Hereditary hypochromic microcytic anemia associated with loss-of-function DMT1 gene mutations and absence of liver iron overload
Funding information: This work was supported in part by grant n. 17552 from the Associazione Italiana per la Ricerca sul Cancro (AIRC).
Hypochromic microcytic anemias are due to altered erythroblastic iron availability reducing the hemoglobin (Hb) content of red cells.1 In addition to the most common causes of hereditary microcytic anemias (thalassemias, thalassaemic hemoglobinopathies and sideroblastic anemias), several atypical forms have been identified.1 Such disorders are associated with inherited changes of genes (TMPRSS6, transferrin, DMT1, STEAP3, ALAS2, GLRX5, and ceruplasmin) involved in iron metabolism.1 However, the scarce number of cases identified has not yet allowed their precise molecular and clinical characterization.
DMT1 is an integral membrane protein acting as a proton-coupled divalent metal transporter. It is expressed at the apical border of duodenal enterocytes where it allows the uptake of dietary/nonheme iron. In other tissues (especially in erythroid precursors), DMT1 mediates the transfer of iron, internalized by transferrin, from the endosomes to cytoplasm.2 Recently, it has been suggested that DMT1 allows the metal import into the mitochondria in addition to the previous described so-called “kiss-and-run” fusion mechanism by which endosomes and lysosomes directly transfer iron to these organelles.3 Pivotal studies performed in mice and rats identified a DMT1 missense mutation as responsible for a microcytic phenotype. Subsequently, DMT1 mutations have been described in six patients with hypochromic microcytic anemia. All subjects but one presented liver iron overload.4 Importantly, except for two cases, no DMT1 immunoblotting analyses were performed.
Here, we report the identification of a patient with microcytic hypocromic anemia due to two novel DMT1 alterations. The patient presented transfusion-dependent anemia until she was 3 years old (Supporting Information Table S1). Then, recombinant erythropoietin (rEpo) treatment was started at 15 μg/week, avoiding any further blood transfusions (Figure 1A). The rEpo dose was increased over-time and, at present, it is 150 μg/week administered subcutaneously. The child is healthy, her growth curves are constantly between 50th-75th centiles and satisfactory Hb levels have been maintained. Liver iron concentration (LIC) was calculated from liver T2* measured by magnetic resonance imaging at the age of 6, 10, and 11 years with no liver and/or cardiac iron overload detected (Figure 1B).
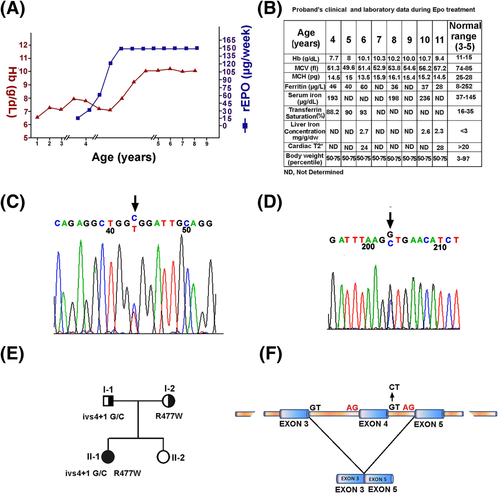
(A) Values of hemoglobin (Hb, g/dL) in relation to recombinant erytropoietin (rEPO) treatment. (B) Proband's clinical and laboratory data during rEpo treatment. (C) Genomic sequence of DMT1 exon 15 of the proband identifying the R477W mutation; (D) Genomic sequence of DMT1 exon 4 intron 4 junction of the proband identifying ivs4 + 1 G/C change; (E) Pedigree of the family with 2 new DMT1 mutations; (F) Schematic representation of DMT1 exon 4 skipping in the proband
Exons and exon-intron boundaries sequencing of DMT1 gene allowed the characterization of our patient as a compound heterozygote for two new DMT1 mutations. We found a C > T transition at nucleotide 1429 of exon 15 (R477W) (Figure 1C) and a G > C substitution at position +1 bp of the splice-donor site within intron 4 (ivs4 + 1 G/C) (Figure 1D). The R477W was inherited from proband's mother, while ivs4 + 1 G/C was of paternal inheritance (Figure 1E). The mutation R477W was not found in single nucleotide polymorphism (SNP) databases (htpp://www.ncbi.nlm.nih.gov/SNP/) nor detected by sequencing the corresponding exon in 50 healthy subjects. A different change on R477 residue (R477Q) has been detected in a patient with BRAFV600E-mutant metastatic melanoma. Particularly, this was one of the four DMT1 mutations acquired upon treatment of melanoma patients with Vemurafenib, a Raf kinase inhibitor. The study suggests that mutations in DMT1 could be associated to the drug resistance.5 The amino acid substitution R477W found in our patient is predicted to be damaging by Polyphen multicriteria software (http://www.bork.embl-heidelberg.de/PolyPhen/). To investigate whether ivs4 + 1 G/C mutation might affect RNA maturation by inactivating the splice-donor site, DMT1 mRNAs (from PBLs) was analysed. cDNA amplification/sequencing of the DMT1 mRNA region between exons 2 and 8 produced in controls a single transcript (522 bps), while 2 transcripts were obtained from the proband cDNA (Supporting Information Figure S1A). The improperly spliced RNA lacks exon 4 but maintains the correct reading frame (Figure 1F). The putatively translated protein should lack 42 amino acids from residues 62 to 103 that might result into uncorrected protein insertion/activity into plasma membrane (Supporting Information Figure S1B). The proband exon 4-including DMT1 transcript was reduced to 50–60% of the DMT1 mRNA level of a control subject (not shown). To evaluate the effect of mutations on DMT1 protein content, we prepared cell extracts from the EBV-transformed lymphoblasts of the proband and her relatives and analysed them by gel-electrophoresis/immunoblotting. A 30–40% reduction of the protein was evidenced in the proband and her father compared to I-2 (Supporting Information Figure S2A). No signals associated to a smaller DMT1 isoform were evidenced suggesting that mRNA lacking exon 4 was not efficiently translated or, alternatively, that the mutated protein was unstable (Supporting Information Figure S2B).
So far, the major clinical features of the six previously described patients include hypocromic and microcytic anemia, high serum iron and transferrin saturation, increased serum soluble transferrin receptor, with normal or slightly increased serum ferritin. Liver iron overload was reported in all but one of the described cases. Recent studies have shown a mitochondrial membrane localization for DMT1, suggesting a role in iron uptake by mitochondria, where the first steps of heme synthesis occur. Mice lacking hepatic DMT1 (Dmt1liv/liv mice) that do not show measurable changes in liver iron suggest that DMT1 is dispensable for the overall hepatic iron economy.6 In particular, DMT1 mutations do not alter liver NTBI (non transferrin-bound iron) uptake and faintly reduce TBI incorporation. We suggest that liver iron accumulation might not be directly due to DMT1 mutation, and perhaps be just a consequence of transfusions or of anemic-state per-se. It is also possible that recombinant Epo treatment may prevent potential liver damage. In conclusion, although very rare, DMT1 deficiency must be taken into consideration in the differential diagnosis of neonatal microcytic hypochromic anemia. Indeed, an early identification and an immediate rEpo treatment improve the management of the disease and may avoid transfusions.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study received approval from the Institutional Review Board of University of Campania, Italy.
CONFLICT OF INTEREST
The authors declare no competing financial interests.




