Clinical characteristics and prognostic factors in multiple myeloma patients with light chain deposition disease
Abstract
Light chain deposition disease (LCDD) is characterized by monotypic immunoglobulin depositions which will eventually lead to loss of organ function if left untreated. While the kidney is almost always affected, the presence and degree of LCDD in other organs vary. Ten to thirty percent of LCDD patients have underlying Multiple Myeloma (MM), yet outcome and prognostic markers in this particular patient group are still lacking. Here, we analyzed 69 patients with MM and biopsy proven LCDD and report on renal and extra-renal involvement and its impact on prognosis as well as renal response depending on hematologic response. Coexisting light chain diseases such as AL amyloid and cast nephropathy were found in 30% of patients; those with LCDD and concurrent amyloid tended to have shorter survival. Cardiac involvement by LCDD was seen in one-third of our patients and was associated with shorter overall survival; such patients also had a significantly higher risk of treatment-related mortality (TRM) after stem cell transplant (SCT) compared to LCDD patients without cardiac involvement. This study highlights that MM patients with LCDD present with different clinical features compared to previously reported LCDD cohorts. Rapid initiation of treatment is necessary to prevent progressive renal disease and worse outcome. Coexisting light chain diseases and cardiac involvement are more common than previously reported and confer worse clinical outcome, emphasizing the need for careful patient careful patient evaluation and treatment selection.
1 INTRODUCTION
Monoclonal immunoglobulin deposition disease is a disorder associated with abnormal clonal proliferation of plasma cells or, less frequently, B lymphocytes that leads to light chain deposition disease (LCDD), heavy chain deposition disease (HCDD) and combined light and heavy chain deposition disease (LHCD).1 LCDD represents the most common disease group within this spectrum and, if left untreated, can lead to a rapid decline in organ function.2-5 The kidney is the most commonly affected organ and renal failure requiring dialysis is present in approximately 20% of patients at presentation,2, 6 while extra-renal LCDD seems to be less common and includes cardiac, lung, liver or nerve involvement.7-11 Though historically LCDD conveyed a rather poor prognosis, more recent reports suggest that early detection and rapid initiation of treatment can improve clinical outcome.12-18 The majority of LCDD patients in previous studies had an underlying plasma cell dyscrasia with multiple myeloma (MM) reported in up to 10-30% of patients according to current international myeloma working group criteria (IMWG).2, 19-21 However, no data are available as to whether the outcome of LCDD is influenced by the presence of MM. In this study, we reviewed and analyzed 69 patients with LCDD who presented to the Myeloma Institute in Little Rock Arkansas, between 1997 and 2015. Eighty-five percent of our patients had clinical MM representing one of the largest cohort of such patients reported. The aim of our study was to determine clinical features and prognostic factors in MM patients who present with LCDD. Of special interest was to determine to what extent renal impairment, extra-renal involvement and presence of other concomitant light chain diseases, such as amyloid and cast nephropathy (CN) influence clinical outcomes. Furthermore, as previous reports on other systemic light chain diseases, especially amyloid disease, have suggested increased treatment related mortality after stem cell transplant, we investigated whether this holds true in LCDD.22, 23
2 METHODS
The University of Arkansas for Medical Science (UAMS) Myeloma data base was searched to identify patients with biopsy proven light chain deposition disease (LCDD) at clinical presentation after obtaining IRB approval. The medical records were retrospectively reviewed to obtain demographic, clinical presentation, organ involvement, laboratory, treatment information, and clinical outcome of these patients.
2.1 Clinical and laboratory analysis
All patients underwent detailed clinical staging at initial registration, including full blood count, analysis of blood chemistry, and standard MM-related serological and urinary measurements including free light chain assay (FLc), serum electrophoresis (SPEP), and urine electrophoresis (UPEP). Immunofixation analyses of serum and urine were performed to define the nature of the monoclonal protein present in serum and/or urine. Proteinuria was measured from 24 hour urine collection on presentation. Nephrotic range proteinuria was defined as 24 h proteinuria of ≥ 3.5 g. Glomerular filtration rate (GFR) was estimated using the Modification of Diet in Renal Disease (MDRD) formula. Cardiac testing with EKG as well as echocardiogram were performed routinely prior to initiation of treatment in each patient. For gene expression profiling (GEP), plasma cells were enriched by anti-CD138 immunomagnetic bead selection of mononuclear cell fractions of bone marrow aspirates and was performed with the Affymetrix U133Plus2.0 microarray platform (Santa Clara, CA) using methods previously described.24
2.2 Histology analysis
Patients with clinical suspicion of LCDD either due to renal or extrarenal manifestation underwent biopsy of the clinically suspected organ. All biopsies were allocated for ancillary studies including routine light microscopy (LM), immunofluorescence (IF), and electron microscopy (EM). Tissues were fixed in 10% buffered formalin and processed routinely for LM, while, for EM, samples were fixed in glutaraldehyde. Congo red staining was performed in each case to evaluate for amyloidosis. Tissues were obtained in Michel's fixative for direct immunofluorescence (IF) and slides stained with antisera against IGG, IGA, IGM, C3, C1q, and kappa and lambda light chains on each case. A piece allocated for EM was routinely processed, stained and scoped, and followed by digital photographs.
