Bloodstream infections caused by Klebsiella pneumoniae in onco-hematological patients: clinical impact of carbapenem resistance in a multicentre prospective survey
Conflict of interest: Nothing to report
Abstract
The aim of this study was to identify risk factors for mortality in patients suffering from hematological malignancies (HMs) with bloodstream infections (BSIs) caused by Klebsiella pneumoniae (KP). We conducted a prospective cohort study on KP BSI in 13 Italian hematological units participating in the HEMABIS registry–SEIFEM group. The outcome measured was death within 21 days of BSI onset. Survivor and non-survivor subgroups were compared and Cox regression analysis was conducted to identify independent predictors of mortality. A total of 278 episodes of KP BSI were included in the study between January 2010 and June 2014. We found that 161 (57.9%) KP isolates were carbapenem resistant (CRKP). The overall 21-day mortality rate was 36.3%. It was significantly higher for patients with CRKP BSI (84/161, 52.2%) than for those with BSI caused by carbapenem susceptible KP (CSKP) (17/117, 14.5%; P < 0.001). Septic shock (HR 3.86), acute respiratory failure (HR 2.32), inadequate initial antimicrobial therapy (HR 1.87) and carbapenem resistance by KP isolates (HR 1.85) were independently associated with mortality. A subanalysis was conducted in only 149 patients with CRKP BSI who had received ≥48 hr of adequate antibiotic therapy, and combination therapy was independently associated with survival (HR 0.32). Our study shows that in recent years carbapenem resistance has dramatically increased in HM patients with KP BSI in Italy and is associated with a worse outcome. The optimal management of such infections and the definition of new empirical/targeted antimicrobial strategies in HM patients can still be considered unmet clinical needs. Am. J. Hematol. 91:1076–1081, 2016. © 2016 Wiley Periodicals, Inc.
Introduction
During recent decades significant improvements have been made in the treatment of hematologic malignancies (HMs). However, several types of infections often complicate the treatment course of HM patients; bloodstream infections (BSIs) are the most common and most severe infectious complications, with a reported incidence ranging from 11% to 38% and a 40% rise in crude mortality rates 1. In recent years, a clear trend has been reported in the epidemiology of severe infections, in particular BSIs; a shift has been shown from Gram-positive to Gram-negative bacteria in HM patients, with mortality rates significantly higher in patients with BSI caused by Gram-negative bacteria compared to those with BSI caused by Gram-positive bacteria 1, 2. Furthermore, a worrisome and extensive emergence of antimicrobial-resistance in Gram-negatives has been recently reported in HM patients 1-4. In particular, resistance to carbapenems caused by Klebsiella pneumoniae (KP) has become a significant problem in several countries 5, 6, and has recently been reported as one of the major emerging causes of severe and fatal infections in HM patients in Italy 2, 7, 8. Girmemia et al. recently described episodes of infections caused by carbapenem-resistant KP (CRKP) in an Italian cohort of 112 stem cell transplant (SCT) recipients. These authors reported an incidence of 0.4% and 2% in autologous-SCTs and allogeneic-SCTs, respectively 8. However, up until now data on the clinical impact of carbapenem resistance in the general HM population (i.e. not only SCTs) with KP BSI are scarce.
The aim of this study was to identify risk factors for mortality in HM patients with BSIs caused by KP. Particular attention was given to defining the impact of carbapenem resistance by the KP isolates on mortality.
Methods
We conducted a prospective cohort study in 13 Italian hematological wards of tertiary care centers or university hospitals participating in the Haematologic Malignancies Associated Bloodstream Infections Surveillance (HEMABIS) registry—Sorveglianza Epidemiologica Infezioni Fungine in Emopatie Maligne (SEIFEM) group throughout Italy from January 2010 to June 2014. Antibacterial prophylaxis was administered to patients at all participating centers according to the Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) criteria 9.
All episodes of BSI caused by KP (KP-BSI) that occurred in hospitalized HM patients were included. Data collected from the hospital charts and the laboratory database included patient demographics, disease and disease stage at time of KP BSI, type of SCT (autologous or allogeneic), and outcome of infection; for each bacterial isolate, antimicrobial susceptibility was recovered and analyzed. All information was entered in case report forms and then recorded in a specific database. Recurrent infections were excluded; only the first episode per patient was included in our registry.
