A randomized and double-blind controlled trial evaluating the safety and efficacy of rituximab for warm auto-immune hemolytic anemia in adults (the RAIHA study)
Conflicts of interest: Marc MICHEL and Bertrand GODEAU have received research support from Roche. Roche Pharmaceuticals played no role in designing the study, collecting and analyzing the data, writing the paper or decision to submit for publication.
ClinicalTrials.gov identifier: NCT01181154
Abstract
This Phase 3 multicentre randomized double-blind and placebo-controlled trial aimed to compare the efficacy and safety of rituximab (RTX) to placebo for treating newly diagnosed warm autoimmune hemolytic anemia (wAIHA) in adults receiving prednisone. Adults with a confirmed diagnosis of wAIHA who previously received corticosteroids for less than 6 weeks could be included. At inclusion, all patients received prednisone at a daily dose of 1 mg/kg for 2 weeks, and then tapered according to a pre-defined recommended reduction scheme. Besides prednisone, eligible patients received 2 infusions of RTX or placebo at a fixed dose of 1,000 mg 2-week apart. The primary endpoint was overall response rate (complete response [CR] + partial response [PR]) in an intent-to-treat (ITT) analysis at 1 year. A total of 32 patients (17 females [53%], mean age at inclusion 71 ± 16 years) were enrolled and randomized. In all, 27 patients were followed for at least 1 year and their data were evaluable for response. With an ITT analysis, the overall response rate at 1 year was 75% [95%CI: 47.6-92.7] with 11 CR and 1 PR with RTX versus 31% [11.0-58.7] (5 CR) with placebo (P = 0.032). At 2 years, 10/16 patients with RTX versus 3/16 with placebo still showed CR (P = 0.011). Overall, eight severe infections occurred during follow-up, six with placebo and two with RTX (P = 0.39). At 2 years, six patients with placebo had died, but none with RTX (P = 0.017). Compared to placebo, RTX combined with prednisone may be effective and safe for treating newly-diagnosed wAIHA in adults. Am. J. Hematol. 92:23–27, 2017. © 2016 Wiley Periodicals, Inc.
Introduction
Autoimmune hemolytic anemia (AIHA) is a rare autoimmune disease in which autoantibodies directed toward red blood cell membrane antigens lead to their accelerated destruction 1. AIHA can affect both children (mainly < 5 years old) and adults. The estimated prevalence is 0.17/1,000 people and annual incidence 1 to 3/100,000 people 1, 2. Among adults, the mean age at time of diagnosis is about 55 years, and the peak incidence occurs around the seventh decade 3, 4, with a slight predominance of females (sex ratio 1.5 to 2) 1, 5. Among the different subtypes of AIHA, warm autoimmune hemolytic anemia (wAIHA) represents 60 to 70% of all AIHA cases in adults 2, 5. The overall mortality with adult wAIHA ranges from 8 to 15% 3, 4.
The management of wAIHA has long been and is still mainly empirical or based on a few retrospective uncontrolled studies 5-7. Only recently were results reported from two prospective studies including one randomized controlled open trial 8, 9. Corticosteroids (prednisone or prednisolone), the cornerstone of therapy, are commonly given as first-line treatment at an initial daily dose of 1 to 1.5 mg/kg 2, 6, 7. With corticosteroids, an initial clinically significant response (i.e., increase in hemoglobin [Hb] level > 10 g/dL with at least a 2-g increase from baseline) is achieved in 75% to 80% of cases, with a median time to achieve at least a partial response (PR) of 15 days 7. Except for the few patients who are truly refractory to corticosteroids, the major issue is that approximately 60% of patients achieving an initial response become corticosteroid-dependent. For patients with no response to corticosteroids or showing relapse when corticosteroids are decreased or stopped, classical alternatives include splenectomy or immunosuppressive agents such as azathioprine, mycophenolate mofetil, or ciclosporin 7. Rituximab (RTX), the well-known chimeric monoclonal antibody that targets CD20 antigen on B lymphocytes, was first shown to be highly effective for refractory wAIHA in children 10. For adults, a few retrospective studies first showed that RTX could have a high efficacy (i.e., ≥80% response) for both primary and secondary wAIHA 11, 12, which led to its increased off-label use in many countries as second-line treatment and as an alternative to splenectomy 5, 6. These promising data were then confirmed in two prospective studies 8, 9, including one randomized open-label Phase 3 trial conducted in Denmark, showing a 75% response rate at 12 months with RTX + prednisolone versus 36% with prednisolone alone 9. Predictors of response to RTX have not clearly been identified, but young age and a short interval between diagnosis and RTX administration have been associated at least in some studies with good response 12, 13. The safety profile of RTX in adult'immune cytopenias in general is usually good, and adverse events such as late-onset neutropenia or secondary hypogammaglobulinemia have been only rarely reported in this setting 13, 14.