2.3 Statistical analysis
Clinical outcome, including progression free survival (PFS) and overall survival (OS) were assessed with Kaplan-Meier analysis and the log-rank test was used to identify differences in stratified analysis. For transplant-related mortality, Fisher's exact test was used to assess differences in outcome between patients with and without cardiac light chain deposition disease. All analysis was done in R 3.2.2.25
3 RESULTS
3.1 Patient characteristics
Table 1 presents the baseline characteristics of our 69 patient cohort. All 69 patients had biopsy proven LCDD. Sixty seven patients had an evaluable bone marrow (BM) biopsy and of these, 85% had ≥ 10% clonal plasma cell infiltration with clinical MM. Light chain concentrations were substantially higher in patients with ≥ 10% BM plasmacytosis compared to those with <10% plasma cell infiltration. The mean age at diagnosis was 58.4 years, and males were affected twice as often. Clonal Kappa light chain production was seen in 61% of cases and most patients had light chain disease only (54%). GEP data were available in 50 patients, revealing low risk in 46.26 Eighty-six percent of patients underwent treatment with at least one novel therapeutic agent and 80% of patients received a SCT. Renal insufficiency with a GFR of less than 60 mL/min was seen in 84% of patients, whereby 38% had a GFR of <30 mL/min qualifying for chronic renal disease stage IV and V and 23% of patients were dialysis dependent. Patients with a GFR of <30 mL/min or dialysis dependence at diagnosis tended to present with higher light chain concentrations. In addition to LCDD, 17% of patient had concurrent light chain amyloid (AL) and 13% had cast nephropathy (CN). Of interest, biopsy proven cardiac LCDD was found in 33% of patients, exceeding frequencies of 2-20% previously reported.12, 13, 19, 27
| Characteristic | |
| Median age in years (range) | 58.4 (32-84) |
| Sex (male/female) n | 46/23 (ratio 2:1) |
| B2M | 13.3 (2.3-54.8) |
| Albumin | 3.4 (2-4.7) |
| Hb g/dL (range) | 10.8 (7.6-15.7) |
| LDH | 188 (119-377) |
| GEP 70 Low Risk (%) | 46/50 (92%) |
| GEP 70 High Risk (%) | 4/50 (8%) |
| Treatment | |
| IMID | 60/69 (87%) |
| Thalidomide | 49/69(71%) |
| Revlimid | 11/69 (16%) |
| Protesome Inhibitor (Velcade) | 41/69 (59%) |
| Stem Cell Transplant (SCT) | 55/69 (80%) |
| Bone marrow findings | |
| ≥ 10% clonal plasma cells | 57/67 (85%) |
| Light chain concentration mg/dL: Mean, Median (Range) | 500.2, 217.0 (3.1-4460) |
| < 10% clonal plasma cells | 10/67 (15%) |
| Light chain concentration mg/dL: Mean; Median (Range) | 22.6, 4.75 (2.21-67.2) |
| GFR | |
| Mean GFR of entire cohort | 34.5 ml/min |
| Light chain concentration mg/dL: Mean, Median (Range) | 442.7, 190 (2.21-4460) |
| GFR>60 ml/min | 11/69 (16%) |
| Light chain concentration mg/dL: Mean, Median (Range) | 98.3, 20 (2.21-343) |
| GFR 30-60 ml/min | 16/69(23%) |
| Light chain concentration mg/dL: Mean, Median (Range) | 381.5, 106 (5.43-1470) |
| GFR 15-30 ml/min | 11/69(16%) |
| Light chain concentration mg/dL: Mean, Median (Range) | 667, 710.5 (43.10-1400) |
| Light chain concentration mg/dL: Mean, Median (Range) | 340.3, 247.5 (3.1-1030) |
| GFR<15 ml/min | 15/69 (22%) |
| Hemodialysis (%) | 16/69 (23%) |