The outcome measured was death within 21 days of the first positive blood culture. Survivor and non-survivor subgroups were compared in order to identify predictors of mortality. To investigate predictors of mortality and the role of combination therapy in such infections, a subanalysis was conducted that included all patients with CRKP BSI who had received ≥48 hr of antibiotic therapy (empirical and/or targeted) with at least one drug the KP isolate was susceptible to.
The ethics committee of each participating site approved use of the HeMABIS Registry. Informed consent was obtained from each patient.
Definitions
The following terms were defined prior to data analysis:
KP BSI was defined as an infection manifested by the presence in at least 1 blood culture that grew a KP strain.
Neutropenia was defined as an absolute neutrophil count (ANC) <500 neutrophils/μL at the onset of BSI; neutropenia was considered prolonged if the duration was ≥10 days and severe if ANC was <100 neutrophils/μL.
KP BSI was considered hospital-acquired if the index culture had been collected >48 hr after admission and signs and symptoms of infection were absent at admission. If cultures had been collected ≤48 hr after the admission date, the isolate was classified as healthcare-associated or community-acquired 10.
Empirical antimicrobial therapy schemes were selected by attending physicians according to American and European guidelines for the treatment of febrile neutropenia 11-13; i.e., in most cases with anti-pseudomonal b-lactam agents (i.e., carbapenems, piperacillin/tazobactam or cefepime) usually combined with an amynoglicoside. Most patients with previous evidence of CRKP infection/colonization were also empirically treated by associating an antimicrobial agent to which the previous isolate was susceptible. The initial treatment was classified as inadequate if the infecting pathogen demonstrated resistance (as determined by in vitro susceptibility testing) to the administered antimicrobial(s); conversely, initial antimicrobial therapy was considered adequate if it contained at least one drug to which the KP isolate was subsequently demonstrated susceptible.
Post-antibiogram antibiotic therapy was defined as monotherapy if it included treatment with only one of the following drugs: gentamicin, colistin or tigecycline; all the other antibiotic regimens (including another active drug or a carbapenem) were defined as combination therapy.
Septic shock as well as severe sepsis were defined according to the Surviving Sepsis Campaign criteria 14.
Statistical analysis
Continuous variables were compared with Student's t test for normally distributed variables and the Mann-Whitney U test for non-normally distributed variables. Categorical variables were evaluated using the χ2 or two-tailed Fisher's exact test. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to evaluate the strength of any association that emerged. Values are expressed as means ± standard deviation (SD) (continuous variables) or as percentages of the group from which they were derived (categorical variables). Two-tailed tests were used to determine statistical significance; a P value of <0.05 was considered significant. Cox regression analysis was conducted to identify independent risk factors for 21-day mortality. Variables emerging from univariate analysis with P values of <0.1 were included in the Cox regression model. The Kaplan-Meier method was used for survival analysis. All statistical analyses were performed using the Intercooled Stata program, version 11, for Windows (Stata Corporation, College Station, Texas, USA).
Results
A total of 278 episodes of KP BSI were included in the study. Of these, 161 (57.9%) KP isolates were carbapenem-resistant. The rate of carbapenem-resistance among KP isolates significantly increased from 21.4% in 2010 to 75.9% in 2013 (P < 0.001) (Fig. 1), and it was 61.1% during the first six months of 2014. Overall, 6666 patients diagnosed with acute myeloid/lymphatic leukemia (3106) or who had undergone SCT (3560) had been admitted to participating centers during the study period; data on the total number of patients admitted for other HMs were not available. Overall, in these patients, the incidence rate of KP BSI was 4.02% (268/6666); it was 6.63% (206/3106) for patients with acute myeloid/lymphatic leukemia and 1.74% (62/3560) for SCT recipients.
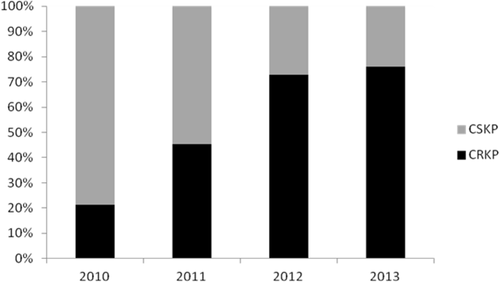
Percentages of resistance to carbapenems among Klebsiella pneumoniae isolates throughout the study period.
