Hyper-CVAD plus nelarabine in newly diagnosed adult T-cell acute lymphoblastic leukemia and T-lymphoblastic lymphoma
Abstract
Nelarabine, a water soluble prodrug of 9-β-D-arabinofuranosylguanine (ara-G), is a T-cell specific purine nucleoside analogue. Given its activity in relapsed and refractory T acute lymphoblastic leukemia (T-ALL) and T lymphoblastic lymphoma (T-LBL), we sought to define its role in the frontline treatment of adult patients. Therefore, we conducted a single arm phase 2 study to determine the safety and efficacy of nelarabine in combination with hyper-CVAD in newly diagnosed patients. For induction/consolidation, patients received eight cycles of hyper-CVAD alternating with high-dose methotrexate and cytarabine plus two cycles of nelarabine given at a dose of 650 mg/m2 intravenously daily for 5 days. This was followed by thirty months of POMP maintenance chemotherapy with two additional cycles of nelarabine given instead of cycles 6 and 7 of POMP maintenance. Sixty-seven patients, including 40 with T-ALL and 26 with T-LBL, were enrolled. Complete response rates in both T-ALL and T-LBL were 87% and 100% respectively. Grade 3 to 4 neurotoxic adverse events were reported in 5 patients. There were 21 relapses (31%) including 2 after allogeneic stem cell transplantation. Median duration of follow-up was 42.5 months. The 3-year complete remission duration (CRD) and overall survival (OS) rates were 66% and 65%, respectively. Compared to our historic hyper-CVAD data, there was no survival benefit with the addition of nelarabine. In conclusion, hyper-CVAD plus nelarabine was well tolerated and active in the frontline treatment of adult T-ALL/LBL patients.
1 INTRODUCTION
Historically, the outcome of adult patients with newly diagnosed T-acute lymphoblastic leukemia (T-ALL) and T-lymphoblastic lymphoma (T-LBL) has been poor with low complete response (CR) rates and short median overall survival (OS) ranging from 11 to 17 months.1-4 With the use of intensive chemotherapy regimens, maintenance therapy, and intensive central nervous system (CNS) prophylaxis, CR rates have significantly improved reaching 90% to 95% with long-term disease-free survival rates of 56% to 65%.5-9 Despite this improvement, OS rates have not exceeded 67% with 30% to 40% of the patients relapsing underscoring the need for better therapeutic strategies.5-8
Nelarabine, a prodrug of 9-β-D-arabinofuranosylguanine (ara-G), is an ideal T-cell specific purine nucleoside analogue. Upon demethoxylation and intracellular phosphorylation, it is converted to ara-G triphosphate preferentially accumulating in T-lymphoblasts inhibiting DNA replication.10-13 Numerous studies have confirmed its antitumor activity in both adult and pediatric patients with relapsed/refractory T-ALL/LBL leading to its accelerated approval in October 2005 by the Food and Drug Administration for this indication.14-18 In this heavily pretreated population, nelarabine induced CR in 31% to 36% of adult patients with a 1-year OS rate ranging from 24% to 28%.14, 15
Given its activity in relapsed disease, the role of nelarabine combined with intensive chemotherapy in the frontline setting has been of significant interest. In the pediatric experience, newly diagnosed T-ALL patients with slow early response to treatment achieved a 5-year event-free survival of 69% with intensive chemotherapy plus nelarabine identical to that achieved by rapid early responders who did not receive nelarabine.19
There is limited data on the use of nelarabine in the frontline setting in adult patients. Therefore, we conducted a single-arm phase 2 study to evaluate the safety and efficacy of hyper-CVAD plus nelarabine in the frontline treatment of adult patients with T-ALL/LBL. We previously published preliminary results of this study and here we report an update on the outcome of 67 patients treated at our institution.20
2 MATERIALS AND METHODS
2.1 Patient population
Adult patients with previously untreated or minimally pretreated (failure to one induction course or achieving CR after ≤ 2 cycles) T-ALL/LBL were enrolled (NCT00501826). The study was conducted according to the standards of Good Clinical Practice, per institutional research policies and procedures, and in accordance with the Declaration of Helsinki. Approval was obtained from The University of Texas M.D. Anderson Cancer Center's institutional review board.
