Optimizing the pretransplant regimen for autologous stem cell transplantation in acute myelogenous leukemia: Better outcomes with busulfan and melphalan compared with busulfan and cyclophosphamide in high risk patients autografted in first complete remission: A study from the acute leukemia working party of the EBMT
This study was presented orally at the 59th annual meeting of the American Society of Hematology, Atlanta USA December 8-12 2017
Abstract
Autologous stem cell transplantation remains a clinical option to consolidate some adult patients with acute myelogenous leukemia (AML) in first complete remission (CR1). In a small cohort of patients, we have previously shown better outcomes following Busulfan and Melphalan (BUMEL) over Busulfan and Cyclophosphamide (BUCY). To identify the subpopulations that might get the highest benefit with BUMEL, we designed a larger study. All adult patients with primary AML and available cytogenetics, autografted from January 2000 to December 2016 in CR1, were included: 1137 patients received BUCY and 512 BUMEL. All factors differing in distribution between the 2 conditioning groups were introduced in multivariate analyzes. In a primary analysis, we found an interaction between conditioning and the poor risk group defined as poor cytogenetics and/or presence of the FLT3-ITD mutation. During analysis of the poor risk group, 176 patients received BUCY and 62 BUMEL. BUMEL was associated with a lower RI at 5 years (53% versus 69%, HR: 0.52, P = .002), a better Leukaemia-free survival (LFS) (42% versus 25%, HR: 0.54, P = .002) and a better OS (54% versus 36%, HR: 0.61, P = .02). During analysis of the non poor risk group, 961 patients received BUCY and 450 BUMEL. At 5 years, the RI was 50% and 47%, the LFS 45% and 48% and the OS 56% and 60% respectively, with no significant difference. We conclude that BUMEL is the preferable conditioning regimen for the poor risk leukemic patients, while in AML patients without poor risk cytogenetics or FLT3 both conditioning regimens are valid.
1 INTRODUCTION
During the past decades, autologous stem cell transplantation (ASCT) has been widely used as consolidation therapy in patients with acute myelogenous leukemia (AML) in first or second complete remission (CR).1 While allogeneic stem cell transplantation (allo-HSCT) has become more feasible over time thanks to the availability of alternative donors and the use of reduced intensity conditioning, ASCT has become less popular mainly because of the high incidence of relapse post transplant (RI).2
Yet, allo-HSCT is associated with higher rates of nonrelapse mortality (NRM), graft-versus-host disease (GVHD), and infection. Moreover, patients who undergo allogeneic transplant tend to have a poorer quality of life compared with patients who undergo ASCT.3 Recent data regarding ASCT have shown possible improvements with a better selection of patients such as good and intermediate 2 risk patients as per the 2010 ELN classification, and patients in molecular remission.4-8 In addition, there have been attempts at improving the high dose conditioning regimen pre ASCT by replacing oral Busulfan (BU) with the intravenous formulation9-11 and by combining BU with high dose Melphalan (BUMEL).Two previous studies, from the Italian GITMO transplant group12, 13 have suggested that the combination of Busulfan with high-dose Melphalan (BUMEL) might be associated with an improved outcome over the historical combination of BU with Cyclophosphamide (BUCY) and the European Society for Blood and Marrow Transplantation (EBMT) in a recent preliminary study5 confirmed a better outcome post BUMEL. In this latter study, the population of patients unfortunately was too small to identify possible relevant prognostic factors. In an effort to better investigate prognostic factors and in an attempt to detect patients that might get the highest benefit from BUMEL, we designed a larger study including all adult patients with de novo AML and available cytogenetics, autografted from January 2000 to December 2016 in CR1 in EBMT centers, following either BUMEL or BUCY reaching a total of 1137 patients. We believe such studies may be of importance at a time when ASCT for AML may also take advantage and become an ideal platform for other tools such as immune therapies14 including CAR-T cells15 for better in vivo purging and achievement of molecular remission and/or effective maintenance therapy post ASCT.
