Long term impact of hyperleukocytosis in newly diagnosed acute myeloid leukemia patients undergoing allogeneic stem cell transplantation: An analysis from the acute leukemia working party of the EBMT
Abstract
Up to 20% of acute myeloid leukemia (AML) patients present initially with hyperleukocytosis, placing them at increased risk for early mortality during induction. Yet, it is unknown whether hyperleukocytosis still retains prognostic value for AML patients undergoing hematopoietic stem cell transplantation (HSCT). Furthermore, it is unknown whether hyperleukocytosis holds prognostic significance when modern molecular markers such as FLT3-ITD and NPM1 are accounted for. To determine whether hyperleukocytosis is an independent prognostic factor influencing outcome in transplanted AML patients we performed a retrospective analysis using the registry of the acute leukemia working party of the European Society of Blood and Marrow Transplantation. A cohort of 357 patients with hyperleukocytosis (159 patients with white blood count [WBC] 50 K-100 K, 198 patients with WBC ≥ 100 K) was compared to 918 patients without hyperleukocytosis. Patients with hyperleukocytosis were younger, had an increased rate of favorable risk cytogenetics, and more likely to be FLT3 and NPM1 mutated. In multivariate analysis, hyperleukocytosis was independently associated with increased relapse incidence (hazard ratio [HR] of 1.55, 95% confidence interval [CI], 1.14-2.12; P = .004), decreased leukemia-free survival (HR of 1.38, 95% CI, 1.07-1.78; P = .013), and inferior overall survival (HR of 1.4, 95% CI, 1.07-1.84; P = .013). Hyperleukocytosis retains a significant prognostic role for AML patients undergoing HSCT.
1 INTRODUCTION
A significant subset of newly diagnosed acute myeloid leukemia (AML) patients, estimated at 5%-20%, present initially with an elevated white blood count (WBC) exceeding 100,000/mL.1-5 Patients presenting in like fashion are treated emergently as a substantial body of evidence published over the past three decades conclusively shows that these patients are at a significant risk for early death during initial induction therapy.5-9 Risk factors for hyperleukocytosis (HL) include younger age and leukemias skewed toward monocytic differentiation.10-12 Central to the pathogenesis of the increased mortality seen in hyperleukocytosis patients are the aggregate detrimental effects of leukostasis, tumor lysis syndrome, and disseminated intravascular coagulation. While hyperleukocytosis is frequently defined at the 100 000 WBC threshold, adverse hyperleukocytosis associated phenomenon are also seen at lower WBC counts.13 However, it remains unclear whether the inferior prognosis associated with hyperleukocytosis results from the high tumor burden or rather is an inherent feature of a unique subtype of AML. To date, no studies have examined the long term outcome of AML patients with hyperleukocytosis who underwent hematopoietic stem cell transplantation (HSCT). In this analysis we address this question and analyze a large cohort of patients with HL who underwent HSCT in first remission and compare their clinical outcome with non-HL patients.