| Light chain concentration mg/dL: Mean, Median (Range) | 695.2, 160 (3.49-4460) |
| Mean (range) Proteinuria (g/24hrs) | 3.5 (0.1-18.5) |
| Nephrotic range proteinuria (%) | 21/69 (30%) |
| Clonal subtype | |
| IgA | 2 (3%) |
| IgG | 28 (41%) |
| IgM | 1 (1.5%) |
| Light chain only | 37 (54%) |
| Kappa LC | 42 (61%) |
| Lamda LC | 27 (39%) |
| Extrerenal LCDD (%) | 23/69 (33%) |
| Heart | 22 (32%) |
| Lung | 0 (0)% |
| Liver | 0 (0)% |
| GI tract | 1 (1.5%) |
| LCDD with concomittant Amyloid | 12/69 (17%) |
| LCDD with concomittant Cast Nephropathy | 9/69 (13%) |
- B2M: Beta2-Microglobulin, Hb: Hemoglobin, LDH: Lactate Dehydrogenase, GEP: Gene Expression Profiling, GFR: Glomerular Filtration Rate
3.2 Histology
Light microscopy (LM) in renal LCDD showed mild to moderate nodular mesangial expansion in glomeruli with thickening and wrinkling of the tubular basement membranes (Supporting Information Figure 1a). Linear, either kappa or lambda, light chain restricted staining of glomerular and tubular basement membranes was seen on Immunofluorescence (IF) and was highly diagnostic of LCDD (Supporting Information Figure 1b). Electron microscopy (EM) demonstrated the presence of corresponding typical dark powdery electron dense deposits along the inner aspects of glomerular basement membranes and outer aspects of tubular basement membranes (Supporting Information Figure 1c). While cardiac biopsies failed to reveal LM abnormalities, IF demonstrated characteristic linear, either kappa or lambda light chain restricted staining in the interstitial space EM depicted those light chain deposits as dark granular electron densities (Supporting Information Figure 1c).
3.3 Overall survival, hematologic response, and renal function as a prognostic marker
3.3.1 Overall survival and impact on novel agents
Of the 69 evaluated patients, 42 (61%) had died at last follow up. Causes of death were as follows: unknown, 18 (43%); progression of MM disease, 8 (19%); sepsis/infectious complications, 10 (24%), of which 6 (14%) occurred within 100 days of high dose chemotherapy suggesting treatment related death; secondary malignancy 2 (5%); complications of renal failure, 2 (5%); stroke, 1 (2,5%); and subdural hematoma, 1 (2.5%). Median OS from diagnosis for the whole cohort was 5.16 years (Supporting Information Figure 2a). The use of novel agents in this cohort did not seem to improve OS. The median survival was not improved by the application of novel agents (6.17 years versus 6.9 years for those not receiving novel drugs).
3.3.2 Hematologic response predicts improvement of renal function
Multiple previous reports have shown that depth and speed of onset of response to treatment correlate with renal outcome.12 Among 53 evaluable patients achieving a complete response (CR) or very good partial response (VGPR), compared to those who achieved less than a VGPR, GFR improved significantly by 4.85 mL/min within 12 months and decreased for non-responders by 7.54 mL/min within the same time frame (Figure 1a, p = 0.02). Patients on hemodialysis at baseline were excluded. The Proteinuria also improved substantially, though not significantly, for patients with a CR/VGPR (1.2 g/24 h) compared to those who did not achieve at least a VGPR (0.059 g/24 h) (Figure 1b, p = 0.2).