The aim of this study was to confirm, with a randomized double-blind placebo-controlled Phase 3 trial, the efficacy and safety of RTX used as first-line therapy combined with prednisone for adult' wAIHA.
Methods
Study design
This was a Phase 3 prospective randomized (1/1 ratio), double-blind, placebo-controlled, multicentre trial to assess the efficacy of RTX for treating newly-diagnosed wAIHA (Supporting Information). The study was conducted over a 4.5 year period (2011–2015) in France through the network of the national referral center for adult'immune cytopenias and promoted and funded by the Direction de la Recherche Clinique and the Assistance Publique-Hôpitaux de Paris with the support of Roche Pharmaceuticals, which provided the vials of RTX and placebo. Patients were included over a 2.5 year period and followed for 2 years after inclusion.
Patients
Inclusion criteria were (1) age ≥ 18 years at inclusion; (2) wAIHA defined at time of diagnosis by Hb level ≤ 10 g/dL with signs of hemolysis (at least haptoglobin level < 4 mg/l), and a positive direct antiglobulin test (DAT) result (IgG or IgG + complement C3d pattern); (3) wAIHA diagnosed and treated < 6 weeks before inclusion; (4) serum gammaglobulin level >5 g/l at inclusion; (5) absence of detectable lymph nodes on total body CT-scan (performed before inclusion if not performed at wAIHA diagnosis); (6) Evans' syndrome accepted with presence of the other inclusion criteria and platelet count > 30 × 109/l at inclusion; (7) females of childbearing age with negative pregnancy test results and effective contraceptive method within at least 6 months after inclusion; and (8) informed and written consent.
The main exclusion criteria were (1) prior treatment with RTX; (2) wAIHA treated for > 6 weeks with corticosteroids; (3) ongoing or recent (< 2 weeks) treatment with immunosuppressants (other than corticosteroids); (4) associated non-Hodgkin lymphoma (except for stage A chronic lymphocytic leukemia); (5) systemic lupus erythematosus with extra-hematologic manifestations requiring treatment; (6) any other associated cause of hereditary or acquired hemolytic anemia; (7) negative DAT result, DAT with C3d positivity alone and/or definite diagnosis of cold agglutinin disease; (8) positive HIV and/or hepatitis C virus and/or HBV test result; (9) neutrophil count < 1.0 × 109/l at inclusion.
Randomization and masking
Eligible patients were randomized in a 1:1 ratio (double-blind) to receive two infusions of RTX or placebo at a fixed dose of 1,000 mg 2-week apart, on Days 1 and 15 after randomization (Fig. 1). Allocation to treatment groups involved a central computerized randomization procedure; the senior statistician was not involved in the study conduct or monitoring. Patients, investigators, and study nurses were blinded to treatment assignments during the study. RTX and placebo vials were made and provided by Roche and were totally indistinguishable.