2 METHODS
2.1 Patients
This study is a retrospective, multicenter analysis. Data were provided by the Acute Leukemia Working Party (ALWP) of the EBMT group registry. The EBMT registry is a voluntary working group of more than 600 transplant centers that are required to report all consecutive stem cell transplantations and follow-ups on an annual basis. Audits are routinely performed to ensure the accuracy of data. Since 1990, registry patients provided informed consent authorizing the use of their personal information for research purposes. The ALWP of the EBMT group approved this study.
Eligibility criteria for this analysis included 1649 adult AML patients (age ≥18 years), all with available cytogenetics, receiving an autograft in CR1 in the period from January 2000 to December 2015 after either BUCY (n = 1137) or BUMEL (n = 512). BU was administered either IV (35%) for a total of 9.6 mg/kg (2%) or 12.8 mg/kg (33%) or per os for a total dose of 8mg/kg (6%) or 16 mg/kg (54%). MEL was administered as a single IV dose of 120 mg/m2 in 11% and 140 mg/m2 in 89% of the patients. Patients with M3 AML were excluded from the analysis. Cytogenetics abnormalities were classified according to MRC criteria.16
2.2 Statistical analysis
The primary end points of this study were Leukaemia-free survival (LFS) and overall survival (OS) at five years post transplant. Secondary endpoints included disease Relapse Incidence (RI) and NRM. OS was defined as the time between the date of transplant and death. LFS was defined as survival without relapse.
Cumulative incidences of RI and NRM were calculated from the date of transplant to the date of relapse or death in remission, respectively, with the other event being the competing risk. LFS was defined as the interval from transplant to either relapse or death in remission. Follow-up values reported correspond to patients alive when the analysis was performed.
Variables considered were patient age, sex and Karnofsky score, cytogenetics risk groups, presence of the molecular markers NPM1mutated and FLT3-ITD or mutation, year of transplant, number of induction courses to reach CR, time from diagnosis to transplant, source of stem cells (Bone Marrow versus Peripheral Blood), pretransplant conditioning (BUCY versus BUMEL) and causes of death. Univariate comparisons were done using the log-rank test for OS, LFS, and the Gray's test for RI and NRM. Multivariate analyzes were performed using Cox proportional hazards model for all endpoints. To avoid confounding factors, all factors with different distribution between the 2 types of conditioning and factors known as potentially related to the outcome were included in the final regression model, and we did not proceed to any variable selection. Interactions between conditioning and other prognostic factors were systematically tested.
All tests were two-sided. The type I error rate was fixed at 0.05 for determination of factors associated with time to event outcomes.
Statistical analyzes were performed with SPSS 24.0 (IBM Corp., Armonk, New York) and R 3.4.0 software packages (R Development Core Team, Vienna, Austria).
3 RESULTS
The follow up of alive patients was 43 months in the BUCY group and 59 months in the BUMEL group.
3.1 Overall patient population
Supporting Information Table S1 describes the two patient populations. The distribution of patient and disease characteristics was even. In particular, cytogenetics were similar in the BUCY and BUMEL patient groups with respectively 21% and 19% of the patients in the good risk, 74% and 74% in the intermediate risk and 5% and 7% in the adverse risk categories respectively. Of note however, more patients receiving BUMEL needed two induction courses to reach CR1 (so called slow remitters 59% versus 52%, P = .02) and had an interval from initial diagnosis to transplant of almost one month longer (5.5 versus 4.7 months, P < 10−3). A molecular AML marker was detected in a total of 429 BUCY and 347 BUMEL patients. More BUMEL than BUCY patients were in molecular CR1 at time of ASCT (89% versus 76%, P = .003).
Following BUMEL and BUCY, the LFS at five years were 46.9% and 41.6% and the OS 59.7% and 53.1%, not significantly different (P = .1 and P = .16). The RI were 47.7% and 53.2% (P = .07) and the NRM 5.1 and 4.9% (P = ns). The incidence of secondary allo-HSCT post relapse after ASCT was identical in the two groups (38.9% of the BUCY group and 38.7% of the BUMEL group).