2 METHODS
2.1 Study design and data collection
This is a retrospective analysis based on the registry data of the acute leukemia working party (ALWP) of the European Society of Blood and Marrow Transplantation (EBMT). The EBMT is a voluntary working group comprising more than 500 transplant centers that are required to report all consecutive stem cell transplantations and follow-ups once a year. Audits are routinely performed to determine the accuracy of the data. This study was approved by the ALWP institutional review board. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent authorizing the use of their personal information for research purposes. Eligibility criteria for this analysis included adult non-M3 AML patients over 18 years of age who underwent a first allogeneic stem cell transplantation at first complete remission between 2005 and 2015. Hyperleukocytosis was defined as a WBC count of over 50 000 WBC/µL. Cytogenetic risk was assessed according to the European LeukemiaNet criteria.14 Intensity of conditioning was determined according to EBMT published criteria.15 Stem cell grafts consisted of either bone marrow (BM) or G-CSF mobilized peripheral blood (PB). All donors were HLA-matched according to standard criteria (locus-A, -B, -C, DRB1, -DQB1). Patients who had undergone a previous stem cell transplantation were excluded from the analysis. Grading of acute and chronic graft-versus-host disease (GVHD) was performed using established criteria.16, 17
2.2 Statistical analysis
The clinical end points evaluated were leukemia-free survival (LFS), relapse incidence (RI), nonrelapse mortality (NRM), acute and chronic GVHD, GVHD-free/relapse-free survival, defined as events including grade 3-4 acute GVHD, systemic therapy-requiring chronic GVHD, relapse, or death in the first post-HCT year (GRFS), and overall survival (OS). LFS was defined as survival with no evidence of relapse or progression. Relapse was defined as the reappearance of ⩾5% BM blasts and/or extramedullary lesion due to specific blast cell infiltration. NRM was defined as death without evidence of relapse or progression. OS was defined as the time from ASCT to death, regardless of the cause. The statistical analysis was performed for three groups of patients according to presentation WBC count: WBC < 50 K, 50 K ≤ WBC < 100 K, and WBC ≥ 100 K. Cumulative incidence curves were used for RI and NRM in a competing risks setting, since death and relapse are competing. Probabilities of OS and LFS were calculated using the Kaplan–Meier estimate. Univariate analyses were done using the Gray's test for cumulative incidence functions and the log rank test for OS and LFS. Multivariate analyses were performed by stepwise selection of variables associated with P < .15 in univariate analysis. All tests were two-sided with the type I error rate fixed at 0.05. Statistical analyses were performed with SPSS 19 (SPSS Inc, Chicago, Illinois, USA) and R 2.13.2 (R Development Core Team, Vienna, Austria) software packages.
3 RESULTS
3.1 Patients, disease, and transplant characteristics
We examined data on 1275 patients transplanted between 2005 and 2015 from 98 reporting centers (Supporting Information Appendix). Table 1 summarizes the baseline demographic and clinical characteristics of the analyzed cohort. Patients with hyperleukocytosis were younger compared to their nonhyperleukocytosis counterparts, and were more likely to be FLT3-ITD and NPM1 mutated. Comparing the three groups of patients according to WBC count (<50 K vs. 50 K-100 K vs. ≥100 K), the number of cycles to reach first complete remission (CR1) was similar but HL patients more often received myeloablative conditioning compared to non-HL patients. Adverse risk cytogenetics were more common in non-HL patients, whereas patients with WBC ≥ 100 K more frequently harbored favorable risk cytogenetics.
| Clinical Parameter | WBC<50 (n = 918) | 50<WBC<100 (n = 159) | WBC>100 (n = 198) | Pa |
|---|---|---|---|---|
| Follow up duration in m, median (range) | 31.25 (1-121.48 ) | 32.77 (2.36-108.36 ) | 35.97 (0.69-123.48 ) | |
| Age in y, median (range) | 52.2(18.1-72.1) | 49.1(18.8-70.6) | 48.8(18.6-72.1) | .016 |
| Gender, n (%) | ||||
| Male | 487 (53.11) | 82 (51.57) | 95 (47.98) | .419 |