Eligible patients had to have an Eastern Cooperative Oncology Group performance status of ≤ 3 and adequate renal and hepatic function [serum creatinine ≤ 2 mg/dL (≤ 2.5 mg/dL acceptable if due to disease), total bilirubin ≤ 2 mg/dL (≤ 5 mg/dL acceptable if due to disease), aspartate aminotransferase and alanine aminotransferase ≤ 4 x upper limit of normal]. All patients provided a written informed consent prior to study enrollment. Patients were diagnosed per the WHO classification criteria and categorized into immunophenotypic subtypes (early, thymic, or mature) as detailed previously.21, 22 Early T-cell precursor (ETP) ALL/LBL was defined by: (1) lack of CD1a and CD8 expression, (2) absent or weak CD5 expression, and (3) expression of at least 1 myeloid (CD11b, CD13, CD33, CD117) or stem cell (CD34, HLA-DR) marker.23 A subset of patients were categorized as non-ETP not otherwise specified (NOS) based on presence of CD5 and CD8 expression, however lack of data on CD1a expression prohibited further subtyping. Complex cytogenetics was defined as ≥3 chromosomal abnormalities in ≥2 metaphases.
2.2 Study design and treatment regimen
This is a single-arm phase 2 study designed to determine the efficacy, measured by CR rate, CR duration (CRD), and OS, and safety of adding nelarabine to hyper-CVAD in newly diagnosed patients with T-ALL/LBL. Therapy consisted of the standard hyper-CVAD regimen as previously described.5 Patients received eight induction-consolidation cycles of hyper-CVAD (cycles 1, 3, 5, 7) alternating with high-dose methotrexate (MTX) and cytarabine (cycles 2, 4, 6, 8) followed by two cycles of nelarabine [650 mg/m2 intravenously (IV) daily for 5 days] every 21 to 35 days (Supporting Information Figure S1). After treating 30 patients, the protocol was amended to introduce nelarabine earlier in induction after cycles four and five, in an attempt to prevent early relapses and improve survival (Supporting Information Figure S1).
All patients received CNS prophylaxis consisting of eight intrathecal treatments as previously described.5 Treatment of active CNS leukemia and the indication for therapeutic cranial irradiation have been published.5 Patients with initial bulky mediastinal disease (≥ 7cm) or residual mediastinal disease at the end of the induction/consolidation portion of therapy were considered for local consolidative mediastinal irradiation (XRT; 30–39 Gy over 4 to 5 weeks) after recovery from intensive chemotherapy and prior to the start of maintenance therapy.
Patients received thirty months of 6-mercaptopurine, methotrexate, vincristine, and prednisone (POMP) maintenance chemotherapy as previously detailed.5 Two additional cycles of nelarabine were given instead of cycles 6 and 7 of POMP maintenance as early intensification. MTX (100 mg/m2 IV on day 1) plus pegylated-asparaginase (2000 IU/m2 IV on day 2; dose reduced to 1000 IU/m2 for age >60 years) and hyper-CVAD were given instead of cycles 18 and 19 of POMP maintenance as late intensification (Supporting Information Figure S1). Supportive care measures based on standard institutional practice were provided to all patients.
2.3 Response criteria
For T-ALL, CR was defined as ≤ 5% blasts in the bone marrow (BM) with normalization of peripheral blood counts (ANC ≥ 1 × 109/L and platelet count ≥ 100 × 109/L) and complete resolution of extramedullary disease. Partial response (PR) was defined similarly except for the presence of 6 to 25% BM blasts. For T-LBL and extramedullary disease, CR was defined as complete resolution of all known disease and PR was defined as ≥ 50% reduction in tumor size assessed by computed tomography (CT).
