The changing face of adult posttransplant lymphoproliferative disorder: Changes in histology between 1999 and 2013
Funding information: Health Resources and Services Administration, Grant/Award Number: 234-2005-370011C
Abstract
Posttransplant lymphoproliferative disorder (PTLD) typically presents with either polymorphic or monomorphic histology. While both are the end result of immunosuppressive therapies, their origins are felt to be different with different prognoses and responsiveness to therapy, resulting in 2 different malignancies. We attempted to confirm reports suggesting that the relative frequency of these 2 histologies is shifting over time. We analyzed 3040 adult PTLD cases in the UNOS OPTN database from 1999 to 2013. Changes in PTLD cases over time were analyzed for histology, time from transplant to diagnosis, and patient EBV serostatus. We found that the relative proportion of polymorphic versus monomorphic histology has changed with an increase in the proportion of monomorphic cases with time (1999-2003, 54.9% vs. 45.1%; 2004-2008, 58.3% vs. 41.7%; 2009-2013, 69.7% vs. 30.3%; P = <.001). The change is driven by a gradual increase in the number of monomorphic PTLD with a steady number of polymorphic PTLD. The change is most strongly seen in transplant recipients who were EBV serostatus positive at the time of transplant. Potential causes are changes in immunosuppressive regimens with increased tacrolimus use (P = .009) and increased survival among transplant patients leading to later occurrence of PTLD (P = .001) that have occurred during the time frame analyzed. As organ transplantation has evolved over time, PTLD has coevolved. These changes in histology have important implications regarding the origin and clinical management of PTLD.
1 INTRODUCTION
An increased risk for developing malignancy after organ transplantation has been recognized for many years.1-3 One common malignancy, posttransplant lymphoproliferative disorder (PTLD), represents a heterogeneous group of lymphoid proliferations, occurring in the setting of pharmacologic immunosuppression after organ transplantation. These proliferations range from benign polyclonal lymphoid collections to aggressive malignant lymphomas and may involve T cells, B cells, and/or plasma cells.4-9 These conditions differ not only in their clinical presentation but also in their management, making an accurate diagnosis critical for patient care. The most common histologies are polymorphic and monomorphic B cell PTLD. Both polymorphic and monomorphic PTLD present with nodal effacement of the lymph node architecture or extranodal disease. Polymorphic PTLD shows a full range of B cell maturation and may be polyclonal and involve T cells and plasma cells. In contrast, monomorphic PTLD is composed of monomorphic sheets of monoclonal transformed B cells. Epstein-Barr Virus (EBV) tumor status and PTLD histology are closely linked, with polymorphic PTLD typically involving EBV and monomorphic PTLD frequently being EBV negative.5, 7, 10-14 Genetic alterations in PTLD driven by EBV are dramatically different than that of EBV negative PTLD suggesting a different developmental pathway for these 2 histologies.15 The genetic differences are reflected in different clinical presentations with polymorphic PTLD occurring sooner after organ transplantation and monomorphic PTLD occurring later.7, 16, 17 Patient EBV serostatus at the time of organ transplant also plays a role in PTLD development with increased risk of PTLD and EBV positive histology being more common in patients who are EBV seronegative.18-21 Other than the fact that polymorphic and monomorphic PTLD occur in organ transplant patient, by virtue of their viral status, histology, genetic alterations, and patient populations, these 2 PTLD histologies should be considered independent entities.
Older reports suggested that EBV positive and polymorphic histology was more common than EBV negative and monomorphic histology.22-24 More recently, reports have suggested that PTLD histology is changing from mostly EBV positive polymorphic to EBV negative monomorphic.7, 15 In one early report of 133 PTLD patients, prior to 1991 only 2% were EBV negative versus 23% from 1991 to 2000.7 A more recent report on adult PTLD patients at our center by Luskin et al noted that the proportion of EBV negative PTLD had increased from 10% by 1990–1995 to 48% by 2008–2013 with corresponding increases in monomorphic PTLD.15 In an effort to determine if the changes described in these single center reports are truly universal, we obtained PTLD data from the United Network for Organ Sharing (UNOS). In this report we describe changes in PTLD histology over time and a potential link to changes in immunosuppression agents and patient survival after organ transplantation.