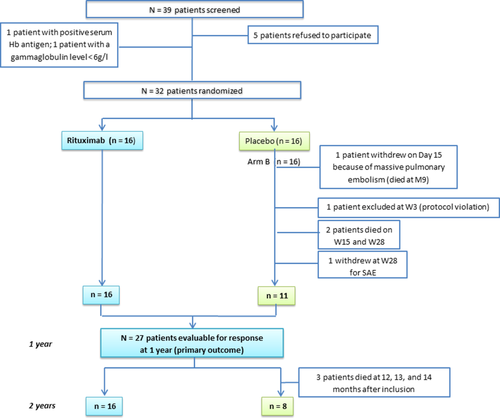
Flow of patients in the trial. Notes: Hb = haemoglobin, W = week, M = month, SAE = serious adverse event. [Color figure can be viewed at wileyonlinelibrary.com]
Procedures
Premedication with 100 mg intravenous methylprednisolone was systematically administered before RTX or placebo as recommended. At inclusion, whatever the dose and type of corticosteroids treatment received before entering the study, all patients were given prednisone at a daily dose of 1.0 mg/kg for at least 2 weeks. After Day 15, with at least PR achieved (defined by Hb level > 10 g/dL with at least a 2 g increase from baseline), the dose of prednisone had to be tapered by 10 mg every 10 days up to 20 mg, then by 5 mg every 10 days from 20 to 10 mg, then by 2.5 mg from 10 to 5 mg and then stopped after 10 days with lasting response. With lack of response within 2 weeks after inclusion, the daily dose of prednisone could be increased up to 1.5 or 2 mg/kg at the investigator's discretion. In the absence of at least PR within 6 weeks after inclusion despite an increased dose of prednisone, the use of other drugs such as danazol or immunosuppressors and/or an indication for splenectomy were left to the investigator's discretion. Each treatment and dosage was specifically reported in the case report form, and the patient was considered a non-responder in the intent-to-treat (ITT) analysis. The need for transfusion was left to the investigator's discretion and the number of red-blood packed cells transfused was recorded. There was no recommendation in the protocol regarding systematic antibiotic prophylaxis with trimethoprim-sulfamethoxazole, but pneumococcal vaccine was advised. Laboratory and adverse toxic events were graded according to National Cancer Institute Common Terminology Criteria v4.0. Severe infection was defined as requiring hospitalization and/or intravenous antibiotics and/or resulting in death.
Outcomes
The primary outcome was the efficacy of RTX by comparing the overall response rate (PR + complete response [CR]) at 1 year in both groups.
Secondary objectives were PR, cumulative dose of steroids, and number of transfusions and hospitalizations at 1 year and incidence and severity of adverse events in both groups and CR/PR at 2 years.
CR was defined as Hb level ≥11 g/dL (women) or 12 g/dL (men) without features of ongoing hemolysis (including normal haptoglobin level), without any ongoing treatment for wAIHA on two different occasions 4-week apart in the absence of any recent transfusion. CR could be considered even with a positive DAT result.
PR was defined as Hb level ≥ 10g/dL with at least a 2 g increase from baseline (i.e., at wAIHA diagnosis) without any other treatment than prednisone given at a daily dose ≤ 10 mg or recent transfusion.
Failure was considered CR or at least PR not achieved at 1 year or if the patient had received any therapy (other than prednisone and transfusions) known to be active in wAIHA (danazol, immunosuppressor) or had undergone splenectomy within the year after inclusion.
CD19+ B lymphocytes in peripheral blood were assessed at baseline and then repeatedly at Weeks 12, 28, and 52. Investigators were blinded to results until the end of the study.
Statistical analysis
The sample size was calculated considering an 80% overall response rate (CR + PR) at 1 year with RTX versus 20% with placebo. We needed 32 patients (16 in each group) for 90% power with a 2-sided significance level of 5%. Analysis was performed on an ITT basis. Data are presented as mean ± SD or median (interquartile range [IQR]) for continuous variables, depending on their distribution. Categorical variables are presented as number (%). Primary outcome was analyzed on an ITT basis and is presented with 95% binomial confidence intervals (95% CIs). A per-protocol analysis was also used for data for patients who reached the end of follow-up. Comparisons between groups involved Fisher's exact test for categorical variables and Mann–Whitney non-parametric test for continuous variables. Univariate analysis of factors associated with 1-year response revealed no factors except treatment, so we did not perform multivariate analysis. P <0.05 was considered statistically significant. All analyses involved use of Stata 12.0.
Ethics
The protocol was approved by our Institutional Review Board (Comité de Protection des Personnes Ile de France IX) and written informed consent was obtained from all patients in accordance with the Declaration of Helsinki. The study was registered at ClinicalTrials.gov (NCT01181154).