Table 1 shows the clinical and epidemiological characteristics of cohort patients according to carbapenem-resistance of the KP isolate. Compared to patients with carbapenem-susceptible KP (CSKP) BSI, those with CRKP BSI were more likely older, had indwelling peripherally inserted central catheters (PICCs), suffered from acute myeloid leukemia, had previous CRKP culture-positive surveillance rectal swabs, and received antibiotic prophylaxis, in particular with fluoroquinolones. Conversely, patients who had indwelling centrally inserted (both short- and long-term) venous catheters (CVCs) and those who suffered from non-Hodgkin's lymphoma and/or had undergone hematopoietic stem cell transplantation, more likely had a BSI episode caused by CSKP.
| Variables | CRKP-BSI (n = 161) | CSKP-BSI (n = 117) | P values |
|---|---|---|---|
| Demographic information | |||
| Male sex | 85 (52.8) | 66 (56.4) | 0.55 |
| Age ≥ 55 years | 92 (57.1) | 60 (51.3) | 0.33 |
| Patients' characteristics | |||
| Indwelling CVC | 97 (60.2) | 90 (76.9) | 0.003 |
| Short-term CVC | 28 (17.4) | 36 (30.8) | 0.008 |
| PICC | 40 (24.8) | 11 (9.4) | 0.001 |
| Long-term CVC | 29 (18.1) | 43 (36.7) | <0.001 |
| Parenteral nutrition | 21 (13.1) | 15 (12.8) | 0.95 |
| Diabetes mellitus | 25 (15.5) | 10 (8.5) | 0.08 |
| Chronic hepatic failure | 3 (1.8) | 1 (0.8) | 0.48 |
| Chronic renal failure | 7 (4.3) | 4 (3.4) | 0.69 |
| ANC < 500/mmc | 132 (81.9) | 100 (85.4) | 0.44 |
| ANC < 100/mmc | 118 (73.3) | 74 (63.2) | 0.07 |
| ANC < 500/mmc for at least 10 days | 97 (60.2) | 61 (52.1) | 0.17 |
| Corticosteroid treatment | 71 (44.1) | 44 (37.6) | 0.27 |
| Chemotherapy | 129 (80.1) | 92 (78.6) | 0.76 |
| Radiotherapy | 5 (3.1) | 7 (5.9) | 0.24 |
| Indwelling urinary catheter | 27 (16.8) | 14 (11.9) | 0.26 |
| Hematological malignancy | |||
| Acute myeloid leukemia | 119 (73.9) | 61 (52.4) | <0.001 |
| Chronic myeloid leukemia | 1 (0.6) | 0 | 0.39 |
| Acute lymphatic leukemia | 12 (7.4) | 14 (11.9) | 0.20 |
| Chronic lymphoid leukemia | 1 (0.6) | 1 (0.8) | 0.82 |
| Non Hodgkin's lymphoma | 18 (11.2) | 25 (21.4) | 0.02 |
| Hodgkin's lymphoma | 2 (1.2) | 3 (2.6) | 0.41 |
| Multiple Myeloma | 3 (1.9) | 3 (2.6) | 0.69 |
| Myelodysplastic syndrome | 2 (1.2) | 5 (4.2) | 0.11 |
| Stage of Hematological Disease | |||
| Newly diagnosed/Relapsed after 1st remission | 64 (39.7) | 20 (17.1) | <0.001 |
| Complete remission | 21 (13.1) | 36 (30.8) | <0.001 |
| Refractory/Relapsed after 2 or more remissions | 56 (34.8) | 47 (40.2) | 0.35 |
| Hematopoietic stem cell transplantation | 26 (16.1) | 36 (30.8) | 0.003 |
| Autologous | 9 (5.6) | 9 (7.7) | 0.48 |