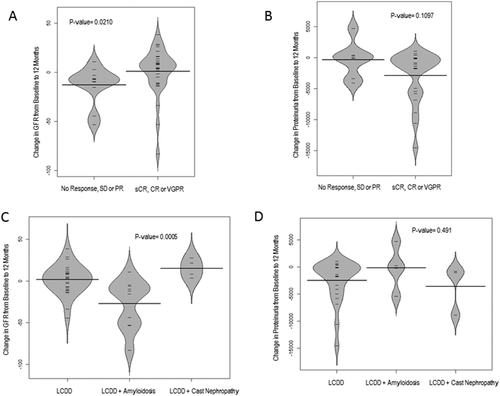
Change of GFR and proteinuria within 12 months according to hematologic response and coexisting light chain disease. Hematologic response with a sCR/CR and VGPR was significantly associated with an improvement in GFR of 4.9 mL/min compared to non-responders, who had a worsening in GFR of 7.5 mL/min by 12 months (A, p = 0.02). Proteinuria likewise improved for responders by 1.2 g/24 h compared to 0.06 g/24 h for non-responders (B, p = 0.1). Change in GFR and proteinuria furthermore depended on coexisting light chain diseases. Patients with LCDD and coexisting amyloid had worsening in GFR (–13.7 mL/min) compared to LCDD only (+3.8 mL/min) and LCDD + cast nephropathy (+15 mL/min) (C, p = 0.0005). Improvement in proteinuria was also less for the LCDD + amyloid group (0.003 g/24 h for LCDD+ amyloid, 0.8 g/24 h for LCDD only, and 1 g/24 h for LCDD and cast nephropathy) yet result was not significant (D, p = 0.49)
3.3.3 Renal function at diagnosis as a prognostic marker for outcome
Severe renal impairment at diagnosis has been shown to be an important adverse prognostic marker for OS despite potential improvement in renal function.28 Our data also suggests that severe renal disease with a GFR < 30 mL/min at LCDD diagnosis shows a tendency to inferior OS. OS was 61.4% for GFR > 30 mL/min compared to 44.9% for patients with GFR < 30 mL/min and 31.2% for patients on dialysis (p = 0.069) (Figure 2). We excluded patients with concurrent AL or cast nephropathy, as previous studies suggested greater renal impairment and inferior clinical outcome.5, 20
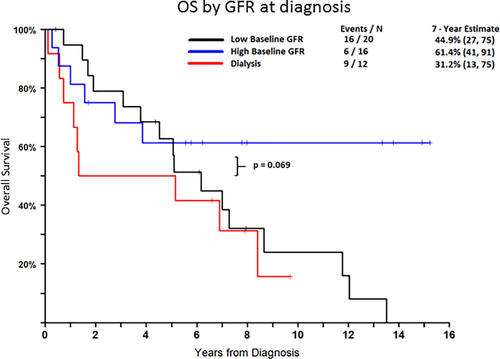
Patient survival according to GFR at diagnosis. Patients with low baseline GFR or dialysis dependence at presentation have a tendency to worse long term outcome. Seven year survival for patients with GFR < 30 mL/min or dialysis dependence was 44.9% and 31.2%, respectively compared to 61.4% for patients who presented with GFR > 30 mL/min (p = 0.069). Patients with amyloid or cast nephropathy were excluded from this study. [Color figure can be viewed at wileyonlinelibrary.com]
3.4 LCDD and concomitant light chain diseases
Little work exists on LCDD with concurrent other light chain diseases. While there are few reports describing concurrent LCDD and cast nephropathy,20 reports on outcome in patients with concomitant amyloid are lacking. Interestingly, this scenario was not uncommon in our patient population: 12 of 69 LCDD patients were found to have concurrent biopsy proven AL, 2 of these patients had multiple sites of amyloid involvement (n = 12, 17%). Affected organs included kidney (n = 4), gastrointestinal tract (n = 4), heart (n = 3), and subcutaneous tissue diagnosed by fat pad biopsy (n = 3) (Supporting Information Table 1). Interestingly, 11 of the 12 concurrent amyloid and LCDD patients showed lambda restriction (92%). As only 27 LCDD patients in our cohort had clonal lambda disease, the incidence of amyloid in these lambda restricted LCDD patients was 40% (11/27).
Renal response to treatment in patients with concurrent LCDD and AL was significantly worse compared to LCDD patients without amyloid, and GFR decreased by 14 mL/min within 12 months (Figure 1c, p = 0.0005). Improvement in proteinuria was also less common in the LCDD plus AL group, though not significant (Figure 1d). The worsening of GFR in LCDD plus AL patients was independent of hematologic response and patients who achieved a CR/VGPR still appeared to have worsening in GFR, albeit patient number was small for this analysis (n = 4, Supporting Information Figure 3a and b).