Results
Patients
A total of 39 patients were screened over the inclusion period; five refused to participate and two patients could not be enrolled, the first because of HBV positivity and the second because of gammaglobulin level < 6 g/l (Fig. 1). As planned, a total of 32 patients (17 females [53%], mean age at inclusion of 71 ± 16 years) from 13 participating centers fulfilling the eligibility criteria were enrolled and randomized (n = 16 in each group). The baseline characteristics (mean age, sex ratio, hemoglobin level, parameters of hemolysis, number of packed red cells transfused received before inclusion) were comparable between the groups (Table 1). Three patients (age 90, 88, and 87 years) with placebo died within the first year of follow-up and none with RTX (P = 0.226) (Fig. 1); one had prematurely withdrawn from the study after a diagnosis of massive pulmonary embolism on Day 15 just before receiving the second infusion of 100 mg methylprednisolone and placebo. One patient was excluded at Week 3 because of a protocol violation, and another patient with placebo was prematurely withdrawn at Week 28 for severe anemia, which was considered a severe adverse event by the investigator. Overall, 27 patients were followed for at least 1 year and their data were evaluable for response (Fig. 1).
| Rituximab | Placebo | P value | |
|---|---|---|---|
| Age (years) | 70.1 ± 16.6 | 71.4 ± 16.5 | 0.678 |
| Females, no. (%) | 7 (44%) | 10 (63%) | 0.288 |
| Hemoglobin level (g/dL) at diagnosis | 7.09 ± 1.26 | 7.14 ± 1.25 | 0.77 |
| Reticulocytes count (×109/l), median [range] | 215 [124–275] | 244 [166–434] | 0.144 |
| Lactate dehydrogenase level (UI/l), median [range] | 642 [437–936] | 478 [325–595] | 0.168 |
| Total bilirubin level (μmol/l),median [range] | 34 [28–69] | 50 [34–66] | 0.274 |
| Haptoglobin level (mg/l), median [range] | 0.08 [0.01–0.1] | 0.05 [0.01–0.1] | 0.658 |
| Patients transfused at least once before inclusion, no. (%) | 8 (50%) | 5 (31%) | 0.340 |
| No. of packed red cells administered by treatment arm, mean | 2.0 | 1.3 | 0.397 |
- Data are mean ± SD unless indicated.
Efficacy, primary, and secondary outcomes
At 1 year, with an ITT analysis (primary outcome), the overall response rate (CR + PR) was 75% [47.6–92.7%] (11 CR and 1 PR) with RTX versus 31% [11.0–58.7%] (5 CR) with placebo (P = 0.032).
A per-protocol analysis based on the pattern of response at 1 year among evaluable patients showed 12/16 responses with RTX versus 5/11 with placebo (P = 0.224).
At 2 years, 10/16 patients (63%) with RTX versus 3/16 with placebo showed CR (P = 0.029 with an ITT analysis). Relapse free survival in the two groups is shown on Fig. 2. The mean cumulative dose of prednisone at 1 year was 4.32 ± 2.27 g with RTX versus 5.94 ± 3.16 g with placebo (P = 0.14), and the mean number of weeks on prednisone therapy was 20.1 versus 24.3 (P = 0.31). In terms of hospitalizations, beyond that for RTX infusions and within the first 12 months of follow-up, four patients with RTX had to be hospitalized (n = 10 hospitalizations) versus 10 patients with placebo (n = 23 hospitalizations) (P = 0.073). The median duration of hospital stay was 13 days [IQR 6–26] with RTX versus 28 days [19-61] with placebo (P = 0.076). Four patients with RTX received at least one transfusion of packed red cells from Day 1 and Week 52 (mean number of packed red cell units 4.0 ± 2.82) versus five patients with placebo (mean number of packed red cell units 5.6 ± 4.15).
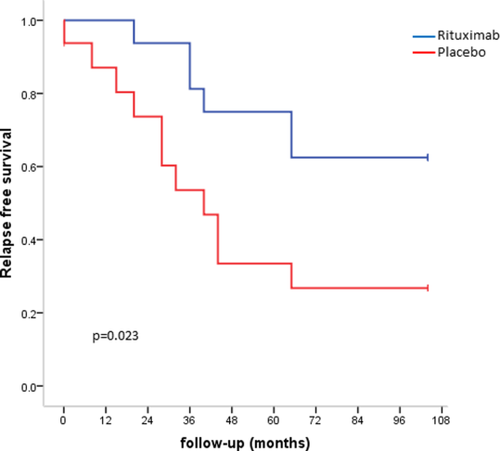
Relapse free survival rates in patients treated with RTX or placebo estimated by the Kaplan-Meïer method and compared by the log rank test. Events are primary non response, relapse and death. [Color figure can be viewed at wileyonlinelibrary.com]
The DAT results were negative at Week 52 for 8/13 (61.5%) RTX patients with available data as compared to only 2/10 (20%) placebo patients. For both groups, all patients with negative DAT results at Week 52 achieved CR. After the end of follow-up (month 24), DAT results were negative for 9/14 (64%) RTX patients versus only three placebo patients.
