 | 100 | 83.8 |
| Yes | 76 | 3.4 | 33.2(5.9) | 48.2(6.5) | 0 | 0 | 16.2 | ||
| Day 33 MRD | |||||||||
| < 0.01% | 298 | 37.8 | 91.9(1.6) | < .001 | 95.1(1.3) | < .001 | 100 | 20.0 | 6.2 |
| 0.01% -< 0.1% | 326 | 41.3 | 87.8(2.3) | 91.0(1.9) | 0 | 65.3 | 24.8 | ||
| 0.1% -< 1% | 93 | 11.8 | 72.6(5.2) | 82.0(4.3) | 0 | 14.6 | 19.3 | ||
| ≥ 1% | 72 | 9.1 | 51.8(6.2) | 64.5(6.1) | 0 | 0 | 49.7 | ||
| Week 12 MRD | |||||||||
| < 0.01% | 617 | 85.2 | 89.8(1.4) | < .001 | 93.9(1.0) | < .001 | 100 | 88.6 | 46.4 |
| 0.01% -< 0.1% | 66 | 9.1 | 78.2(5.6) | 86.8(4.4) | 0 | 11.4 | 16.4 | ||
| ≥ 0.1% | 41 | 5.7 | 55.5(8.3) | 71.1(7.4) | 0 | 0 | 37.3 | ||
- EFS indicates event-free survival; BM, bone marrow; CNS, central nervous system; HR, high risk; IR, intermediate risk; MRD, minimal residual disease; OS, overall survival; SR, standard risk; WBC, white blood count.
- a Patients with successful investigation of the respective criteria were included.
Patients were stratified according to clinical risk of relapse, based on the National Cancer Institute (NCI) risk criteria,11 7-day prednisone response (poor prednisone response was defined as ≥ 1 × 109/L blasts in peripheral blood on day 8), Day 15 and Day 33 BM response and cytogenetic subtypes. Standard risk (SR) patients with M3 marrow (≥ 25% blast) on Day 15 would be upstaged to intermediate risk (IR), and IR with M3 marrow would be upstaged to high risk (HR).
Minimal residual disease (MRD) was piloted at Beijing Children's Hospital and Hong Kong. BM was tested for MRD on Day 33 and Week 12 of treatment by Flow cytometry and/or PCR quantitative method for immunoglobulin and T-cell receptor antigen gene rearrangements.12, 13 The required sensitivity and quantitative range of MRD testing was 0.01%. SR patients with Day 33 MRD ≥ 0.01% and < 1% were upstaged to IR, and patients with Day 33 MRD ≥ 1% or Week 12 MRD ≥ 0.1% were upstaged to HR.
To reduce complications during early and late intensification of BFM protocol, the dose intensity of early intensification was reduced by 50% in SR patients. The delayed intensification was modified according to Children's Oncology Group (COG) delayed intensification with 25%-33% reduction of dexamethasone and doxorubicin dose.14 Prophylactic Cranial irradiation was omitted in all patients except those with CNS disease at presentation. CNS2 patients received two additional doses of intrathecal chemotherapy during induction. A randomized question was tested in the maintenance phase whether a one week rest of mercaptopurine and methotrexate during the vincristine-dexamethasone pulse might be associated with less toxicity and interruption of treatment. Allogeneic hematopoietic stem cell transplant (HSCT) was indicated for patients with induction failure (Day33 BM blast ≥ 5%) or Ph+/BCR-ABL1 positive or infants with MLL rearrangement. For those with MRD testing, Week 12 MRD ≥ 0.1% was also eligible for HSCT in first remission.
2.3 Statistical analysis
Event-free survival (EFS) was estimated from date of diagnosis until date of one of the following events: relapse, refractory disease, second malignancy or death from any reason. Overall survival (OS) was defined as time from diagnostic date through the date of death due to any reasons. Relapse-free survival (RFS) was estimated from the date of diagnosis until the date of relapse at any site. If no event occurred, the observation time was censored at the last follow-up. Patients abandoned treatment without serious toxicities or lost to follow-up in complete remission (CR) were censored at the last contact. Survival rates were estimated by the Kaplan-Meier method,15 and differences in OS and EFS were compared with two-tailed log-rank test.16 Cox proportional hazards model was used for multivariate analyses.17 Cumulative incidence of relapse (CIR) for competing events was constructed by the method of Kalbfleisch and Prentice,18 and compared using Gray's test.19 The Fisher's exact test was used to compare the difference of categorical variables among the groups. The Kruskal-Wallis or Wilcoxon Mann-Whitney tests were used to compare the difference of continuous variables among the multiple or two groups, respectively. Statistical analyses were performed using R software (Version 2.15.2; R Foundation for Statistical Computing, Vienna, Austria) and SPSS 16.0 (SPSS Inc., Chicago, Illinois).