| Allogeneic-Matched Related | 4 (2.5) | 2 (1.7) | 0.66 |
| Allogeneic-Matched Unrelated | 8 (4.9) | 14 (11.9) | 0.03 |
| Allogeneic-Mismatched | 7 (4.3) | 9 (7.7) | 0.23 |
| Characteristics of BBSI | |||
| Hospital acquired | 148 (91.9) | 101 (86.3) | 0.13 |
| Healthcare-associated | 13 (8.1) | 16 (13.7) | 0.13 |
| Primary site of BSI | |||
| Urinary tract | 17 (10.6) | 10 (8.5) | 0.57 |
| Respiratory tract | 19 (11.8) | 3 (2.6) | 0.004 |
| Surgical wound | 3 (1.8) | 0 | 0.13 |
| CVC | 22 (13.6) | 15 (12.8) | 0.83 |
| Unidentified | 102 (63.3) | 84 (71.8) | 0.13 |
| Antibiotic prophylaxis | 107 (66.5) | 46 (39.3) | <0.001 |
| Co-trimoxazole | 15 (9.3) | 10 (8.5) | 0.82 |
| Fluoroquinolones | 92 (57.1) | 33 (28.2) | <0.001 |
| Antifungal prophylaxis | 97 (60.2) | 62 (52.9) | 0.22 |
| Antibiotic resistance by KP isolate | |||
| Third generation cephalosporins | 161 (100) | 28 (23.9) | <0.001 |
| Fluoroquinolones | 161 (100) | 38 (32.9) | 0.72 |
| Piperacillin/tazobactam | 161 (100) | 37 (31.6) | <0.001 |
| Amikacin | 148 (91.9) | 13 (11.1) | <0.001 |
| Gentamicin | 56 (34.8) | 17 (14.5) | <0.001 |
| Tigecycline | 39 (24.2) | 0 | - |
| Colistin | 22 (13.6) | 0 | - |
| Inadequate initial antimicrobial therapy | 126 (78.3) | 26 (22.2) | <0.001 |
| 21-day mortality | 84 (52.2) | 17 (14.5) | <0.001 |
- Abbreviations: CVC, central venous catheter; PICC, peripherally inserted central catheter; ANC, neutrophil count.
The overall 21-day mortality rate was 36.3% (101/278); however, it was significantly higher for patients with CRKP BSI (84/161, 52.2%) than for those with BSI caused by CSKP (17/117, 14.5%; P < 0.001). Survival curve analysis confirmed the higher risks of mortality associated with CRKP BSI (P < 0.001) (Fig. 2).
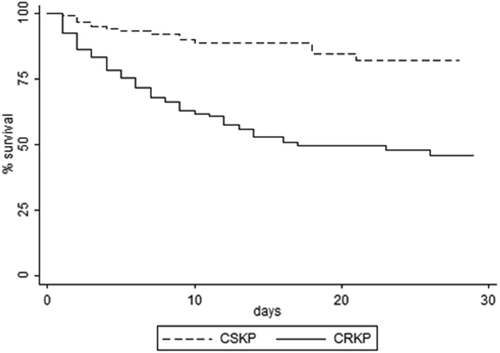
Kaplan-Meier survival estimates among patients with BSI caused by carbapenem resistant (CR) and carbapenem susceptible (CS) Klebsiella pneumoniae (KP).
Risk factors for 21-day mortality in patients with KP BSI
In a univariate analysis (described in Table 2), variables associated with 21-day mortality were: age ≥ 55 years, severe and/or prolonged neutropenia, corticosteroid treatment, acute myeloid leukemia, new diagnosis/relapse after first remission as stage of HM disease, respiratory tract as primary site of KP BSI, septic shock, altered state of consciousness, acute renal and/or respiratory and/or hepatic failure, inadequate initial antimicrobial therapy, and carbapenem resistance by KP isolate. On the contrary, non-Hodgkin's lymphoma and/or the complete remission stage of HM disease were significantly associated with survival.