2 METHODS
We performed a retrospective cohort study of all patient in UNOS diagnosed with PTLD between 1999 and 2013. As secondary analyses, we also looked at time trends in the broader transplant community over the same time period. Data on PTLD and organ transplant patients was obtained from UNOS. Due to the small number of PTLD patients reported to the UNOS database prior to 1999, only data from 1999 to 2013 was analyzed (Based on Organ Procurement and Transplantation Network data as of October 2014). Organ recipients over the age of 18 who developed PTLD between 1999 and 2013 after kidney, liver, lung, heart, pancreas, or intestine transplantation were included in our analysis. Our analysis was further limited to those with either monomorphic or polymorphic PTLD histology. Patient demographic and transplant data, PTLD histology, and immunosuppression were analyzed. While data was available on the EBV serostatus of the patients, no data was available on EBV status of the tumor. Characteristics of PTLD patients within the UNOS database were summarized using descriptive statistics. We stratified our analysis by PTLD histology (polymorphic vs. monomorphic) and used Wilcoxon rank sum test to analyze continuous variables and Chi square test for categorical variables. Furthermore, we used univariate and multivariable logistic regression to measure the association of factors with the development of polymorphic compared to monomorphic PTLD histology. All statistical analyses were performed using STATA 13 (College Station, TX).
3 RESULTS
3.1 Patient demographics
Between 1999 and 2013, 375 561 total new adult organ transplants were performed. Of all new organ transplants performed during this time period, there were 59.3% kidney, 22.0% liver, 7.6% heart, 5.4% lung, and 5.7% other organs and/or multi-organ transplants. PTLD occurred in 3040 patients (0.81%) (Table 1). The median age of PTLD patients was 48·2 years with 69% male and 31% female. Over the years studied, there was a statistically significant increase in the relative proportion of lung transplants over time, although it was a numerically small increase (4.5% in 1999 up to 7.1% in 2013). Using a logistic regression model, on average the relative proportion of lung transplants increased by ∼0.21% per year (P = <.001). The cumulative incidence of PTLD differed by organ transplanted (kidney, 0.57%; liver, 0.87%; heart, 1.55%; lung, 1.65%; other/combination, 1.29%; P = <.001)
| Characteristic | N (%) |
|---|---|
| Age—years | |
| Median | 50 |
| Range | 18–81 |
| Sex | |
| Female | 943 (31) |
| Male | 2097 (69) |
| Organ transplanted | |
| Kidney | 1803 (59) |
| Liver | 669 (22) |
| Heart | 231 (8) |
| Lung | 164 (5) |
| Other/combination | 173 (6) |
| PTLD histology | |
| Monomorphic | 1878 (62) |
| Polymorphic | 1162 (38) |
| Time between transplant and PTLD diagnosis—years | |
| Median | 4.1 |
| Interquartile range | 1.0–8.5 |
| Maintenance Immunosuppression | |
| Tacrolimus | 1752 (58) |
| Cyclosporine | 1049 (35) |
| Mycophenolate | 1464 (48) |
| Azathioprine | 794 (26) |
| Treated for acute rejection | |
| Yes | 350 (12) |
| No | 2690 (88) |
| T cell-depleting antibody induction | |
| Yes | 874 (29) |
| No | 2166 (71) |
3.2 Changes in PTLD histology
The histology of PTLD has changed from 1999 to 2013 (Figure 1A). Categorizing into 5 year time periods for the date of PTLD diagnosis, the relative proportion of monomorphic versus polymorphic PTLD changed between 1999 and 2013, with number of cases of monomorphic PTLD increasing relative to the number of cases of polymorphic PTLD. (1999–2003, 54.9% vs. 45.1%; 2004–2008, 58.3% vs. 41.7%; 2009–2013, 69.7% vs. 30.3%; P = <.001). Over this time period, the number of monomorphic PTLD cases increased (1999–2003, 460 cases; 2004–2008, 597 cases; 2009–2013, 821 cases), while the number of polymorphic PTLD cases remained relatively stable (1999–2003, 378 cases; 2004–2008, 427 cases; 2009–2013, 357 cases) (Figure 1B).