| Female | 430 (46.89) | 77 (48.43) | 103 (52.02) | |
| Missing | 1 | 0 | 0 | |
| WBC at diagnosis, median (range) | 5.3 (0.2-49.9) | 68 (50-99.1) | 164.1 (100-780) | |
| Time diagnosis to transplant in days, median (range) | 151 (55-393) | 140 (67-369) | 143 (63-375) | .065 |
| ELN cytogenetic risk category, n (%) | ||||
| Favorable | 72 (7.84) | 13 (8.18) | 28 (14.14) | <10-4 |
| Intermediate I | 515 (56.1) | 108 (67.92) | 126 (63.64) | |
| Intermediate II | 175 (19.06) | 22 (13.84) | 25 (12.63) | |
| Adverse | 156 (16.99) | 16 (10.06) | 19 (9.6) | |
| FLT3-ITD status, n (%) | ||||
| Wild type | 647 (70.48) | 58 (36.48) | 69 (34.85) | <10-4 |
| Mutated | 271 (29.52) | 101 (63.52) | 129 (65.15) | |
| NPM1 status, n (%) | ||||
| Wild type | 670 (72.98) | 65 (40.88) | 78 (39.39) | <10-4 |
| Mutated | 248 (27.02) | 94 (59.12) | 120 (60.61) | |
| FLT3-ITD /NPM1 combined status, n (%) | ||||
| FLT3 wt/NPM1 wt | 582 (63.4) | 42 (26.42) | 49 (24.75) | <10-4 |
| FLT3 wt/NPM1 mut | 65 (7.08) | 16 (10.06) | 20 (10.1) | |
| FLT3 mut/NPM1 wt | 88 (9.59) | 23 (14.47) | 29 (14.65) | |
| FLT3 mut/NPM1 mut | 183 (19.93) | 78 (49.06) | 100 (50.51) | |
| Donor type | ||||
| Matched sibling donor | 523 (56.97% ) | 109 (55.05% ) | 88 (55.35% ) | .844 |
| Matched unrelated donor | 395 (43.03% ) | 89 (44.95% ) | 71 (44.65% ) | |
| Number of induction cycles to reach CR1, n (%) | ||||
| 1 induction | 668 (75.65) | 112 (75.17) | 151 (79.89) | .437 |
| >1 induction | 215 (24.35) | 37 (24.83) | 38 (20.11) | |
| Missing | 35 | 10 | 9 | |
| BM derived graft, n (%) | 192 (20.92) | 34 (21.38) | 56 (28.28) | .075 |
| PB graft, n (%) | 726 (79.08) | 125 (78.62) | 142 (71.72) | |
| Conditioning regimen, n (%) | ||||
| Myeloablative | 463 (50.44) | 95 (59.75) | 116 (58.59) | .02 |
| Reduced intensity | 455 (49.56) | 64 (40.25) | 82 (41.41) | |
- a P value of a test of the null hypothesis that all the groups are the same.
- Abbreviations: WBC, white blood cells; ELN, European LeukemiaNet; NPM1, nucleophosmin1; FLT3-ITD, FMS-like tyrosine kinase-3 internal tandem duplication; CR1, first complete.
3.2 Overall survival and relapse incidence
The cumulative incidence of relapse at 3 years was 24% (95% CI: 22% to 27%). LFS and OS at 3 years were 61% (95% CI: 58%-64%) and 66% (95% CI: 63%-69%), respectively. A univariate cox regression model (Supporting Information Table S1) demonstrated that RI was significantly increased in HL patients [29% for WBC 50 K-100 K (95% CI: 22%-37%), and 30% for WBC > 100 K (95% CI: 23%-36%) vs. 22% for non-HL patients (95% CI: 19%-25%); P = .013]. The three year LFS rate tended to be lower among patients with hyperleukocytosis but this did not reach statistical significance [55% (95% CI: 48%-63%) vs. 63% (95% CI: 59%-66%); P = .066). A similar trend was also noted for OS (Figure 1). We also note that when we analyzed patients with hyperleukocytosis (>50K WBC) with regard to their FLT3-ITD/NPM1 mutational status we found that the 52 patients with FLT3-ITD mut/NPM1wt had a significantly increased risk of RI compared to the other subgroups (Supporting Information Table S2).
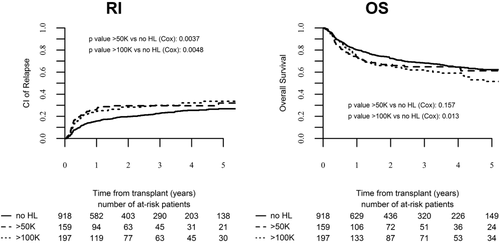
Relapse incidence and overall survival of transplanted AML patients stratified per initial WBC count
3.3 NRM and GVHD
Overall, 57 (13%) patients died of nonrelapse etiologies after HSCT. Leukemia and GHVD constituted the most common causes of patient death both in non-HL and HL patients (Supporting Information Table S3). Overall, grade II-IV acute GVHD was seen in 24% (95% CI: 22% to 27) of the patients analyzed in this cohort, whereas grade III-IV acute GVHD was experienced by 8% (95% CI: 6%-10%) of patients. The cumulative incidence of chronic GVHD at 3 years was 42% (95% CI: 39%-45%), and the rate of extensive chronic GVHD was 22% (95% CI: 19%-24%). As shown in Supporting Information Table S1, a univariate cox regression model indicated that the cumulative incidence of grade II-IV acute GVHD, chronic GVHD, and extensive chronic GVHD were comparable in each of the three groups (<50 K vs. 50 K-100 K vs. ≥100 K). The 3 year incidence of GRFS was significantly increased in patients with a WBC count < 50 K compared to HL patients (45% vs. 39% vs. 36%, respectively; P = .022).