3 RESULTS
3.1 Patients’ characteristics
A total of 2231 out of 2427 (91.9%) patients with newly diagnosed ALL were enrolled in this study (Supporting Information Table S2). Among the ten hospitals, four hospitals recruited 100% of patients (high recruitment group, n = 1574), and the other six hospitals enrolled 70.2% to 84.7% of patients (low recruitment group, n = 657). The four centers with high recruitment included not only the developed areas such as Beijing and Hong Kong, but also the less economically developed area in southwestern China, Chongqing. Meanwhile, the low recruitment group also consisted of several centers in Beijing and Shanghai. The reason for incomplete recruitment was due to financial reason, and was related to the insurance scheme with variable reimbursement rate. Comparing the high and low recruitment groups, there was no difference in the patients’ characteristics to suggest selection bias: age, gender, initial WBC counts, immunophenotyping, genetic fusion products, and survivals (Supporting Information Table S3). The patients'characteristics of the total evaluable population according to risk groups were shown in Table 1.
3.2 Treatment outcome
At a median follow-up of 47.3 months (range 0–85.8 months), the estimate of 5-year OS and EFS for the whole group were 85.3% ± 0.8% and 79.9% ± 0.9%, respectively (Figure 2). Univariate predictors of outcome were presented in Table 1. CNS status was identified as a significant predictor of survival, and the outcome for CNS2 or CNS3 was inferior to CNS1 (P < .001). Analysis of survival was also performed according to the cytogenetic subtypes (Supporting Information Figure S2). The TEL-AML1 fusion was detected in 16.3% of patients, and was associated with the best outcome (5y-OS: 93.0% ± 1.5%; 5y-EFS: 89.5% ± 1.8%). BCP-ALL patients with MLL rearrangement had 5-year OS and EFS rates similar to those of patients with T-cell ALL. As the tyrosine kinase inhibitor was not included for treatment of BCR-ABL1+ ALL in the protocol, the outcome of patients with BCR-ABL1 was the worst among the various subgroups, with a 5y-OS of 53.7% ± 5.8% and 5y-EFS of 48.0% ± 5.5%. With the exception of infants and post-transplantation toxicities, there was no obvious change in the prognostic influence of those factors. After exclusion of the 15 infants in the analysis, the 5-year EFS and OS for the 2216 patients over age of one year were 80.2% ± 0.9% and 85.4% ± 0.8%, respectively.
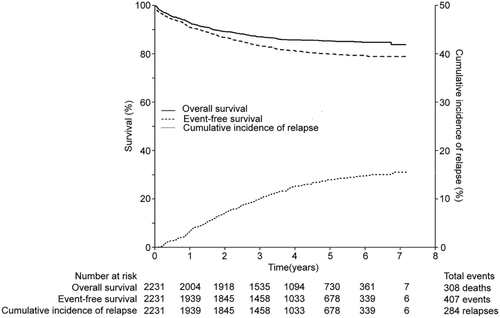
Overall survival (OS), event-free survival (EFS) and cumulative incidence of relapse (CIR) for all patients
3.3 Events
Treatment outcome was summarized in Table 2. CR was achieved on day 33 in 94.1% of evaluable patients. Among the 76 patients with induction failure, 54 achieved delayed remission after early intensification, and 22 patients had not achieved CR by the start of fourth HR course (resistant disease). Nineteen patients (0.9%) did not complete induction treatment due to financial difficulty and returned hometown for further treatment. Eighty-nine patients (4.0%) in CCR without serious toxicities gave up treatment because of financial difficulty, and 54% of them during early intensification phase and 35% during consolidation phase.