The reported causes of death did not differ as well between the two groups. The major cause of death was leukemia related. Seven cases of liver veno-occlusive diseases (VOD/SOS) were reported as cause of death in the BUCY group versus 2 in the BUMEL group. Secondary malignancies as causes of death were reported in 13 patients receiving BUCY and 4 receiving BUMEL.
In multivariate analysis, the use of BUMEL had a significant favorable impact on relapse (HR:0.82; 95% CI: 0.70-0.97; P = .02), LFS (HR: 0.84; CI:0.72-0.98; P = .03) and OS (HR: 0.84; 0.70-0.99; P = .04) (Supporting Information Table S2). Other prognostic factors were cytogenetics risk groups and FLT3 status; younger patient age was a good prognostic factor for NRM, LFS and OS. In addition, we found an interaction between conditioning and adverse cytogenetic group as well as the presence of FLT3 ITD. There was no interaction with other variables.
We therefore studied separately patients in the poor risk group defined as having poor cytogenetic and/or presence of the FLT3-ITD mutation and the other included in the nonpoor risk group.
3.2 Analysis of the poor risk group (poor cytogenetics and/or presence of FLT3-ITD)
One hundred seventy six patients received BUCY and 62 BUMEL (Table 1). The distribution of the characteristics of patients receiving BUCY and BUMEL was even except for the presence of FLT3-ITD (66.5% in the BUCY group vs 43.6% in the BUMEL group, P = .001) and the duration of the interval from diagnosis to transplant which was slightly longer in the BUMEL group (5.7 months versus 4.6 months, P < 10−4). By multivariate analysis (Table 2), BUMEL was associated with a lower RI (HR: 0.52 (0.34-0.79); P = .002), a better LFS (HR: 0.54 (0.36-0.80); P = .002) and a better OS (HR: 0.61 (0.40-0.94); P = .02). At five years, the RI post BUMEL versus BUCY was 53% versus 69%, the LFS 42% versus 25%, the OS 54% versus 36% respectively (Figure 1). The NRM were identical (5.2% versus 5.6%, P = .8).
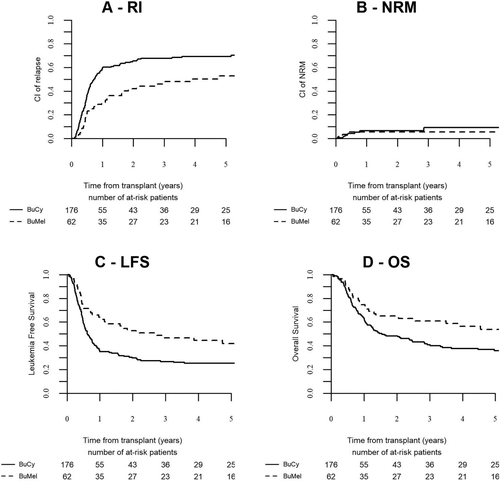
Outcome of AML patients autografted in first remission with BUCY or BUMEL as pretransplant regimen. Poor risk group defined by cytogenetics and/or the presence of a FLT3-ITD mutation. (A) Relapse incidence, (B) Non relapse mortality, (C) Leukemia free survival, (D) Overall survival
| BuCy | BuMel | Test P-value | |
|---|---|---|---|
| N | 176 | 62 | |
| Follow-up median (range) months | 48(0–175) | 52(1–55) | |
| Age at Tx_median (range) (IQR) | 52(18.6–74.6)(41.6–60.2) | 53(27.4–68.8)(44.7–59) | .85 |