3.4 Impact of hyperleukocytosis on outcome according to donor type
To determine whether donor type, namely matched sibling donors (MSD) versus matched unrelated donor (MUD), influenced the outcome of HL patients, a univariate analysis was performed. As shown in Supporting Information Table S4, in patients receiving grafts from MSD, grade III-IV acute GVHD was significantly increased in HL patients compared to non-HL patients (12% vs. 6%) while there was a trend toward increased RI in HL patients with WBC > 100 K which did not reach statistical significance (33% vs. 24%, P = .054). In MUD, chronic GVHD rates were notably lower in HL patients (both >50 K and >100 K) compared to their non-HL counterparts (38% vs. 30% vs. 48%). RI was statistically marginally inferior in HL patients with WBC > 50 K (25% vs. 20%, P = .071). Supporting Information Tables S5 and S6 depict the results of multivariate analyses revealing that in MSD, HL of over 50 K was significantly associated with an increased risk of relapse compared to non-HL patients (HR, 1.6; 95% CI, 1.03-2.49; P = .035), a finding also observed in MUD (HR, 1.72; 95% CI, 1.005-2.94; P = .047). GRFS and the incidence of extensive chronic GVHD was also increased in HL patients with MSD whereas it was not significantly different among HL patients transplanted from URD.
3.5 Multivariate analysis of factors impacting on clinical outcome
To assess the effect of hyperleukocytosis on patient outcome following transplant, we performed a multivariate analysis using the following covariates in the regression modeling: WBC at diagnosis, ELN cytogenetic risk category, FLT3-ITD status, patient age, donor type, and number of induction cycles to reach CR1. As illustrated in Table 2 and Figure 1 the analysis confirmed that increasing WBC count had a significant effect on clinical outcome. A WBC count of over 100 K had an adverse effect on RI (HR, 1.55; 95% CI, 1.14-2.12; P = .004), LFS (HR, 1.38; 95% CI, 1.07-1.78; P = .01), and OS (HR, 1.4; 95% CI, 1.07-1.84; P = .013). Of note, the effect of hyperleukocytosis on OS was limited only to HL of over 100 K as a WBC count of 50 K-100 K was not found to significantly affect OS (HR, 1.25; 95% CI, 0.91-1.7; P = .15). GRFS rates were inferior in HL patients both in patients with HL > 50K (HR, 1.3; 95% CI, 1.02-1.65; P = .03), and those with HL of over 100K (HR, 1.38; 95% CI, 1.11-1.71; P = .002).