| Risk group | ||||
|---|---|---|---|---|
| Treatment outcome | All (%) | SR (%) | IR (%) | HR (%) |
| N | 2231(100) | 868(100) | 893(100) | 470(100) |
| Induction remission | 2100 (94.1) | 853 (98.3) | 875 (98.0) | 372 (79.1) |
| Induction failure | 76 (3.4) | 0 | 0 | 76 (16.2) |
| Resistant disease | 22 (1.0) | 0 | 0 | 22(4.7) |
| Induction Death | 36 (1.6) | 10 (1.2) | 14 (1.6) | 12 (2.6) |
| Give up in induction | 19 (0.9) | 5 (0.6) | 4 (0.4) | 10 (2.1) |
| CCR | 1725 (77.3) | 752 (86.6) | 719 (80.5) | 254 (54.0) |
| Remission Death | 64 (2.9) | 13 (1.5) | 30 (3.4) | 21 (4.5) |
| Relapse | 284 (12.7) | 75 (8.6) | 102 (11.4) | 107 (22.8) |
| BM relapse | 230 (10.3) | 61 (7.0) | 76 (8.5) | 93 (19.8) |
| CNS relapse | 31 (1.4) | 7 (0.8) | 16 (1.8) | 8 (1.7) |
| TEST relapsea | 15 (1.1) | 7 (1.4) | 3 (0.6) | 5 (1.6) |
| BM + CNS relapse | 3 (0.1) | 0 | 2 (0.2) | 1 (0.2) |
| BM + TEST relapsea | 3 (0.2) | 0 | 3 (0.5) | 0 |
| Other relapse | 2 (0.09) | 0 | 2 (0.2) | 0 |
| Secondary malignancy | 1 (0.04) | 0 | 1 (0.1) | 0 |
| Give up in CCR | 80(3.6) | 13 (1.5) | 23 (2.6) | 44 (9.4) |
| 5-year OS (%) | 85.3 ± 0.8 | 91.5 ± 1.0 | 87.7 ± 1.2 | 68.1 ± 2.3 |
| 5-year EFS (%) | 87.9 ± 1.2 | 81.6 ± 1.5 | 59.9 ± 2.6 | 79.9 ± 0.9 |
- BM, bone marrow; CCR, continuous complete remission; CNS, central nervous system; EFS indicates event-free survival; HR, high risk; IR, intermediate risk; OS, overall survival; SR indicates standard risk; TEST, testicular.
- a Males only.
CIR at five years (5y-CIR) was 15.3% ± 0.9%. The most frequent site of relapse was BM. The 5y-CI of isolated CNS relapse and combined relapses with CNS involvement was 1.7% ± 0.3% and 1.8% ± 0.3%, respectively. Only one patient with IR risk developed an acute myeloid leukemia as a secondary neoplasm after first CR of 21.6 months.
3.4 Toxicity
Death during induction happened in 36 patients (1.6%), 27 due to infection and nine due to bleeding. Death due to nonrelapse causes after archiving CR happened in 64 patients (2.9%): 21 at early intensification, 14 at consolidation, 10 at delayed intensification, 11 during maintenance and another eight after stem cell transplantation. The cause of remission death was mainly attributed to severe infections (73.4%), and other causes included bleeding and multiple organ failure. Graft-versus-host disease was the main cause of death after transplant.
Septicemia was the major cause of treatment-related mortality, and infective pathogen was identified in 58.8% of these died cases (47/80). The majority were bacterial infections (59.6%), 25.5% were fungal, and 14.9% were viral. Other significant treatment-related toxicities included seizures, pancreatitis, thrombosis and osteonecrosis.
3.5 Comparison of the MRD group and non-MRD group
In the two centers piloting MRD stratification (the MRD group, n = 855), MRD monitoring had upstaged 246 (28.8%) cases from SR to IR, 18 (2.1%) patients from SR to HR, and 19 (2.2%) patients from IR to HR. Consequently, patients in the MRD group could be classified as MRD-SR, MRD-IR, and MRD-HR in 26.0%, 55.1%, and 18.9%, respectively, which was significantly different with that of the non-MRD group (n = 1376, SR: 46.9%, IR: 30.7% and HR: 22.4%, P < .001). Comparing treatment outcome of MRD group and non-MRD group (Supporting Information Figure S3 and Table S4), OS was similar in both groups (86.5% ± 1.2% vs 84.6% ± 1.0%, P = .273), but EFS was significantly better in the MRD group (82.4% ± 1.4% vs 78.3% ± 1.2%, P = .038). The CIR was significantly lower in the MRD group (10.7% ± 1.3% vs 18.0% ± 1.2%, P < .001).
We observed that the MRD group had more patients with favorable subtypes as compared with non-MRD group (Supporting Information Table S5), namely TEL/AML1 positive, good prednisone responders, non-M3 marrow on Day 15 induction. In multivariate analysis for prognosis (Supporting Information Table S6), sex, age, WBC counts, Immunophenotype, CNS status, BCR-ABL1, TEL-AML1, MLL rearrangement, prednisone response, BM blast on day 15 and 33, as well as the MRD-based classification were used as covariates, all of which reached a P value <0.1 in the respective univariate analysis. Adjusted for these factors, the MRD-based classification remained as an independent prognostic factor for both RFS and EFS.
3.6 HSCT
Forty of 470 HR patients received allogeneic HSCT in the first remission (26 with HLA-identical sibling donor, 11 with haploidentical donor, and two with matched unrelated donor). Stem cell source was BM in 15 cases, cord blood in 15 and peripheral blood in 10. The time interval from diagnosis to transplant was 7.2 months (2.5 - 20.0 months). Eight patients died from transplant-related complications. The SCT group had the trend of better OS (79.7% ± 6.4% vs 66.9% ± 2.5%, P = .170) and EFS (75.0% ± 6.8% vs 58.4% ± 2.7%, P = .065) than the chemotherapy HR group (Supporting Information Figure S4). The CIR in HSCT group was 10.0% ± 2.3%, which was significantly lower than those HR patients treated with chemotherapy (29.7% ± 6.6%, P = .002). Details of randomization for maintenance in SR and IR patients will be reported separately. There was no statistical difference in the outcome of the two arms (Supporting Information Figure S5).