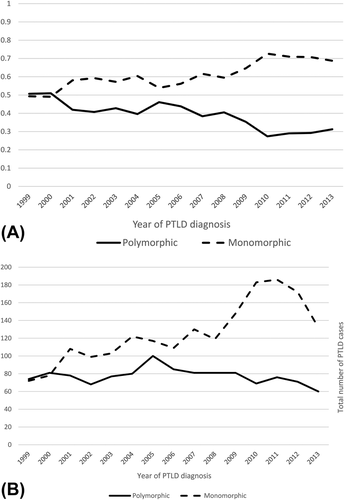
PTLD histology by year. (A) Proportion of monomorphic and polymorphic PTLD. (B) Absolute numbers of monomorphic and polymorphic PTLD
3.3 Time from transplant to PTLD diagnosis
The time from organ transplantation to diagnosis of PTLD has significantly increased from 1999 to 2013. Using 5 year time periods, the median time in months from transplant to PTLD diagnosis has grown (1999–2003, 39.3 [0–181.8]; 2004–2008, 50.4 [0–238.8]; 2009–2013, 55.5 [0–306.8]; P = <.001). When comparing patients diagnosed with PTLD within 3 years of transplant versus those beyond 3 years, in the 1999–2002 time frame about equal number of diagnoses were made. Since 2002 the percent of patients diagnosed greater than 3 years after transplant has steadily grown (Figure 2A) reaching a high in 2013, when 64% of patients were diagnosed greater than 3 years after organ transplant. This increased time from organ transplant to PTLD diagnosis is correlated with increased monomorphic histology by multivariable logistic regression (Table 2, P = <.001).
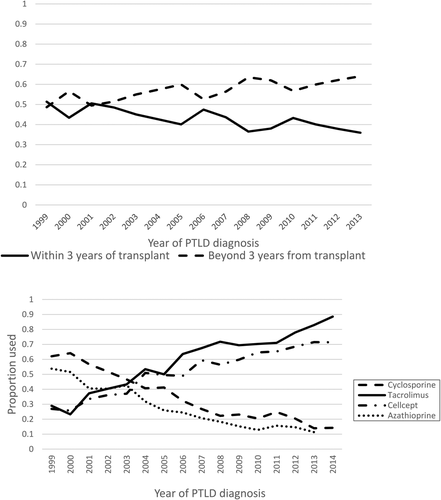
Influences on PTLD histology. (A) Time from transplant to PTLD. By year, the proportion of patients with PTLD diagnosed within 3 years of organ transplantation versus those diagnosed beyond 3 years after organ transplantation. (B) Maintenance immunosuppressive agents. By year, the proportion of patients on cyclosporine, tacrolimus, azathioprine, and mycophenolate
| Characteristic | Multivariate OR 95% CI | P-value | |
|---|---|---|---|
| Age (continuous) | 0.99 | 0.988-0.999 | .048 |
| Sex | |||
| Male | 0.77 | 0.66-0.91 | .002 |
| Organ transplanted | |||
| Kidney | 0.92 | 0.72-1.15 | .002 |
| Liver | 0.87 | 0.67-1.12 | .278 |
| Heart | 1 | — | — |
| Lung | 0.61 | 0.45-0.84 | .837 |
| No. years between transplant and PTLD diagnosis (continuous) | 0.93 | 0.91-0.95 | <.001 |
| Tacrolimus maintenance | 0.82 | 0.69-0.99 | .034 |
| Anti-rejection treatment received | 1.20 | 0.95-1.52 | .136 |
| T cell-depleting antibody induction received | 0.93 | 0.78-1.11 | .424 |
3.4 Changes in immunosuppression and PTLD histology
Immunosuppressive regimens have evolved between 1993 and 2014 with cyclosporine and azathioprine being gradually replaced by tacrolimus and mycophenolate (Figure 2B). These changes are correlated with the shift in PTLD histology. Tacrolimus use was associated with monomorphic histology on univariable logistic regression (OR 0.82; 95% CI [0.71-0.95], P = .009) while cyclosporine (OR 1.15, 95% CI [0.99-1.34], P = .071), azathioprine (OR 1.0, 95% CI [0.59-1.69], P = .99), and mycophenolate (OR 0.89, 95% CI [0.77-1.03], P = .12) use was not found to be preferentially associated with a PTLD histology. Whether these drugs are directly causative of the changes in PTLD or are surrogate for changes in immunosuppressive protocols during these years in not known.