| Outcome | Hazard ratio (95% CI) | P |
|---|---|---|
| Relapse incidence | ||
| WBC < 50 (reference) | 1 | |
| WBC 50-100 | 1.64 (1.17-2.3) | .003 |
| WBC ≥ 100 | 1.55 (1.14-2.12) | .004 |
| WBC ≥ 100 vs. WBC 50-100 | 0.94 (0.63-1.41) | .78 |
| Induction cycles > 1 | 1.46 (1.13-1.89) | .003 |
| ELN favorable cytogenetic risk (reference) | 1 | |
| Intermediate I | 1.75 (1.01-3.04) | .044 |
| Intermediate II | 2.34 (1.29-4.24) | .004 |
| Adverse | 3.31 (1.83-5.97) | <.001 |
| Unrelated donor vs. matched sibling | 0.75 (0.57-0.97) | .032 |
| Leukemia-free survival | ||
| WBC < 50 (reference) | 1 | |
| WBC 50-100 | 1.33 (1- 1.77) | .049 |
| WBC ≥ 100 | 1.38 (1.07- 1.78) | .013 |
| WBC ≥ 100 vs. WBC 50-100 | 1.03 (0.73-1.46) | .83 |
| Induction cycles > 1 | 1.36 (1.1 −1.69) | .003 |
| ELN favorable cytogenetic risk (reference) | 1 | |
| Intermediate I | 1.62 (1.06-2.47) | .024 |
| Intermediate II | 1.89 (1.19-3.01) | .006 |
| Adverse | 2.59 (1.63-4.11) | <.001 |
| Overall survival | ||
| WBC < 50 (reference) | 1 | |
| WBC 50-100 | 1.25 (0.91-1.7) | .15 |
| WBC ≥ 100 | 1.4 (1.07-1.84) | .013 |
| WBC ≥ 100 vs. WBC 50-100 | 1.12 (0.77-1.62) | .52 |
| ELN favorable cytogenetic risk (reference) | 1 | |
| Intermediate I | 1.84 (1.14-2.95) | .011 |
| Intermediate II | 2.13 (1.27-3.56) | .003 |
| Adverse | 3.06 (1.83-5.12) | <.001 |
As summarized in Table 3, grade II-IV acute GVHD, chronic GVHD, and extensive chronic GVHD rates were not significantly influenced by hyperleukocytosis of any degree. A focused analysis comparing the clinical outcome of patients with hyperleukocytosis of over 50 K to patients without hyperleukocytosis confirmed that in HL patients relapse (HR, 1.59; 95% CI, 1.24-2.05; P = .0002), LFS (HR, 1.36; 95% CI, 1.1-1.67; P = .003), and OS (HR, 1.33; 95% CI, 1.07-1.67; P = .01) rates were significantly worse compared to non-HL patients.
| Outcome | Hazard ratio (95% CI) | P |
|---|---|---|
| Nonrelapse mortality | ||
| WBC < 50 (reference) | 1 | |
| WBC 50-100 | 0.83 (0.47-1.46) | .52 |
| WBC ≥ 100 | 1.06 (0.67-1.67) | .8 |
| WBC ≥ 100 vs. WBC 50-100 | 1.27 (0.65-2.48) | .47 |
| Age, 10 year increment | 1.25 (1.05-1.47) | .008 |
| Unrelated donor versus matched sibling | 1.57 (1.09-2.25) | .014 |
| Grade II-IV acute GVHD | ||
| WBC < 50 (reference) | 1 | |
| WBC 50-100 | 0.9 (0.62-1.3) | .58 |
| WBC ≥ 100 | 1.05 (0.76-1.46) | .73 |
| WBC ≥ 100 vs. WBC 50-100 | 1.17 (0.74-1.83) | .48 |
| ELN favorable cytogenetic risk (reference) | 1 | |
| Intermediate I | 1.8 (1.07-3.03) | .025 |
| Intermediate II | 1.83 (1.03-3.24) | .036 |
| Adverse | 1.73 (0.97-3.1) | .062 |
| Chronic GVHD | ||
| WBC < 50 (reference) | 1 | |
| WBC 50-100 | 0.9 (0.66-1.22) | .52 |
| WBC ≥ 100 | 0.83 (0.63-1.1) | .19 |
| WBC ≥ 100 vs. WBC 50-100 | 0.92 (0.63-1.34) | .66 |
| Unrelated donor versus matched sibling | 1.41 (1.14-1.74) | .001 |
- Abbreviations: WBC, white blood cells; GVHD, graft versus host disease.
4 DISCUSSION
In this report of a large cohort of transplanted AML patients with hyperleukocytosis, we show that patients with hyperleukocytosis have a distinct clinical course resulting in increased RI and inferior leukemia free survival, GFRS and OS, all of which were prognostically independent of other standard clinical, cytogenetic, and molecular risk factors for adverse outcome in AML.
