Nilotinib induced bone marrow CD34+/lin–Ph+ cells early clearance in newly diagnosed CP-chronic myeloid leukemia
Funding information: Novartis
Chronic myeloid leukemia (CML) is a clonal disorder characterized by Philadelphia (Ph) chromosome and/or BCR/ABL tyrosine-kinase (TK) presence. Target therapy with imatinib has greatly improved its outcome while deeper and faster responses are reported with the second-generation TKIs dasatinib and nilotinib. Sustained responses may enable TKI discontinuation. However, a fraction of treatment-free remission patients, including those in long-term complete molecular response (MR), may experience disease recurrence1 possibly due to persistence of quiescent leukemic precursor cells [ie, leukemic progenitor cells (LPCs) CD34+/CD38+/lin– and leukemic stem cells (LSCs) CD34+/CD38–/lin–].
Time-course and mechanisms of LPCs/LSCs clearance during TKI treatment have not been clearly established yet, although in vitro data suggests that quiescent LSCs are indeed not sensitive to BCR/ABL inhibition.2, 3 In vivo preliminary data suggest that residual CD34+ Ph+ cells are very rarely detected in nilotinib-treated patients in complete cytogenetic response (CCyR), compared to imatinib-treated ones.4 Moreover, in five patients in CCyR at 3 months of nilotinib-treatment, undetectable CD34+ Ph+ cells were reported.4 Despite the very limited number of patients reported, these in vivo data suggest that the rapid inhibitory activity of nilotinib on CML burden may affect stem cells as well.
The PhilosoPhi34 (EudraCT: 2012–005062-34) is a multi-center, prospective single-arm study conducted on behalf of the Rete Ematologica Lombarda (REL).
Its primary endpoint was to evaluate the rate of residual CD34+/lin–Ph+ cells in the bone marrow (BM) of CCyR patients after 6 months of nilotinib-treatment by cell selection system and fluorescence in situ hybridization (FISH) analysis.
Secondary endpoints evaluated were: clearance of CD34+/lin–Ph+ cells in the BM of CCyR patients at 3 and 12 months of treatment; CCyR rate and the MRs in peripheral blood (PB) at 3, 6, and 12 months of treatment, respectively.
The protocol design also included an exploratory study aimed at evaluating the Gene Expression Profiling (GEP) of selected CD34+/lin– cells at diagnosis and at 12 months of treatment using Affymetrix GeneChip Instruments and Software Systems, and Affymetrix GeneChip Human Genome HTA 2.0. This exploratory study was planned to initially include 30 consecutive patients.
A total of 87 patients were enrolled and their median time on-study was 26 months at data analysis. Of the enrolled patients, two discontinued nilotinib by 3 months of treatment while five additional patients discontinued treatment between 6 and 8 months (see Supporting Information Table 2).
Of the 84 6-month evaluable samples, 79 showed CCyR and 78 were adequate for FISH analysis; 7 negative tests were excluded since less than 200 nuclei were analyzed. Only 5/71 (7%; CI 95%: 2.3%-15.7%) evaluable FISH tested positive. Of the 84 3-month evaluable samples, 76 showed CCyR and 75 were adequate for FISH analysis (10 negative tests excluded); only 8/65 (12.3%; CI95%: 5.5%-22.8%) evaluable FISH tested positive. Of the 80 12-month evaluable samples, 79 showed CCyR and 78 were adequate for FISH analysis (9 negative tests excluded); none of the 69 evaluable FISH tested positive (0.0%; CI95%: 0.0%-5%) (Figure 1A).
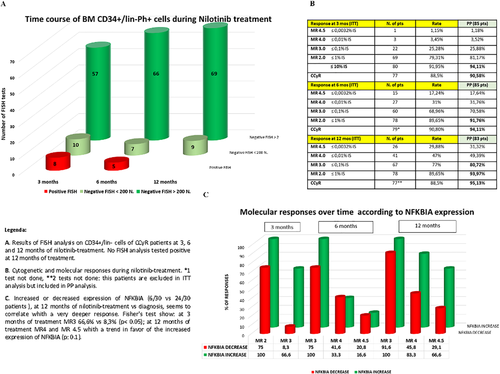
Main results of the PhilosoPhi34 study [Color figure can be viewed at wileyonlinelibrary.com]
The 3-month MR of the FISH-positive patients (at 3 and 6 months) tested ≥ 0.175% international scale (IS).