Furthermore, 9 of the 69 LCDD patients (13%) had biopsy proven renal cast nephropathy (CN) and showed kappa restricted predominance (89%). Renal function was severely compromised in this patient population, with 4 patients requiring dialysis at diagnosis (44%), and mean GFR in the remaining five patients was decreased at 21.73 mL/min compared to 43 mL/min for patients with LCDD without CN (p = 0.02). Yet, LCDD plus CN patients, who did not require dialysis at diagnosis had a significant improvement in GFR with MM treatment (14.8 mL/min, Figure 1c, p = 0.0005) and also substantial improvement in proteinuria (1 g/24 h, Figure 1d).
Overall survival in LCDD plus AL patients tended to be inferior in comparison to subjects with only LCDD, though not significant, was worse compared to LCDD patients. Seven year OS in the LCDD + AL group was 38.1% compared to 46.8% in the LCDD group (p = 0.28) (Supporting Information Figure 4). Seven year estimates for OS between patients with LCDD and those with LCDD plus CN had similar 7-year OS at 46.8% and 54.7%, respectively (Supporting Information Figure 4, p = 0.81).
3.5 Cardiac involvement in LCDD
3.5.1 Clinical features and biomarkers in patients with cardiac LCDD
Based on their clinical presentation and physician's discretion, 24 patients underwent cardiac biopsy, of which 22 proved to have cardiac LCDD, indicating that at least 33% of the patients included in this study had cardiac LCDD (Supporting Information Table 2). All of the patients had some abnormality on electrocardiogram (ECG) and most had an abnormal echocardiogram. The most frequent ECG abnormalities were atrial fibrillation/atrial flutter in 8/22 patients (36%), prolonged QT interval in 7/22 patients (32%), and sinus bradycardia in 3/22 patients (14%). Evaluation with echocardiogram showed that most patients had an increased septal thickness (range 13mm-19mm) with diastolic impairment (grade I-II), which was found in 13 patients (59%). A reduction in ejection fraction (EF < 50%) was only found in four (18%) patients (range 28%-49%). Follow up data showed that 2 of the 4 patients presenting with low EF were able to normalize their cardiac function with anti-myeloma therapy, while septal thickness and diastolic impairment did not improve substantially in any of the patients. BNP and pro-BNP levels were significantly higher in patients with cardiac LCDD compared to those without cardiac involvement (Figure 3b) and could serve as potential markers for disease detection. BNP levels were analyzed in 20 cardiac LCDD and 34 non-cardiac LCDD patients and median value was 873 pg/mL for cardiac LCDD compared to 136 pg/mL for non-cardiac LCDD patients (p = 0.004). Pro-BNP levels were measured in 17 cardiac and 24 non-cardiac LCDD patients and similarly showed significantly elevated levels in cardiac LCDD patients (3260 pg/mL versus 700 pg/mL, p = 0.041).
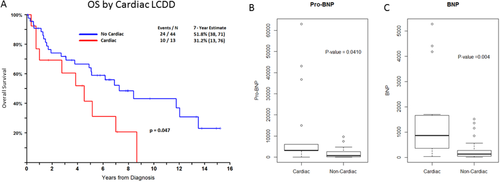
Patient survival according to cardiac involvement of LCDD and relevance of pro-BNP and BNP as biomarkers for cardiac disease. Cardiac LCDD conferred a significant worse survival compared to patients with LCDD, who had no cardiac involvement. Seven year estimates were 31.2% for patients with cardiac involvement compared to 51.8% for LCDD patients without cardiac involvement (p = 0.047). Pro BNP and BNP were significantly higher in cardiac LCDD patients compared to non-cardiac LCDD patients (Fig B, pro BNP- 3260 pg/mL for cardiac LCDD patients and 700 for non-cardiac LCDD patients, p = 0.04. Fig C, BNP-873 pg/mL for cardiac LCDD patients and 137 pg/mL for non-cardiac LCDD patients, p = 0.004). [Color figure can be viewed at wileyonlinelibrary.com]
3.5.2 Cardiac LCDD confers worse OS
Most interesting is that patients with cardiac LCDD had inferior clinical outcome; thus, the 7-year OS estimate in patients with cardiac LCDD was 31.2% compared to 51.8% for non-cardiac LCDD (p = 0.047, Figure 3a). Patients with concomitant biopsy proven amyloid of any organ (n = 9) were excluded in this analysis, given the trend of inferior outcome with LCDD plus AL.