2.4 Disease monitoring and immunophenotyping
For patients with BM involvement, BM evaluation was performed on day 14 of cycle 1 then weekly until remission. Once in CR, BM evaluation was repeated every 4 months. For T-LBL, response was assessed by CT scans after cycle 1 and then repeated every 1 to 2 months until achievement of best response to therapy. Minimal residual disease (MRD) was assessed in remission BM samples using multiparameter flow cytometry with a sensitivity of 0.01%. For MRD analysis, a panel of markers including CD1a, CD2, CD3 (surface and cytoplasmic), CD4, CD5, CD7, CD8, CD10, CD13, CD19, CD33, CD34, CD38, CD45, CD52, CD117, HLA-antigen D related (HLA-DR), myeloperoxidase, and terminal deoxynucleotidyl transferase were assessed on at least 200,000 aspirated nucleated BM cells.
2.5 Safety assessment
Adverse effects were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. Guidelines for dose modifications were previously detailed.5 Nelarabine was dose reduced to 650 mg/m2 IV on alternate days (1, 3, 5) for patients who develop grade 2 therapy-related neurotoxicity or have grade 1 or 2 neurotoxicity prior to nelarabine courses. Nelarabine was discontinued for grade ≥ 3 neurotoxicity except for somnolence, malaise, fatigue, or lethargy that resolve within 24 hours
2.6 Statistical analysis
Differences in the distribution of variables among subgroups were analyzed using x-squared and Mann-Whitney U tests (or Fisher exact tests). OS was calculated from start of therapy until date of death or last follow-up. CRD was measured from date of CR until relapse, death, or last follow-up; death in CR was a censored event. OS and CRD curves were plotted using Kaplan-Meier method and compared using the log-rank test.
3 RESULTS
3.1 Patient characteristics
From August 2007 to May 2016, 67 patients with T-ALL/LBL were enrolled; 40 patients (60%) had T-ALL, 26 (39%) had T-LBL, and 1 (1%) had biphenotypic disease. Patient characteristics are summarized in Table 1. Median age was 37 years (range, 18–78) with male predominance. At diagnosis, four patients (6%) had CNS involvement and 34 (51%) had mediastinal disease. Median white blood cell (WBC) count at presentation was 7.8 × 109/L (range, 0.8–309.2) and 11 patients (16%) had a WBC count >100 × 109/L. Of 65 patients with sufficient immunophenotyping data, 25 (37%) were categorized as thymic, 15 (22%) as ETP, 12 (18%) as early non-ETP, 9 (13%) as mature, and 4 (6%) as NOS. Forty-one patients (61%) had diploid and 12 (18%) had complex karyotype by conventional cytogenetics. Mutational analysis demonstrated wild-type TP53 in all 17 (25%) tested patients.
| Parameters | Hyper-CVAD + Nelarabine (N = 67) | Hyper-CVAD (N = 75) | P-value |
|---|---|---|---|
| Median age (years) [range] | 37 [18–78] | 30 [17–74] | .1 |
| Male gender | 51 (76) | 55 (73) | .7 |
| ECOG Performance Status | |||
| 0–1 | 58 (87) | 61 (81) | .24 |
|
2 3 |
9 (13) 0 |
11 (15) 3 (4) |
|
| Diagnosis | |||
| T-ALL | 40 (60) | 46 (61) | .57 |
| T-LBL | 26 (39) | 29 (39) | |
| Biphenotypic | 1 (1) | 0 | |
| Immunophenotype | |||
| Thymic | 25 (37) | 26 (35) | |
| Early T-cell precursor (ETP) | 15 (22) | 7 (9) | .25 |
| Early Non-ETP | 12 (18) | 5 (7) | |
| Mature | 9 (13) | 9 (12) | |
| Not Otherwise Specified | 4 (6) | 0 | |
| Not Available | 2 (3) | 28 (37) | |
| CNS involvement | 4 (6) | 5 (7) | .87 |
| Mediastinal disease | 34 (51) | 54 (72) | .01 |
| Karyotype | |||
| Diploid | 41 (61) | 49 (65) | |
| Complex | 12 (18) | 4 (5) | .18 |
| Miscellaneous | 8 (12) | 11 (15) | |
| ND/IM | 6 (9) | 11 (15) | |
| Median WBC count (× 109/L) [range] | 7.8 [0.8–309.2] | 10.8 [1–397.9] | .56 |
| ≥100 × 109/L | 11 (16) | 9 (12) | .45 |
| Median BM blasts (%) [range] | 9 [0–96] | 47 [0–99] | .29 |
| Prior therapy in CR | 12 (18) | 3 (4) | .007 |
| Overall response rate (CR+PR) | 53 (96) | 69 (96) | |
| Complete response | 51 (93) | 63 (88) | |
| Partial response | 2 (4) | 6 (8) | .56 |
| No response | 2 (4) | 2 (3) | |
| Early death (within first month) | 0 | 1 (1) | |
| Relapse rate | 21 (31) | 28 (37) | .45 |
| Median time to relapse (mths) [range] | 7.3 [1.4–62] | 11 [3–40] | |
| Deaths | 25 (37) | 38 (51) | .11 |
| Median follow-up (mths) [range] | 42.5 [4–110] | 145 [49–267] |
- Abbreviations: T-ALL: T-cell acute lymphoblastic leukemia; T-LBL: T-lymphoblastic lymphoma; ND: not done; IM: insufficient metaphases; BM: bone marrow; CR: complete remission; PR: partial response; mths: months.