| Variables | Non-survivors (n = 101) | Survivors (n = 177) | OR (95% IC) | P values |
|---|---|---|---|---|
| Demographic information | ||||
| Male sex | 58 (57.4) | 93 (52.5) | 1.22 (0.72–2.06) | 0.43 |
| Age ≥ 55 years | 64 (63.4) | 88 (49.7) | 1.75 (1.03–2.98) | 0.02 |
| Patient's characteristics | ||||
| ANC < 500/mmc | 88 (87.1) | 144 (81.4) | 1.55 (0.75–3.39) | 0.21 |
| ANC < 100/mmc | 78 (77.2) | 114 (64.4) | 1.87 (1.04–3.44) | 0.02 |
| ANC < 500/mmc for at least 10 days | 66 (65.4) | 92 (51.9) | 1.74 (1.02–2.99) | 0.03 |
| Corticosteroid treatment | 51 (50.5) | 64 (36.2) | 1.80 (1.06–3.05) | 0.01 |
| Chemotherapy | 80 (79.2) | 141 (79.7) | 0.97 (0.51–1.88) | 0.92 |
| Radiotherapy | 4 (3.9) | 8 (4.5) | 0.87 (1.19–3.36) | 0.82 |
| Hematological malignancy | ||||
| Acute myeloid leukemia | 76 (75.3) | 104 (58.8) | 2.13 (1.21–3.84) | 0.005 |
| Chronic myeloid leukemia | 0 | 1 (0.6) | - | 0.44 |
| Acute lymphatic leukemia | 9 (8.9) | 17 (9.6) | 0.92 (0.35–2.29) | 0.84 |
| Chronic lymphoid leukemia | 1 (0.9) | 1 (0.6) | 1.76 (0.02–138.92) | 0.68 |
| Non Hodgkin's lymphoma | 8 (7.9) | 35 (19.8) | 0.35 (0.13–0.81) | 0.008 |
| Hodgkin's lymphoma | 0 | 5 (2.8) | - | 0.08 |
| Multiple Myeloma | 3 (2.9) | 3 (1.7) | 1.78 (0.23–13.48) | 0.48 |
| Myelodysplastic syndrome | 2 (1.9) | 5 (2.8) | 0.69 (0.06–4.34) | 0.66 |
| Stage of Hematological Disease | ||||
| Newly diagnosed/Relapsed after 1st remission | 41 (40.6) | 43 (24.3) | 2.13 (1.22–3.72) | 0.004 |
| Complete remission | 6 (5.9) | 51 (28.8) | 0.16 (0.05–0.39) | <0.001 |
| Refractory/Relapsed after 2 or more remissions | 42 (41.6) | 61 (34.5) | 1.35 (0.79–2.31) | 0.23 |
| Hematopoietic stem cell transplantation | 17 (16.8) | 45 (25.4) | 0.59 (0.30–1.14) | 0.09 |
| Autologous | 3 (2.9) | 15 (8.5) | 0.33 (0.06–1.21) | 0.07 |
| Allogeneic-Matched Related | 0 | 6 (3.4) | - | 0.06 |
| Allogeneic-Matched Unrelated | 10 (9.9) | 12 (6.8) | 1.51 (0.56–3.98) | 0.35 |
| Allogeneic-Mismatched | 4 (3.9) | 12 (6.8) | 0.57 (0.13–1.94) | 0.33 |
| Septic shock | 58 (57.4) | 13 (7.3) | 17.02 (8.20–36.62) | <0.001 |
| Altered state of consciousness | 42 (41.6) | 7 (4) | 17.28 (7.10–47.56) | <0.001 |
| Acute renal failure | 29 (28.7) | 7 (4) | 9.78 (3.93–27.45) | <0.001 |
| Acute respiratory failure | 63 (62.4) | 17 (9.7) | 15.60 (7.87–31.44) | <0.001 |
| Acute hepatic failure | 18 (17.8) | 2 (1.1) | 18.97 (4.33–170.73) | <0.001 |
| Inadequate initial antimicrobial therapy | 79 (78.2) | 73 (41.2) | 5.11 (2.83–9.39) | <0.001 |
| Carbapenem-resistance by KP isolate | 84 (83.1) | 77 (43.5) | 6.41 (3.41–12.43) | <0.001 |
- Abbreviations: CVC, central venous catheter; ANC, neutrophil count.
A Cox regression (model A) showed the following significant predictors of mortality: septic shock (HR 3.86, 95% CI 2.47–6.03; P < 0.001), acute respiratory failure (HR 2.32, 95% CI 1.45–3.70; P < 0.001), initial inadequate antimicrobial therapy (HR 1.87, 95% CI 1.08–3.22; P = 0.02) and carbapenem resistance by KP isolate (HR 1.85, 95% CI 1.01–3.42; P = 0.04) (Table 3, Model A).
| Variables | HR | (95% IC) | P values |
|---|---|---|---|
| MODEL (A) | |||
| Septic shock | 3.86 | (2.47–6.02) | <0.001 |
| Acute respiratory failure | 2.32 | (1.45–3.70) | <0.001 |
| Initial inadequate antimicrobial therapy | 1.87 | (1.08–2.22) | 0.02 |
| Carbapenem-resistance by KP isolate | 1.85 | (1.01–3.42) | 0.04 |
| MODEL (B) | |||
| Septic shock | 2.64 | (1.57–4.45) | <0.001 |
| Acute respiratory failure | 2.83 | (1.63–4.92) | <0.001 |
| Combination therapy | 0.32 | (0.19–0.54) | <0.001 |
Antibiotic treatment
Almost all cases of CSKP BSI were treated with a beta-lactam (third-generation cephalosporins or carbapenems or a beta-lactam/beta-lactamase inhibitor) ± an aminoglycoside; antibiotic regimens used for CRKP BSI cases are shown in Supporting Information Table SI, according to mortality.