| Time diag to Tx_median (range) (IQR) mo | 4.6(2.8–11.8)(3.7-6.1) | 5.7(2.8–10.4)(4.5–7.2) | <10-3 |
| Year of Tx_median (range) | 2008(2000–2016) | 2007(2000–2016) | .38 |
| Time diag to CR1_median (range) (IQR) days | 39(6–209)(32–51.5) | 43(1–245)(30.5–61.2) | .57 |
| missing | 69 | 24 | |
| Time CR1 to Tx_median (range) (IQR) | 101(2–295)(75–137.5) | 114.5(4–250)(86–156.5) | .18 |
| missing | 69 | 24 | |
| Patient male sex (%) | 75 (43 ) | 32 (52 ) | .22 |
| BM as source of stem cells(%) | 6 (3.4 ) | 1 (1.6 ) | .47 |
| Poor cytogenetics(%) | 59 (33.5) | 35 (56.4) | .001 |
| FLT3-ITD | 117 (66.5 ) | 27 (43.6 ) | |
| NPM1 neg (%) | 35 (45 ) | 8 (33 ) | .32 |
| NPM1 pos | 43 (55 ) | 16 (66 ) | |
| missing | 98 | 38 | |
| One induction to reach CR1(%) | 49 (48 ) | 14 (37 ) | .26 |
| More than 1 induction to reach CR1 | 54 (52 ) | 24 (63 ) | |
| missing | 73 | 24 | |
| not in molecular CR (%) | 15 (22 ) | 2 (11 ) | .29 |
| Molecular CR | 52 (78) | 16 (89) | |
| Subsequent transplant(%) | 45 (26) | 12 (19) | |
| BU oral (%) | 102 (60 ) | 32 (52 ) | .33 |
| BU IV | 69 (40 ) | 29 (48 ) | |
| causes of death (%) | BuCy | BuMel | |
| N | 102 | 28 | |
| Cardiac toxicity | 0 (0 ) | 1 (4 ) | |
| haemorhage | 6 (6 ) | 0 (0 ) | |
| Failure/Rejection | 1 (1 ) | 0 (0 ) | |
| VOD | 3 (3 ) | 0 (0 ) | |
| Infection | 11 (11 ) | 1 (4 ) | |
| IP | 3 (3 ) | 0 (0 ) | |
| GVHD | 1 (1 ) | 4 (15 ) | |
| Original disease | 65 (66 ) | 17 (65 ) | |
| second malignancy | 5 (5 ) | 1 (4 ) | |
| other transp related | 3 (3 ) | 2 (8 ) | |
| unknwon | 4 | 2 |
| Relapse | NRM | LFS | OS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | CI | P | HR | CI | P | HR | CI | P | HR | CI | P | |
| BUMEL vs BUCY | 0.52 | 0.34-0.79 | .002 | 0.86 | 0.25-2.97 | .82 | 0.54 | 0.36-0.80 | .002 | 0.61 | 0.40-0.94 | .02 |
| Patient age at Tx (per 10 y) | 1.08 | 0.94-1.25 | .26 | 2.52 | 1.33-4.78 | .004 | 1.14 | 0.99-1.31 | .053 | 1.23 | 1.05-1.43 | .009 |
| Female vs male | 0.78 | 0.56-1.09 | .15 | 1.28 | 0.43-3.84 | .66 | 0.82 | 0.59-1.13 | .22 | 0.83 | 0.58-1.18 | .30 |
| Year of Tx | 0.97 | 0.92-1.01 | .17 | 0.97 | 0.83-1.12 | .65 | 0.97 | 0.92-1.01 | .16 | 0.97 | 0.92-1.02 | .21 |
| Time from diag. To Tx (mo) | 1.002 | 0.92-1.09 | .95 | 0.96 | 0.71-1.29 | .79 | 0.99 | 0.92-1.08 | .97 | 1.002 | 0.91-1.10 | .97 |
| FLT3-ITD vs dversecytogenetics | 0.85 | 0.60-1.21 | .37 | 1.34 | 0.42-4.32 | .62 | 0.87 | 0.62-1.22 | .44 | 0.86 | 0.59-1.24 | .41 |
The major cause of death was leukemia related. 3 liver veno-occlusive diseases (SOS) were reported as cause of death in the BUCY and none in the BUMEL group.