3.2 Efficacy and response rates
Twelve patients (18%; T-ALL, N = 10; T-LBL, N = 2) received prior hyper-CVAD therapy and were in CR at the time of enrollment. Among the remaining 55 patients, the overall response rate was 96% of which 51 patients (93%) achieved CR and 2 (4%) achieved PR. There was no difference in the CR rate in ETP versus non-ETP subtypes (93% and 94%, respectively; P = .93), with a median time to CR of 1.2 months (range, 0.4–8.4). Among the T-ALL patients who achieved CR (N = 36), 83% became MRD negative, of which 47% achieved MRD negativity at the time of CR.
After a median follow-up of 42.5 months (range, 4–110), 42 patients (63%) remain alive of which 37 (55%) are still in CR; including two patients taken off study due to MRD relapse and chronic pleural effusion precluding MTX administration (Supporting Information Figure S2). Eleven patients (7 ETP, 3 early non-ETP, 1 thymic with complex karyotype) received allogeneic stem cell transplantation (SCT) after achieving first CR (CR1) and remain alive post SCT. Of these patients, 9 remain in CR and 2 relapsed post SCT.
Twenty-one patients (31%) relapsed (T-ALL, N = 13; T-LBL, N = 8) with a median time to relapse of 7.3 months (range, 1.4–62). All patients achieved CR prior to relapse including 10 T-ALL (77%) patients achieving MRD negativity. Sites of relapse included 12 hematologic (peripheral blood and BM), 5 extramedullary (EM; 4 mediastinal and 1 rib), 3 BM plus EM (2 adenopathy and 1 mediastinal), and 1 BM plus CNS in distribution.
Of the 34 patients with mediastinal disease at presentation, 20 (59%) received XRT following induction/consolidation. The remaining 14 patients (41%) did not receive XRT due to relapse (N = 4), noncompliance/refusal (N = 3), withdrawal of consent (N = 2), SCT (N = 2), physician choice (N = 1), congestive heart failure (N = 1), and failure to meet XRT criteria (N = 1). Two relapses, including 1 mediastinal relapse, occurred among those who received XRT compared to 5 relapses, 3 mediastinal in distribution, in patients who did not receive XRT.
Twenty-five patients died (37%), including 1 partial responder and 1 nonresponder dying after third salvage therapy off study. The remaining deaths occurred in the CR group; causes of death were relapse (N = 18), secondary acute myeloid leukemia (AML; N = 3), intracranial hemorrhage (N = 1), and unknown etiology (N = 1), the latter two patients died in CR. Early deaths (within the first month) did not occur. Among the 3 secondary AML patients, 2 had AML and 1 patient had mixed phenotype acute leukemia (myeloid/B-cell lineage) with no evidence of T-cell disease.