3.5 EBV serostatus and PTLD histology
Recipient EBV serostatus at the time of organ transplant was only available in 46·3% of PTLD patients. Of 1451 PTLD patients with known EBV serostatus, 952 (65%) were EBV positive and 504 (35%) were EBV negative. The relative proportion of PTLD patients who are EBV seropositive at the time of transplant versus those that are EBV negative has remained fairly stable over the years studied (Figure 3A). Looking at the effect of EBV serostatus on PTLD histology, there appears to be a large and continuous change from roughly equal monomorphic versus polymorphic PTLD in 1999–2001 to a 2-1 ratio of monomorphic versus polymorphic by 2013 in EBV seropositive patients (Figure 3B). This difference was less evident in patients who were EBV negative (n = 504) at the time of organ transplantation (Figure 3C). Using logistic regression, EBV seropositive patients were more likely to have monomorphic PTLD compared to seronegative patients (OR 0.76 (0.61-0.95), P =.016).
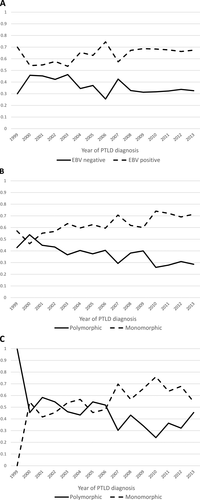
EBV serostatus at the time of transplantation. (A) By year, the proportion of patients who are EBV positive versus EBV negative. (B) The proportion of monomorphic versus polymorphic PTLD in patients who were EBV seropositive by year. (C) The proportion of monomorphic versus polymorphic PTLD in patients who were EBV seronegative by year
Examining patient who received transplant with mismatched EBV status, 189 patients with PTLD were EBV negative at time of transplant and received their organ from an EBV positive donor. Of these 189 patients, 113 (60%) had monomorphic and 76 (40%) had polymorphic PTLD. Of 233 patients with PTLD who were EBV positive at time of transplant and received organ from an EBV negative donor, 156 (67%) had monomorphic and 77 (33%) were polymorphic PTLD. The difference in PTLD histology in patients with mismatched donor-recipient EBV status was not significant (P = .13).
4 DISCUSSION
Posttransplant lymphoproliferative disorder is a unique malignancy in that it is entirely man made and did not exist before the advent of pharmacologic immunosuppression. Pharmacologic immunosuppression created the ground from which PTLD sprouted. Our data suggest that as immunosuppression and transplant protocols have changed over the years, we have altered the conditions that gave rise to PTLD. As a result, it should not be a surprise that PTLD is coevolving. Based on the UNOS data, it appears clear that the histology of PTLD is changing with the proportion of EBV negative monomorphic cases versus EBV positive polymorphic cases increasing over time. The numbers suggest this change in relative proportions is mostly due to increases in the number of monomorphic cases with fairly steady number of polymorphic cases. How are alterations in transplant protocols changing the presentation of PTLD? It has long been suggested that EBV negative PTLD was a distinct entity from EBV positive PTLD rather than a spectrum of related histologies.7, 10 Recent reports confirm that they are indeed different in origin with very different genetic drivers between EBV positive versus EBV negative PTLD.15 EBV negative PTLD carries alterations similar to that seen in typical non-Hodgkin lymphoma and is very different from those seen in EBV positive PTLD.15 So PTLD in fact is at least 2 different malignancies with different oncogenic pathways only linked by the virtue of both arising in immunosuppressed patients. Our data suggest that changes in immunosuppression protocols is altering the environment that produces EBV negative monomorphic PTLD leading to increased incidence. This is supported by our finding that the changes in histology are seen mostly in transplant recipients who are EBV seropositive at the time of transplant. EBV seronegative patients appear not to have seen changes in their PTLD histology. EBV seronegative patients often seroconverts during transplant or develops primary EBV infection and are at highest risk for developing EBV positive polymorphic PTLD.18-21 This histology of PTLD has not increased in numbers as per the UNOS database. Alterations in pharmacologic immunosuppression appear not to have affected this mechanism of PTLD development as there has been little change in the numbers of polymorphic PTLD cases over the years and in patients who were EBV negative at the time of transplantation. EBV negative monomorphic PTLD patients develop their malignancy from mutations similar to that seen in the normal non-transplant population and not through an EBV driven process.15 As such they can occur in any transplant population, both EBV seropositive and seronegative. This process appears to be changing over time.