3.3 Analysis of the non poor risk group (good or intermediate cytogenetics, FLT3 wild type)
The non poor risk group included nine hundred sixty one BUCY and 450 BUMEL patients. Supporting Information Table S3 describes the two patient populations. The distribution of patient and disease characteristics differed in the following: more patients receiving BUMEL needed two induction courses to reach CR1 (59% versus 51%, P = .04) and had an interval from initial diagnosis to transplant of almost one month longer (5.4 versus 4.7 months, P < 10−3). A NPM1 mutation was detected in a total of 145 (45%) BUCY and 62 (54%) BUMEL patients.
Following BUMEL and BUCY, the LFS at five years were 47.6% and 44.6% and the OS 60.5% and 56.4%, not significantly different (P = .74 and P = .68). The RI were 46.9% and 50.2% (P = .57) and the NRM 5.1 and 4.8% (P = .5) respectively (Figure 2). By multivariate analysis, age was a significant prognostic factor for NRM and OS, more recent year of transplant was associated with a better OS and intermediate cytogenetic group was associated with a higher RI, a lower LFS and OS compared to good cytogenetic risk group. The nature of the conditioning regimen had no significant impact.
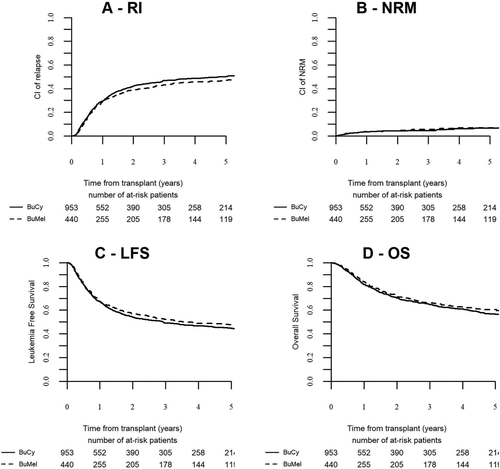
Outcome of AML patients autografted in first remission with BUCY or BUMEL as pretransplant regimen. Nonpoor risk group. (A) Relapse incidence, (B) Non relapse mortality, (C) Leukemia free survival, (D) Overall survival
4 DISCUSSION
ASCT has been widely used in the past to consolidate patients with AML. Data from large historical series1, 17, 18 have indicated for AML autografted in CR1, LFS of 45 to 50% and RI of 40% to 45%. Results of meta analyzes of numerous randomized studies comparing ASCT with allogeneic HLA identical sibling transplants and with conventional maintenance chemotherapy19, 20 have indicated that RI post ASCT as high as it may be, is still lower than following conventional chemotherapy. More recent data have confirmed this finding.21, 22
The high incidence of RI post ASCT, as well as numerous developments in the field of allogeneic transplants such as the use of reduced intensity conditioning and alternative donors which renders this transplant modality feasible in almost all patients explain that the interest in ASCT has faded away. Yet, the quality of life of survivors is better post ASCT.3
ASCT in AML has been recently revisited4, 23 taking into account recent improvements resulting mainly from better selection of patients in the good risk and intermediate risk of the ELN classification, the use of new pretransplant conditioning regimens and a better monitoring of minimal residual disease leading to ASCT preferentially done at time when detection of MRD is negative both in the patients and in the leukapheresis products.
In particular, a recent study from EBMT compared unrelated matched versus autologous transplantation in patients with AML in first molecular remission8: using the 2010 ELN classification, the outcome was superior with ASCT in good risk and equivalent in intermediate 2 risk patients. Patients in the intermediate 1 risk category (including those with FLT3ITD mutation) did better following unrelated donor transplants.
Regarding the pretransplant conditioning regimens, the BUCY combination has been historically the reference with similar outcomes to the combination of Cyclophosphamide and Total body irradiation.24 An important improvement has been the introduction of the IV formulation of BU which has more predictable pharmacokinetics and bioavailability and less toxicity than oral Busulfan. Of interest, a recent retrospective survey from the ALWP of the EBMT10 which included 952 patients with AML who received IVBU before ASCT, reported that OS was 67%, LFS 53%, and RI 40% at 2 years. NRM at 2 years was 7%. The 2-year LFS and RI did not differ significantly between patients transplanted in CR1 and in CR2
High dose Melphalan alone or combined with TBI has been used historically before autografting patients with AML, first in relapse, and later to consolidate CR.25, 26 It has been shown to have a potent immediate anti leukemic activity, however followed by a high rate of relapses.