3.3 Survival
The 3-year probability of CRD and OS for the whole cohort was 66% (95% CI: 52–77) and 65% (95% CI: 51–76), respectively, with a median OS of 82 months (Figure 1A,C). There was a nonsignificant statistical improvement in the CRD (69% vs 63%, P = .54) and OS (71% vs 62%, P = .41) for patients with T-LBL when compared to T-ALL (Figure 1A,C). When analyzed by immunophenotype, no significant differences in CRD or OS were observed among the various subsets (Figure 1B,D). Unlike previous reports, presenting WBC count had no impact on OS (Figure 2A).24 Similarly, there was no difference in OS between the two different nelarabine regimens (Figure 2D). Patients with complex cytogenetics (≥ 3 chromosomal abnormalities) had significantly inferior CRD and OS when compared to patients with noncomplex karyotype (Figure 2B,C).
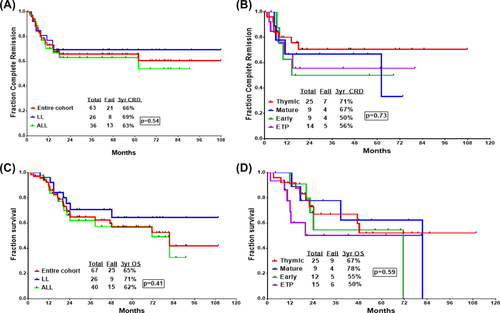
Time-to-event analysis of patients treated with hyper-CVAD plus nelarabine. (A) Complete remission duration (CRD) for the whole cohort. (B) CRD according to immunophenotype. (C) Overall survival (OS) for the whole cohort. (D) OS according to immunophenotype [Color figure can be viewed at wileyonlinelibrary.com]
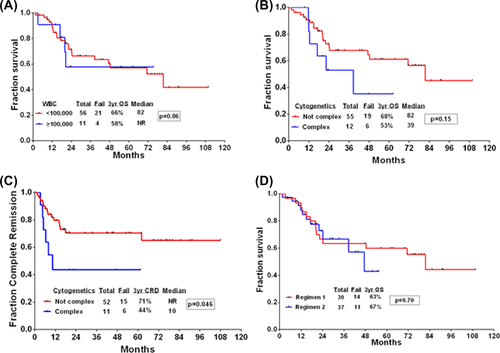
Outcomes of patients treated with hyper-CVAD plus nelarabine. (A) Overall survival (OS) stratified by WBC count upon presentation. (B) OS stratified by karyotype. (C) Complete remission duration stratified by karyotype. (D) OS for patients treated using the two different nelarabine regimens [Color figure can be viewed at wileyonlinelibrary.com]
3.4 Safety and toxicity
Grade ≥ 3 nonhematological treatment emergent adverse events were reported in 91% of patients with the most common toxicities including infections (84%), hypokalemia (46%), and hypophosphatemia (40%) (Supporting Information Table S1). Fifty-five patients (82%) received nelarabine (1 cycle, N = 7; 2 cycles, N = 19; 3 cycles, N = 8; 4 cycles, N = 21). The remaining 12 patients did not receive nelarabine due to: early relapse (N = 4), withdrawal of consent (N = 3), no response (N = 2), SCT (N = 1), patient's choice (N = 1), and death (N = 1). Seventy-two percent of patients experienced at least one form of neurotoxicity. The most common neurotoxicities were peripheral neuropathy (69%), headache (6%), and altered mental status (AMS; 4%). The majority of the neurotoxicities were grade 1 and 2 in severity. Five patients (7%) experienced grade ≥ 3 neurotoxicity including AMS (N = 2), headache (N = 1), peripheral neuropathy (N = 1), and nelarabine-related pain (N = 1). The incidence of neurotoxicity was similar for both nelarabine regimens [regimen 1, N = 24 (80%); regimen 2, N = 24 (65%)].
3.5 Comparison with historical hyper-CVAD data
To help us determine the role of nelarabine in the frontline setting, we compared these results to our historical hyper-CVAD data. Newly diagnosed T-ALL/LBL patients treated at our institution on four prospective clinical trials from 1992 to 2007 with hyper-CVAD alone (ID02–230, CPPDM92-048, DM93-077, and DM99–414) were reviewed. This group included patients treated with the standard and modified hyper-CVAD regimens. Details regarding these regimens were previously described.5 We compared both cohorts with regards to baseline characteristics, CRD, and OS (Table 1). Subset analysis comparing both regimens by diagnosis (T-ALL vs T-LBL) and by immunophenotype (ETP vs Non-ETP) was performed.