Risk factors for 21-day mortality in patients with CRKP BSI
A total of 149 patients (out of 161) with CRKP BSI received ≥48 h of antibiotic therapy (empirical and/or non-empirical) with at least one drug to which the KP isolate was susceptible (12 patients died within 48 h from BSI onset); the 21-day mortality rate among these patients was 48.3% (72/149). In a univariate analysis conducted on these 149 patients, variables associated with 21-day mortality were: severe (62, 86.1% vs. 49, 63.6%; P = 0.001) and/or prolonged neutropenia (53, 73.6% vs. 39, 50.6%; P = 0.004), acute myeloid leukemia (59, 81.9% vs. 52, 67.5%; P = 0.04), respiratory tract as primary site of BSI (13, 18.1% vs. 5, 6.5%; P = 0.03), septic shock (41, 56.9% vs. 4, 5.2%; P < 0.001), altered state of consciousness (32, 44.4% vs. 5, 6.5%; P < 0.001), acute renal (20, 27.8% vs. 5, 6.5%; P < 0.001) and/or respiratory (50, 69.4% vs. 9, 11.7%; P < 0.001) and/or hepatic failure (11, 15.3% vs. 1, 1.3%; P < 0.001), and inadequate initial antimicrobial therapy (64, 88.9% vs. 51, 66.2%; P = 0.001). On the contrary, the complete remission stage of HM disease (3, 4.2% vs. 18, 23.4%; P < 0.001) and/or treatment with a combination of antibiotics (40, 55.5% vs. 69, 89.6%; P < 0.001) were significantly associated with survival.
In the Cox regression analysis (model B) the variable combination therapy was retained as independently associated with survival (HR 0.32, 95% CI 0.19–0.54; P < 0.001), whereas significant predictors of mortality were septic shock (HR 2.64, 95% CI 1.57–4.45; P < 0.001) and acute respiratory failure (HR 2.83, 95% CI 1.63–4.92; P < 0.001) (Table 3, Model B).
Discussion
We found that during the study period most (57.9%) KP isolates causing BSI in patients suffering from HMs were resistant to carbapenems; notably, the rate of carbapenem resistance displayed a worrisome trend, increasing significantly from 21.4% in 2010 to 75.9% in 2013.
These data differ from what has been reported in epidemiological studies on antibiotic resistance in cancer patients published worldwide from 2007 to 2013 and recently reviewed, in which >98% of KP isolates were reported as susceptible to carbapenems 1.
Conversely, the increasing trend towards carbapenem-resistance among KP isolates in HM patients has been recently highlighted in our country. In our previous survey of the HeMABIS registry, conducted from 2009 to 2012, we found a rate of resistance to carbapenems among KP isolates equal to 34.9% 2. In addition, in a single Italian hospital hematology department a significant increase in the incidence of CRKP among all Gram-negatives causing BSIs from 2009–2010 (1.4%) to 2011–2012 (32.1%) has been reported 7. The trend in carbapenem resistance in our population is also in line with that reported in the general Italian population from 2010 to 2013 by Antimicrobial resistance surveillance in Europe (EARSS); however, the overall mean rate of carbapenem resistance among KP isolates during the same time period was more than twice as high in our HM population compared to that reported in the general population in Italy (mean 26.3%), where CRKP strains are endemic 15. This enhanced spread of CRKP in hematological settings might be due to all well known risk factors for infections in HM patients, and in particular the severe and prolonged post-chemotherapy neutropenia, which particularly predisposes these patients to severe infections, including those caused by multidrug resistant (MDR) Gram-negatives. Confirming this hypothesis, neutropenia and/or HM have been recognized as independent risk factors for infection and/or colonization by Klebsiella pneumoniae carbapenemase (KPC)-producing KP strains 16, 17, and chemotherapy/radiation therapy has been reported to be an independent risk factor for CRKP BSI development among CRKP rectal carriers 18. In addition, the recent use of fluoroquinolones, which was significantly more frequent among CRKP patients in our HM cohort compared to CSKP, has been reported as an independent predictor of KPC-KP isolation 17.