Among RTX patients, the number of B cells at baseline was not significantly different for the responders and the six patients who did not respond or relapsed (289/μl [83–464] vs. 173/μl [123–324], P = 0.689). The level of B cell depletion was also similar for patients who did not achieve an initial response and the responders.
Safety
No immediate post-infusion reactions were reported in both groups. Two episodes of grade 3 neutropenia were observed with RTX (Supporting Information Table 2). They occurred 8 weeks and 4 months after the first RTX infusion, with a nadir of neutrophil count 0.93 and 0.49 × 109/l, respectively. These two episodes were transient and asymptomatic.
Two cases of pneumonia occurred with RTX (Supporting Information Table 2). The first patient was a 55-year-old woman who presented acute interstitial pneumonia 6 weeks after the first RTX infusion while on 50 mg prednisone. Blood culture was negative; bronchoscopy with bronchoalveolar lavage analysis showed lymphocytic alveolitis (predominantly TCD8+ lymphocytes) with negative culture but positive PCR results for Pneumocystis jirovecii infection. The diagnosis of pneumocystis pneumonia was retained by the investigator and treatment with trimethoprim-sulfamethoxazole was successful. The patient ultimately underwent splenectomy 9 months after inclusion after AIHA relapse.
Ten severe adverse events concerning seven patients with mostly severe infections were reported with placebo, some fatal, and a massive pulmonary embolism occurred in an 87-year-old woman on Day 15 just before the second infusion (Supporting Information Table 2).
Gammaglobulin level significantly decreased with RTX treatment: mean level at inclusion was 10.8 ± 4.0 g/l versus 8.1 ± 2.2 g/l at Week 52 (P = 0.028), but overall, mean gammaglobulin level at 1 year did not differ among evaluable patients in the RTX and placebo groups (8.1 ± 2.2 vs. 7.7 ± 1.5 g/l, P = 0.499).
Outcome and mortality
The type of treatment, pattern of response and outcome of non-responders in both groups are in Fig. 3. Within 24 months of follow-up, six patients with placebo died (median age 84 years [IQR 71–90]) versus none with RTX (P = 0.017; Fig. 3; Supporting Information Table 2).
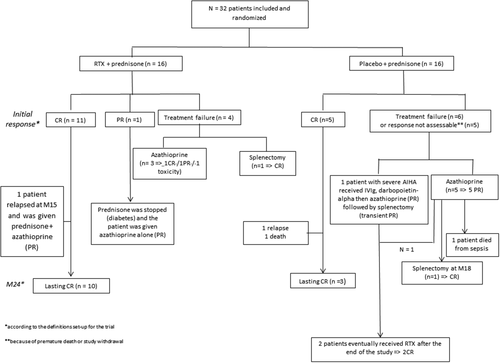
Outcome of patients with rituximab and placebo and other second or third-line treatments.
Discussion
This is the first randomized double-blind placebo-controlled Phase 3 trial to evaluate the efficacy and safety of RTX in newly-diagnosed adult wAIHA. The regimen of RTX was a fixed dose of 1,000 mg 2-week apart as commonly used for rheumatoid arthritis 15 and in our referral center for immune cytopenias 16. The primary endpoint of the study was reached because with the ITT analysis, the overall response rate was significantly higher with RTX than placebo (75% vs. 31%) at 1 year. Interestingly, the overall response rate was the same as that found at 1 year in a previous randomized prospective open trial performed in Denmark, with RTX given at 375 mg/m2 weekly for 4 weeks 9, and close to the 69.2% CR rate at 1 year found in a Phase 2 study with a lower dose of RTX 8.
In our study, the difference between the two groups was even more significant at 2 years in the ITT analysis: 10 patients with RTX who achieved CR at 1 year were still in CR as compared to only 3/16 with placebo. Moreover, the DAT results become negative with RTX treatment in most patients who achieved CR which emphasizes the potential curative effect of a single course of RTX for wAIHA. However, approximately one-third of the patients with placebo who received prednisone alone for 3 months, a duration shorter than historically recommended 7, may achieve CR at 1 year. Due to the sample size, it was not possible to identify some predictive factors of good and CR to corticosteroids.