We found no difference in CRD and OS for the whole cohort with the addition of nelarabine (Figure 3A,B). In patients with T-LBL there was a trend toward superior CRD with the use of hyper-CVAD alone which did not translate into an improvement in OS (Figure 3C,D). However in T-ALL patients, there was a nonsignificant improvement in the CRD with the combination regimen without an improvement in OS (Figure 3E,F). In ETP-ALL/LBL patients, the 3-year OS and CRD was higher using the combination regimen, although these were not statistically significant (P-values, .28 and .67, respectively) (Figure 3G,H).
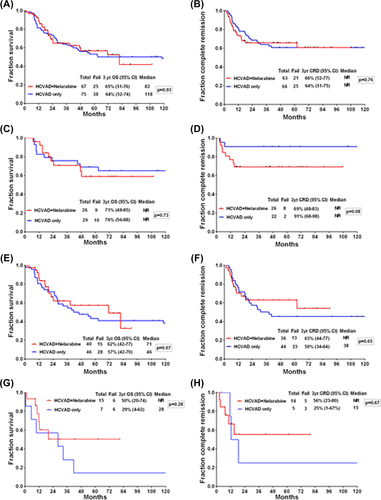
Outcomes of patients treated with hyper-CVAD plus nelarabine compared to historic hyper-CVAD data. (A) Overall survival (OS) per treatment regimen. (B) Complete remission duration (CRD) per treatment regimen. (C) OS for patients with lymphoblastic lymphoma. (D) CRD for patients with lymphoblastic lymphoma. (E) OS for patients with T-cell acute lymphoblastic leukemia. (F) CRD for patients with T-cell acute lymphoblastic leukemia. (G) OS for patients with early T-cell precursor (ETP) immunophyenotype. (H) CRD for patients with early T-cell precursor (ETP) immunophyenotype [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
Hyper-CVAD plus nelarabine is safe and active in the frontline setting with the ability to induce a high CR rate (93%) and durable remissions with no early mortality and a 3-year OS rate of 65%. Earlier administration of nelarabine had no impact on outcome or toxicity. All three patients who developed secondary AML succumbed to the disease indicating the poor prognosis associated with this small subset of patients as previously published by Verma et al.25 Despite the addition of nelarabine, the relapse rate remained high at 31% similar to previous studies.5-7 Outcome of relapsed patients was dismal with 18 deaths (86%) underscoring the need to improve frontline combination regimens.
Intensive chemotherapeutic regimens have significantly improved the outcomes of newly diagnosed adult patients with T-ALL/LBL.5-8 In the study conducted by Thomas et al, hyper-CVAD induced CR in 91% of T-LBL patients with 5-year CRD and OS rates of 62% and 67%, respectively.5 Comparable results have been reported using the Berlin-Frankfurt-Muenster and the UKALL XII/ECOG 2993 protocols.6, 7, 24 Given the similarity to our results, we compared the combination regimen to our historic hyper-CVAD data and found no difference in OS (65% vs 64%), suggesting no added benefit from the use of nelarabine. Similar conclusions have been drawn in the relapsed/refractory setting, since the 1-year OS reported with single-agent nelarabine was identical to that reported with multiagent chemotherapeutic regimens (24–28% vs 24%).14, 15, 26
However, in subset analysis we found a small nonstatistically significant improvement in the CRD (63% vs 50%, P = .65) with the combination regimen in patients with T-ALL. Furthermore, patients with ETP ALL/LBL experienced an improvement in OS and CRD from 29% and 25%, in the hyper-CVAD group, to 50% and 56% in the hyper-CVAD plus nelarabine cohort, however the number of patients were too small to achieve statistical significance. Large-scale randomized studies are needed to determine the role of nelarabine in the frontline treatment of higher-risk adult T-ALL/LBL patients.