Evaluation of Response to Treatment. Five of 87 patients failed treatment; none of them progressed to an accelerated or blastic phase; 2 harbored a mutation. Intention to treat (ITT) cytogenetic analysis was as follows: CCyR 77/87 patients (88.5%) at 3 months; CCyR 79/87 patients (90.8%) at 6 months; CCyR 77/87 patients (88.5%) at 12 months. Figure 1B summarizes the ITT and per protocol (PP) rate of MRs at the different time points.
Gene Expression Profiling of CD34+/lin– Cells. Gene expression profiling (GEP) experiments, for details see Ref. 5, were conducted on the first 30 evaluable patients enrolled. Since the number of selected CD34+/lin– cells collected at diagnosis and at 3, 6, and 12 months showed two opposite trends, the first GEP experiments was performed on the CD34+/lin– cells stored at diagnosis.
Experiment 1. Patients were grouped according to CD34+/lin– cell trends: class 1 (n = 24) showed highly reduced levels of CD34+/lin– cells while class 2 (n = 6) demonstrated increasing levels of CD34+/lin– cells at both 3 and 6 months.
Bioinformatics analysis was performed: 56 transcripts could be selected which differed between the two classes (Supporting Information Table 4). Of note, a large part of the transcripts identified have not been characterized yet; 31/56 transcripts were located on chromosome 15. Among the transcripts with known function, we focused on the NFKBIA gene for its biological relevance in the regulation of apoptotic pathways and cell proliferation. Sample analysis showed an over expression of NFKBIA in class 1 patients, ie those showing decreasing number of C34+/lin– cells over time.
Experiment 2. Although all the 30 patients were in optimal response, in order to assess whether higher NFKBIA expression could be associated with better response to treatment, NFKBIA expression in the CD34+/lin– cells of the 30 patients at diagnosis (class 1) versus 12 months of treatment (class 2) was explored. MRs of patients were stratified according to NFKBIA Log2 expression (absolute value) at diagnosis and at 12 months of treatment: no differences were observed between the two groups.
Since the Log2 fold change indicates that some patients increased NFKBIA expression at 12 months of treatment versus diagnosis, patients were grouped and analyzed according to NFKBIA expression over time with group 1 (n = 6) increasing expression and group 2 (n = 24) decreasing expression at the two time points. The MRs of patients in both groups were compared and results were analyzed using Fisher's test. We observed better MRs at 3 months in patients who increased NFKBIA expression (Figure 1C).
To our knowledge, this is the first in vivo study that highlights the association between increased expression of NFKBIA in CD34+/lin– cells during treatment and a faster and deeper response to treatment. In order to confirm this association, analysis is ongoing to include all patients reaching 12 months of treatment.
Taken together, our results suggest that the faster and deeper CD34+/lin–Ph+ cells clearance during nilotinib-treatment may have an impact on subsequent TFR. Further studies using the more sensitive and patient's specific gDNA Q-PCR6 are ongoing.
ACKNOWLEDGMENTS
Authors thank all REL’ Colleagues for their commitment and also acknowledge Novartis for the Nilotinib supply and for the financial contribution that has partially supported this study. Also J. Welch for proof reading.
Conflict of interest
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
E.P. designed, performed and coordinated the research, collected, contributed, analyzed, and interpreted the data, and wrote the manuscript; M.N. performed the statistical analyses; G.D.C. performed FISH analysis and interpreted data; A.T. designed the study, performed GEP analysis and wrote the paper; M.L. performed GEP analysis; B.D.C. performed the bio statistical analyses (GEP data); G.R. critically revised the paper; R.C. analysed and interpreted data, analysed the results and critically revised the paper. All authors collected data and revised the manuscript.