The rate of mortality among patients with CRKP BSI in our cohort is slightly higher (52.2%) compared to that reported in other studies conducted in general populations 19, 20, but roughly similar to that reported in HM patients 8, 21.
The most important risk factor for mortality in our cohort was septic shock at KP BSI onset; not surprisingly, this variable had been found to be associated with mortality in several previous studies performed on general population patients with CRKP BSI 19, 20, 22. The second variable strongly associated with mortality in our study was acute respiratory failure, which confirms the importance of clinical severity of BSI in influencing the outcome 5, 23.
The inadequacy of empirical antimicrobial regimens has emerged as a predictor of mortality, in line with several previous studies on BSI caused by antibiotic resistant Enterobacteriaceae in the general populations 19, 20, 22-25, as well as in HM patients 1, 2, 26, 27. In addition, a non CRKP-targeted first-line treatment was recently found to be independently associated with increased mortality in allo-SCT patients who developed a CRKP infection 8. Of note, inadequate initial antimicrobial therapy is the only modifiable factor among those independently associated with poor outcome in our cohort. It is possible to speculate that the inadequacy of initial treatment can be judged only retrospectively. In this regard, the use of surveillance data and hospital or unit antibiograms, the individualization of the initial regimen on the basis of prior antibiotic use and prior isolation of resistant pathogens, the use of combinations of antibiotics to cover the most frequent local bacterial isolates, and the optimization of antibiotic doses on the basis of pharmacodynamic principles could represent important criteria for increasing the adequacy of the initial empirical antibiotic choice 28. In addition, the recent increase in the use of laboratory methods for rapid bacterial identification and antibiotic resistance should allow early administration of targeted antimicrobial treatment, thus potentially improving clinical outcome 29.
The resistance to carbapenem by KP isolate also resulted independently associated with mortality in our cohort. In line with this finding, BSIs caused by antibiotic-resistant Gram-negative bacteria have been associated with increased rates of mortality in several previous studies conducted in HM patients with BSI 1, 27, 30, 31. More specifically, resistance to carbapenems has recently been associated with mortality in patients suffering from HM with Gram-negative aerobic bacteraemia 32.
Nevertheless, the optimal treatment for invasive infections caused by CRKP has not yet been established. But, on the basis of observational studies, the use of combination therapy is considered the best therapeutic approach 19, 20, 22 and has been suggested also for HM patients 33. Note that in a recent review and meta-analysis it was reported that there is no evidence-based support for most combination therapies against carbapenem-resistant Gram-negative bacteria, including colistin/carbapenem combination therapy; however, this review was not specifically conducted on CRKP infections/BSIs 34. We analyzed the impact of combination therapy in a subgroup of 149 patients with CRKP BSI who had received ≥48 hr of antibiotic therapy with at least one drug to which the KP isolate was susceptible at the post-antibiogram evaluation. Combination therapy resulted significantly associated with survival in the Cox regression analysis. This finding is in line with what was recently reported by Tofas et al. who reviewed 50 cases of neutropenic patients with hematological diseases complicated with CPKP BSIs and demonstrated that treatment with one active drug (HR for monotherapy versus combination therapy 3.95) was an independent predictor of death 21.
One limitation of our study is that it was performed in a country with a high incidence of CR-KP; therefore, the results may not necessarily be applicable to settings different from ours; in addition, screening for CRKP carriage by rectal swabs was not performed routinely in all participating centers.
In conclusion, our data demonstrate a worrisome increasing frequency in the rate of carbapenem resistance among KP isolates causing BSIs in patients with HMs and a significant impact of these infections on mortality. Understanding the local distribution of pathogens and their susceptibility patterns and of patients' risk factors for CR-KP and its complicated clinical course is urgent and necessary in order to improve the efficacy of therapeutic (empirical and etiological) treatment protocols (and survival) in HM patients.
Author Contributions
E. M. Trecarichi and L. Pagano contributed equally to this article.