4 DISCUSSION
This was the largest multicenter clinical trial in childhood ALL in China. The previously reported single center studies in China were small size and some of them had incomplete recruitment, mainly due to high treatment cost.20, 21 From mid-2000s, the China government started to implement insurance coverage for children with serious illnesses, and then more families could receive treatment according to standard care.22 With this background, this multicenter study group was established. Six centers could not achieve 100% enrollment, however we did not observe any significant difference in the patients’ demographic features between the high and low recruitment groups, which suggested selection bias as unlikely.
In this study, we confirmed some special biological features in Chinese. T-ALL was present in 8.4% in this study, and the previous studies in Chinese reported 8.1% to 9.7% incidence of T-ALL.23, 24 The BFM 2000 study reported that 13.2% of non Ph+ patients had T-ALL,25 and CCG −1900 series enrolled 15.9% of ALL with T- lineage.26 TEL-AML1 incidence in this study was 16.3%, which was lower than the western reports of 21%.27 We noticed that only 6.3% of patients in Chongqing area (the less economic developed region) were TEL-AML1 positive. The presence of TEL-AML1 in childhood ALL might be related to economic development. Further studies to observe the trend in China would be interesting during the era of rapid development.
As compared with the ALL IC-BFM 2002 study,28 our current study had a similar chemotherapy backbone but with reduced intensity in SR. The course of early intensification for the SR group was shortened by 50% from 4 weeks to 2 weeks, and the dose of delayed intensification was reduced by around 25%-33% compared to BFM protocol II. The EFS and CIR for the SR group were better than that of ALL IC-BFM 2002 (87.9% vs 81.0%; 9.7% vs 14.4%).The incidence of death in CR was also lower in SR patients (1.5% vs 2.8%). Patients in HR group in this study adopted a more intensive maintenance treatment of St. Jude Children's Research Hospital.29 The OS and EFS of 68.1% and 59.9% for HR are encouraging. This study adopted strict eligibility criteria for allogeneic HSCT during first CR, and the outcomes of transplanted patients were good. The prophylaxis of CNS leukemia by nonradiation approach was also successful in this study with low CNS relapse rate.
Treatment related mortality was an important cause of treatment failure in developing countries.30, 31 In this study, death happened in 1.6% during induction and 2.9% during remission, which was higher than that of developed countries.32 Infection was the most important cause of death and should be the target for future improvement. Early recognition and intervention for infection may reduce the remission death. Abandonment of treatment was another important cause of treatment failure in developing countries. In this study, 4% of patients abandoned treatment after achieved remission, which was already much lower than previously reported.23 Adopting less intensive treatment and implementing insurance cover would be the most effective strategy.
MRD was widely used for early response assessment but requires standardization and quality control.33 MRD monitoring was piloted in two centers for treatment modification in this study. MRD monitoring had upstaged about 30% of patients from SR to IR/HR to receive a more intensive chemotherapy regimen. The MRD-SR group then became a real “low risk” group with 2.8% CIR despite a reduced intensity SR regimen. MRD-IR constituted the largest proportion of whole group (55.1%), and the CIR could be kept at a relatively low level of 11.3%. MRD stratification was demonstrated to have significant improvement in EFS and CIR. To our knowledge, this was the only study comparing two groups of patients receiving or not receiving MRD guided treatment within the same treatment protocol.
There were several limitations in this study. The recruitment of patients in some centers was not complete and MRD testing could only be applied in two centers. Data collection was also a big challenge as most centers did not have designated data managers. Fortunately the study received grant from government and charity, and honorarium could be offered to staff working off duty to collect data. Despite the above limitation, all the participating centers showed improvement of treatment outcome, and also could follow a standard treatment protocol in the participating centers.
In conclusion, multicenter clinical study was feasible in China. The outcome of this study was satisfactory with acceptable morbidity and mortality. SR group could be cured with less intensive treatment and CNS relapse could be prevented by chemotherapy based treatment. MRD guided treatment appeared to improve EFS and would be extended to all hospitals.
ACKNOWLEDGMENTS
We thank all staff members of the collaborating institutes. Special thanks are due to Statisticians Qian-Qian Wang, Guo-Shuang Feng and data manager Jin Xie.
ROLE OF THE FUNDING SOURCE
The funders had no role in study design, data collection, data analysis, interpretation, or writing of the report. The corresponding author had full access to all the data reported in the manuscript and had final responsibility for the decision to submit for publication.
CONFLICT OF INTERESTS
None declared.