We uncovered 2 possible etiologies related to an increase in monomorphic PTLD histology. First, it is clear that the drugs used in immunosuppression have changed over the past 20 years from cyclosporine and azathioprine based regimens to tacrolimus and mycophenolate or sirolimus based regimens. Induction protocols have also changed as have the amount and duration of the different agents. In our analysis, changes in maintenance immunosuppression have occurred concurrently with changes in PTLD histology, with tacrolimus associated with monomorphic subtype. We did not examine the effect of changes in complex induction protocols and agents on PTLD. It is outside the scope of our analysis to identify which drug or combination may be responsible for this change in PTLD. The UNOS database does not allow one to study drug levels, duration of treatments or account for detailed patient specific changes in treatment. Given that the changes in transplant protocols that are occurring are dramatic, involving new induction and maintenance agents with additional changes in immunosuppression intensity and monitoring, and are all collinear, short of a well-designed very large clinical trial, it would be impossible to know if a single change in immunosuppression is responsible for our findings. In fact, it is likely that changes in PTLD histology are not due to a single change in immunosuppression but rather from the sum total of the alterations in immunosuppression protocols. An alternate mechanism, beyond changes to immunosuppression, might be due to the increasing lifespan of transplant patients. Organ transplant patients are living longer and remaining on immunosuppression longer as a result. By living longer they should be developing more EBV negative monomorphic PTLD as this histology preferentially occurs later after organ transplantation.7, 10, 25 Our data supports this finding in that more recent patients are developing PTLD later after transplantation. One might argue that both changes in immunosuppressive agents and living longer after transplant are not separate mechanisms for PTLD but represent a linked phenomenon. In the end, the cause of the change in PTLD histology is related to changes in our organ transplant protocols whether it is a direct effect from the immunosuppression regimensor an indirect effect from the better outcomes derived from these protocols.
Although our study has some important findings, our analysis has a number of weaknesses. It is retrospective and based on a large registry were PTLD cases may have been underreported. The time frame of the patients is limited to 1999–2013 due to issues with the limited number of PTLD patients prior to 1999. This limited time frame potentially omits important data before and after our analysis resulting in potentially misleading information due to bias in the patient sample. For instance tacrolimus was correlated to monomorphic histology but cyclosporine was not. Given that these 2 variables are near collinear one would have thought both would have been statistically significant. However our 1999–2013 data set is biased towards the era of tacrolimus. As a result the number of cyclosporine patients is smaller in our data set resulting in a statistically insignificant finding. If the data set had been earlier the cyclosporine analysis possibly might have been statistically significant. In addition to the limited time-period, EBV serostatus data was missing in the majority of patients in UNOS and may have influenced our findings on EBV serostatus. Furthermore, immunosuppressive protocols are complex with a large number of variables. Our analysis of immunosuppressive agents was focused on maintenance agents only and may have missed features derived from the details of organ transplant patient care. Certain critical data points such as EBV status of the PTLD tumor itself are absent and other data points likely do not count all patients given the self-reported nature of the database. However, the strength of the database is that the numbers of PTLD patients are large and the changes over time gradual and consistent, thus likely representing real changes in the organ transplant population. While there will be lingering questions of causation for the changes we see in PTLD, the main point of this report that PTLD histology is shifting dramatically over a few short years is firm.
In summary, our analysis shows that there are dramatic changes in the presentation of PTLD over the past 2 decades. PTLD represents a heterogeneous group of disorders that arose with the advent of pharmacologic immunosuppression in organ transplant patients. As immunosuppressive protocols continue to evolve, we will likely continue to see coevolution of PTLD. In the future, as newer immunosuppressive agents and protocols are introduced the end effect on PTLD needs to be closely monitored, not only for the absolute number of cases but also for the characteristics within as shifts in PTLD subtypes may have potential impacts on the choice of therapy and long-term patient outcomes.
ACKNOWLEDGMENTS
This work was supported in part by Health Resources and Services Administration contract 234–2005-370011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.