Regarding the corticosteroid-sparing effect of RTX, although we found low cumulative dose of prednisone and short duration of prednisone treatment with RTX versus placebo within 1 year after inclusion, these differences were not statistically significant. The sample size of the study was not calculated and powered to demonstrate a clear corticosteroid-sparing effect of RTX, and the premature deaths and study withdrawals that occurred with placebo minimized the average exposure to prednisone in this group.
Regarding safety, RTX was generally well tolerated, no immediate reactions were observed, and all 16 patients were alive after 2 years, whereas six deaths occurred with placebo. With RTX, one case of probable pneumocystis pneumonia, based on compatible clinical and radiological presentation and a positive P. jirovecii PCR result with bronchoalveolar fluid was reported. The lack of sensitivity of microscopy demonstration of P. jirovecii infection in non-HIV-infected patients has led to the increased use of PCR-based methods for diagnosis. The limitation is that the positive-predictive value of standard PCR is relatively low because this method cannot always differentiate active pneumonia from colonization 17. Beyond the role of corticosteroids, which are a well-known risk factor of P. jirovecii infection, especially in older patients, such cases after RTX therapy have been reported in other settings 18. The role of RTX, which mainly targets B lymphocytes, in promoting this infection is debated, but some indirect evidence suggests that RTX per se may increase the susceptibility to pneumocystis 19.
In the placebo arm, the number of severe adverse events and especially severe infections and number of deaths (i.e., 6/16 patients) observed within the 2 years of follow-up were higher than expected. The mean age of the patients included (i.e., 71 years) was unexpectedly high as compared with that reported in the largest series from the literature 3, 4 and in the Phase 2 trial performed in Italy 8 and to a lesser extent in the Danish trial 9. In our trial, some older patients with comorbidities who did not achieve CR were exposed to prednisone ± azathioprine on a long-term basis, which could explain at least in part the number of severe infections. In the Danish study, rates of severe adverse events and deaths were comparable in both treatment groups; three cases of pneumonia were reported with RTX + prednisolone versus two with prednisolone alone, with four (12.5%) and three (9.3%) deaths, respectively. In our study, we did recommend to vaccinating eligible patients against pneumococcus, but that was not mandatory (1 patient with placebo died of bilateral pneumococcal pneumonia) and we lacked a recommendation regarding the use of systematic antibiotic prophylaxis.
The strengths of our trial were the randomized and completely blinded allocation, the incorporation of well-defined criteria of response and a reduction scheme of prednisone according to the initial response. Both groups were comparable in demographic data and initial severity of wAIHA. The limitations were the sample size, which was insufficient to detect a significant difference in the cumulative dose of prednisone and blood transfusion requirement as well as the unexpected number of study withdrawals and deaths with placebo.
In conclusion, RTX combined with a relatively short course of prednisone may confer a better benefit-to-risk ratio than prednisone alone for treating adults with newly-diagnosed and primary wAIHA. From these results and those of the previous randomized open-label Phase 3 study, RTX should be considered when available, either as first treatment-line combined with corticosteroids for elderly patients and/or patients with comorbidities or at least as an early second-line option for younger patients not showing a good and rapid initial response to corticosteroids. Both regimens (i.e., 4 weekly infusions at 375 mg/m2 or 2 infusions at 1,000 mg given 2-week apart) seem to lead to the same response-rate but the latter commonly used in autoimmune diseases is simpler in clinical practice. To minimize the risk of P. jirovecii infection, antibiotic prophylaxis with cotrimoxazole is strongly recommended within at least the first 12 months after RTX + prednisone treatment, and pneumococcal vaccine should be considered, especially in older patients.
Acknowledgments
We are grateful to Laura Smales for careful editing of the manuscript. This study was promoted by the Direction de la Recherche Clinique, Assistance Publique-Hôpitaux de Paris and supported by the Etablissement Français du Sang and by Roche
Author Contributions
MM has designed and set up the study (principal investigator), included patients and wrote the manuscript; LT, MH, ME, GLG, LG, SA, BR, ASM, JMM, AJ, LF and MK participated as investigators and included some patients; FRT performed the statistical analysis, BG was involved in the study design and in the writing of the manuscript.