3.5.3 Patients with cardiac LCDD have higher transplant related mortality
Autologous stem cell transplant (ASCT) was performed in 55 of the 69 patients (80%). Reasons not to proceed to SCT in the remaining 14 patients were patient's choice or refusal in 8 patients (57%), late diagnosis with progressive disease and death before transplant in 4 patients (29%) and two patients who were thought not fit for SCT due to age and co-morbidities (14%). Transplant related mortality (TRM- death within first 100 days of SCT) occurred in 6 of these 55 patients (11%), which is a higher rate than the usual rate of approximately 1% seen in the myeloma population at large.4 Five of the six diseased patients in our study had biopsy proven cardiac LCDD suggesting that TRM was 23% in patients with cardiac involvement compared to 2% in LCDD patients without cardiac involvement. Age adjusted Fisher's exact test showed that patients with cardiac LCDD had higher risk of dying from transplant related mortality than non-cardiac LCDD patients (p = 0.03). Of note is that cardiac evaluation prior to transplant in these patients showed a normal ejection fraction (EF > 50%) in all of the diseased cardiac LCDD patients and the only manifestation of an infiltrative cardiac process was increased septal wall thickness (>12 mm) and diastolic dysfunction. Reason for death within the transplant period could not be evaluated in all of these patients, but was noted to be related to infection/sepsis in three of them. Transplant was well tolerated in patients without evidence of cardiac LCDD with only one TRM in a patient with renal LCDD and dialysis dependence.
4 DISCUSSION
This study describes one of the largest series of LCDD patients from a single center to date and presents some unique findings that have not been reported previously. Most previous studies identified LCDD patients based on renal pathology databases and MM patients accounted for maximally 30% of the LCDD patients with the remainder having very low plasma cell involvement on bone marrow (<10%) or other sources of light chain production (e.g., lymphoma). In this study, 85% of patients had clinical MM with the remaining 15% presenting with monoclonal gammopathy of renal significance, indicating that our study population varies greatly from previously reported LCDD studies and shows some unique findings not reported to date.2, 12, 19, 21 We show a high rate of coexisting AL amyloid, which was found in 17% of our overall study population and in 40% of lambda light chain restricted LCDD patients, while previous studies reported only minimal coexisting AL amyloid in LCDD cohorts.6, 19 Our results suggest that coexisting amyloid is not rare in MM with LCDD and especially patients with lambda restriction should be thoroughly scrutinized for amyloid disease as overall survival and renal outcome were worse in this particular population. It is furthermore possible that the number of patients with coexisting amyloid is higher than described as biopsies for amyloid detection were not done in every patient for every relevant organ. Coexisting cast nephropathy was seen in 13% of patients, but in contrast to previous reports did not appear to worsen clinical outcome, which again could be explained by the difference in patient populations and a more aggressive treatment approach in our patient cohort with MM, where ASCT was used in 80% of patients compared to fewer patients in previous studies.8, 20
Renal response to treatment has previously shown to depend on hematologic response and we likewise show an improvement in GFR and proteinuria in patients that achieve a CR/VGPR in comparison to those who do not at least achieve a VGPR.12, 29 Yet, it is of interest that severely impaired GFR (<30 mL/min) at baseline remains an adverse prognostic marker for worse OS independent of hematologic response. Similarly, previous reports have shown that severely decreased renal function at diagnosis remains an independent adverse marker for OS in LCDD19] and in MM patients.28 Patients with poor baseline GFR have a high risk of worsening renal function with likelihood of dialysis requirement in the future,12, 29 which potentially explains the advert impact of poor renal function on outcome.
Furthermore we report a much higher percentage of extra-renal LCDD than previously reported, with the heart being the most common site of involvement.6, 19, 21 Cardiac LCDD was seen in a third of our patient cohort, and clinical features included elevated BNP and pro BNP with abnormal ECG and/or a restrictive pattern on echocardiography with increased septal thickness and diastolic dysfunction. This represents an analogous pattern of diastolic dysfunction and conduction disturbances as seen in cardiac amyloid.30 It is to be emphasized that an even higher percentage of cardiac light chain involvement cannot be excluded, as only 23 of the 69 patients underwent a cardiac biopsy. A thorough and comprehensive cardiac evaluation at disease presentation is necessary to detect patients with possible cardiac LCDD. Patients with cardiac involvement had significant worse clinical outcome than non-cardiac LCDD patient, reflecting that cardiac involvement plays a major role in overall prognosis. Analogous to patients with cardiac amyloid, LCDD of the heart also seemed to confer a significant higher risk of death from transplant compared to non-cardiac LCDD patients,23 indicating that clinicians should carefully evaluate LCDD patients before proceeding to further treatment and stem cell transplantation.