It still remains to be determined whether hyperleukocytotic AML is a distinct clinical entity, nevertheless our data suggest that patients presenting with hyperleukocytosis are significantly more likely to harbor the double mutation phenotype of FLT3mut/NPM1mut compared to their non-HL counterparts (50% vs. 19%, P < .001). Notably, HL patients also had an increased frequency of favorable risk cytogenetics. While decidedly these observations do not constitute formal proof, they do hint at the possible unique biology of HL AML. We note that the data presented is strongly supported by observations made by the Study Alliance Leukemia study group where HL patients were also found to be more likely to be FLT3-ITD (45% vs. 16%) and NPM1 (44% vs. 24%) mutated.18 The Alliance investigators also note that their HL cohort had fewer patients displaying adverse risk cytogenetics which is also in line with our data. Previous research from other groups has also documented an association between hyperleukocytosis and FLT3-ITD.19, 20
Additional salient findings from our study included the following. First, we establish that HL patients are at a significant risk for increased leukemia relapse and inferior leukemia free survival, both of which translated into markedly inferior OS for the group of patients with a WBC count of over 100 K. Importantly, hyperleukocytosis retained its prognostic impact following a multivariate analysis accounting for established adverse risk factors, namely age, cytogenetics, FLT3-ITD, NPM1, and number of induction cycles to reach CR1, thus confirming its prognostic significance. In accord with our findings, the Dutch-Belgian Cooperative Trial Group for Hemato-Oncology and the Swiss Group for Clinical Cancer Research (HOVON/SAKK) categorize patients with normal cytogenetics and a WBC > 100 K as poor risk patients21 based on trial results showing inferior OS and disease free survival for patients with higher WBC counts.22 Interestingly, our data intersect with the results of two major deep sequencing efforts published recently. In the Cancer Genome Project the detrimental effect of incremental leukocytosis on survival was reaffirmed and was seen to be in a magnitude roughly proportional to the effect of complex cytogenetics.23 Indirect inferences pointing to the prognostic role of WBC counts may be also made based on the observation that in their analysis, patients with no identified driver mutations had lower WBC counts than those with identified drivers translating into better clinical outcomes. In the same vein, data from the Cancer Genome Atlas Research Network also indicate that a WBC of over 16 000/ul is associated with inferior survival.24 We note that both studies did not evaluate for transplant related outcomes.
Our analysis brings about several questions. Should AML patients presenting with hyperleukocytosis be referred to transplant upfront (following attainment of first remission) regardless of standard risk factors such as cytogenetics and molecular markers? Although this question cannot be answered conclusively based on data from a retrospective dataset, nevertheless our findings do suggest that these patients have an overall high risk clinical course which even transplant cannot fully overcome, and thus it seems reasonable to assume that HL patients should be referred to transplant on achievement of remission. Dovetailing this question it may possible to ask, given the high rate of relapse following transplant, should patients presenting with hyperleukocytosis be treated preemptively with either donor lymphocyte infusions,25-28 maintenance therapy29-31 or targeted agents32? as is frequently attempted for patients deemed to be at high risk for relapse (e.g. complex cytogenetics, positive MRD studies). Lastly, how does hyperleukocytosis fit into the elaborate molecular based prognostication schemes proposed recently23? Future molecular clinical correlative studies may possibly answer this question.
As with any retrospective analysis we note the inherent limitations of a multicenter registry and note that additional factors for which we did not have a fully annotated dataset, such as the initial induction therapies administered to patients or the minimal residual disease state at initial remission, may have impacted on long term outcome as well.
Simply stated, our analysis shows for the first time that hyperleukocytosis portends an inferior clinical outcome for transplanted AML patients, independent of cytogenetic and molecular risk factors, and constitutes a major determinant of long term outcome. We propose that hyperleukocytosis should be implemented and considered as a major risk factor for relapse after allogeneic HSCT in patients with AML.
Acknowledgments
The authors thank all the European Group for Blood and Marrow Transplantation (EBMT) centers and national registries for contributing patients to the study and data managers for their excellent work. Supporting information is available at the EBMT Web site.
Conflict of interest
Nothing to report.