The BUMEL combination has been advocated by several teams essentially for lymphoid malignancies. For instance, the BUMEL combination with Melphalan at the dose of 140mg/m2 has been shown by Spanish teams to produce better disease control in multiple myeloma26, 27 than high dose Melphalan alone at the conventional dose of 200 mg/m2. BUMEL as preparative conditioning has been initially reported in adult patients with AML by the Gruppo Italiano di Trapianto di Midollo Osseo (GITMO)13: in a retrospective study, 129 AML patients with a median age of 50 years, in first CR received BUMEL as conditioning regimen before ASCT. With a median follow-up of 31 months, the 8-year projected OS and LFS was 62% and 56% respectively for the whole population. The authors concluded that BUMEL is associated with a low toxicity profile (mainly mucositis) and mortality and is an effective conditioning regimen for patients with AML in CR1 undergoing ASCT.
Subsequently, we performed a retrospective study on behalf of the EBMT comparing BUMEL and BUCY in 853 patients with available cytogenetics who underwent ASCT in first remission in the period from 2005 to 20135: Of those, 257 received BUMEL and 596 BUCY. The study showed that Patients autografted with BUMEL did better than those autografted with BUCY with a lower RI (39.5% versus 52.2%) (HR: 0.65; CI: 0.49-0.87; P = .003) a better LFS (55.4% versus 44.6%) (HR: 0.69; CI: 0.53-0.89; P = .005) and a better OS (73.8% versus 63%) (HR: 0.62; CI: 0.47-0.82; P = .0007). There was no difference for NRM (4.5% and 3.2%).
None of these studies however could take advantage of populations of patients large enough to study prognostic factors and possibly identify the subpopulations that might get the highest benefit with BUMEL.
In the present study in a larger patient population, we found an interaction between the conditioning regimen and the patient population risk category; we therefore studied separately the poor risk and the nonpoor risk group which notably led to the finding that only the poor risk group benefitted from the use of BUMEL rather than BUCY. This finding was rather unexpected since the consensus in the past thirty years or so has been that chemosensitive and not chemoresistant tumors get the highest benefit from high dose consolidation with ASCT. One possible explanation of our finding can be that for chemosensitive AML patients, both BUMEL and BUCY achieve similar results; poor risk patients may in fact represent the best target population where one can evaluate the relative tumor cell killing efficacy of the two conditioning regimen and conclude in favor of BUMEL. In this poor risk patient group the rather low two and five years LFS of 30% and 25% respectively post BUCY is indeed in keeping with the historical general consensus that ASCT is not recommended in this patient group. Conversely, the two year LFS of 53% and moreover the 42% LFS at five years with BUMEL, a new heretofore observation, may now challenge this consensus.
We conclude that there is room for improvement of outcome post ASCT using BUMEL as conditioning pretransplant. Further, new targeted therapies are emerging: for instance sorafenib has been recently introduced as maintenance therapy post allogeneic transplantation28 and could in a similar way be introduced post autologous transplants.
Other recent tools are monoclonal antibodies and CART cells.16 ASCT may well become an ideal platform for immunotherapy post transplant. It is conceivable that adult patients with AML may in the near future get benefit from ASCT followed by targeted therapies for both consolidation pre ASCT and maintenance post ASCT, hopefully reducing the relapse rate.
ASCT remains a therapeutic option to consider with no risk of severe chronic GVHD and a predictable better quality of life than allo-HSCT for carefully selected patients. Our study may justify reconsidering randomized studies including revisited and optimized ASCT in AML, using BUMEL as well as new targeted therapies, in patients reaching first molecular remission.
CONFLICT OF INTERESTS
No conflict of interest disclaimed