As previously reported, patients with T-LBL had better outcomes compared to T-ALL.21, 27, 28 The role of SCT in adult T-ALL remains unclear; with some authors recommending SCT in all patients in CR1 and others restricting SCT to higher-risk patients. Immunophenotype has been found to be the most important prognostic factor for T-ALL with early and mature subtypes associated with the worst OS, 28% and 29%, respectively.29 Therefore, in the GMALL studies, SCT in CR1 was recommended to these patients with substantial improvement in the OS of patients within the mature subgroup from 25% without SCT to 59% in transplanted patients suggesting a potential role for SCT in this patient population.30 However, in a recent study by Jain et al, mature T-ALL/LBL patients treated with intensive chemotherapeutic regimens had a 5-year OS of 70% thereby questioning the role of SCT in these patients.21 Here, we report similar results with a 3-year OS of 78% for mature T-ALL/LBL patients.
One major limitation of our study was its single-institution, single-arm, nonrandomized design. Our inability to demonstrate a benefit from the addition of nelarabine in standard risk T-ALL/LBL patients may in part be due to potential differences in patient characteristics, small-sized cohorts, nelarabine dosing schedule, and limitations of the historical control analysis. In the relapsed/refractory setting, single-agent nelarabine given at a dose of 1.5 g/m2 on alternating days in adult patients resulted in CR rates of 31% to 36% compared to 26% in pediatric patients treated at doses ranging from 400 to 900 mg/m2 daily for five days.14-16 This lower response rate was thought to be partly due to the reduced dose schedule used in the pediatric study.16 Despite being heavily pretreated, the incidence of severe neurotoxicity in relapsed/refractory adult patients was low at 7% to 10%.14, 15 However, these patients did not receive sequential or concomitant chemotherapy. Since there was no added neurotoxicity upon combining chemotherapy to nelarabine in pediatric studies, we used the pediatric dosing schedule of nelarabine to avoid the potential cumulative neurotoxicity; which may have negatively impacted our results.16, 19, 31
In conclusion, hyper-CVAD plus nelarabine is safe and active in the frontline setting. Compared to hyper-CVAD alone, there was no survival benefit noted with the addition of nelarabine. The optimal dosing schedule of nelarabine when combined with hyper-CVAD and its role in the treatment of higher-risk patients is yet to be determined. There is an ongoing phase 2 randomized trial (UKALL14) to determine the role of nelarabine, combined with standard induction chemotherapy, in the frontline treatment of adult patients with T-ALL. Novel therapeutic strategies are needed to further improve outcomes of adult T-ALL.
ACKNOWLEDGMENTS
Nelarabine was provided by Glaxo (now Novartis).
AUTHORSHIP CONTRIBUTIONS
CONCEPTION AND DESIGN
Farhad Ravandi, Stefan Faderl, Hagop M. Kantarjian
PROVISION OF STUDY MATERIALS OR PATIENTS
Farhad Ravandi, Hagop M. Kantarjian, Stefan Faderl, Elias Jabbour, Nitin Jain, Deborah Thomas, Tapan Kadia, Gautam Borthakur, Joseph D. Khoury, Jan Burger, William Wierda, Susan O'Brien, Marina Konopleva, Alessandra Ferrajoli, Partow Kebriaei, Bouthaina Dabaja, Steven Kornblau, Yesid Alvarado, Naval Daver, Naveen Pemmaraju, Prithviraj Bose, Philip Thompson, Hind Al Azzawi, Mary Kelly, Preteesh Jain, Guillermo Garcia-Manero, Jorge Cortes
COLLECTION AND ASSEMBLY OF DATA
Rebecca Garris, Mary Kelly, Yasmin Abaza, Joseph D. Khoury, Hind Al-Azzawi.
DATA ANALYSIS AND INTERPRETATION
Yasmin Abaza, Rebecca Garris, Farhad Ravandi, Hagop M. Kantarjian
MANUSCRIPT WRITING
Yasmin Abaza, Farhad Ravandi
FINAL APPROVAL OF MANUSCRIPT
Farhad Ravandi, Yasmin Abaza, Hagop M. Kantarjian and all authors
CONFLICT OF INTERESTS
Authors disclose no relevant conflicts related to this article.




