“All the soarings of my mind begin in my blood:” central nervous system complication of Waldenström macroglobulinemia
A physician or group of physicians considers presentation and evolution of a real clinical case, reacting to clinical information and data (boldface type). This is followed by a discussion/commentary
A 76-year-old man was admitted to our hospital for one month of progressive word finding problems. He was diagnosed with Waldenström macroglobulinemia (WM) two years prior when he presented with fatigue and anemia. The patient carried the MYD88 L265P gene mutation. In the past year, he had developed pancytopenia, elevated serum viscosity, enlarged intra-abdominal lymph nodes, and bilateral pleural effusions. Treatment with monthly infusions of bendamustine and rituximab had been initiated four months before presentation. His absolute lymphocyte count was 1.51 k/uL at the start of therapy, 0.92 k/uL at Cycle 2, 0.75 k/uL at Cycle 3, 1.64 k/uL at Cycle 4, and 1.88 k/uL at four week follow-up after Cycle 4. His counts reached a nadir of 0.24 k/uL midway through the treatment regimen.
The patient's word finding difficulties suggest a lesion in the central nervous system (CNS), specifically in the peri-sylvian region of the left hemisphere, which includes Broca's area (the center for language expression) anteriorly, Wernicke's area (the center for language comprehension) posteriorly, and subcortical white matter fibers (arcuate fasciculus) that connect these two regions. The history of WM, and current immunocompromised state due to the disease itself and chemotherapy, raise a broad differential for his neurological symptoms. The most concerning etiologies include arterial or venous occlusion and cerebral infarction due to serum hyperviscosity, cerebral or meningeal inflammation from WM (Bing-Neel syndrome [BNS]), paraneoplastic or other autoimmune encephalitis, or opportunistic infection.
On examination, he was alert and interactive with mild bradyphrenia. He had impaired fluency with frequent phonemic and semantic paraphasic errors. He could follow simple commands and repeat short but not longer or more complex sentences. He had right-sided brachiofacial weakness. The remainder of his neurological exam was normal. Abnormal laboratory studies included hemoglobin 11.2 g/dL, white blood cells (WBC) 2.09 k/uL with absolute neutrophil count 0.38 k/uL, absolute lymphocyte count 0.54 k/uL and platelet count 142 × 109/L. Serum protein electrophoresis demonstrated a monoclonal spike in the gamma region of 0.34 g/dL and immunofixation confirmed a monoclonal IgM Kappa paraprotein. Serum IgM level was 379 mg/dL. The patient was in a partial response after 4 cycles of bendamustine and rituximab.
The examination supports a mixed aphasia with elements of non-fluency and impaired comprehension that localizes to the left frontal and temporal lobes. The presence of pancytopenia and concurrent immunosuppressive therapy places this patient at elevated risk for progressive multifocal leukoencephalopathy (PML), toxoplasmosis, and primary CNS lymphoma. Furthermore, one should consider CNS abscesses or meningo-encephalitides from fungal (Aspergillus, Cryptococcus), viral (HSV, CMV, VZV) or mycobacterial species. BNS has been described in patients who are responding to therapy for systemic WM 1. Therefore, the patient's response to therapy does not exclude the diagnosis of BNS. With a serum IgM level less than 3,000 mg/dL, hyperviscosity is unlikely. The next step in clarifying the diagnosis is a brain MRI with gadolinium.
MRI showed multifocal well-demarcated nearly confluent areas of abnormal T2 hyperintensity, predominantly in the subcortical white matter of the left frontal, temporal, parietal, and right frontal lobes (Fig. 1A,B). A dominant lesion in the left anterior frontal sub-cortical white matter was edematous and exerted mass effect on adjacent structures. There was modest reduced diffusivity at the periphery of the left frontal lesion (Fig. 1C). There was no abnormal enhancement of this lesion on post-contrast images. As part of staging for WM, the patient underwent a full body positron emission tomography (PET) scan that showed no systemic 18F-FDG-avid lesions. The left frontal lobe lesion showed reduced 18F-FDG uptake (Fig. 1D).
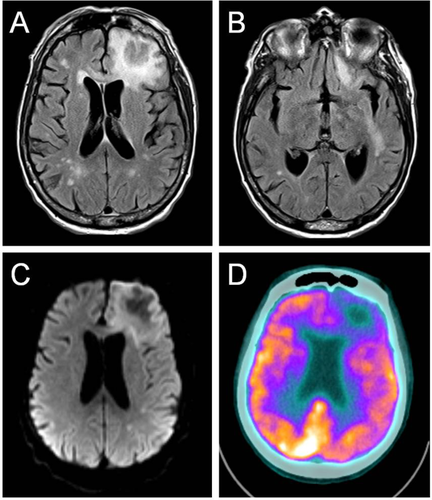
MRI (A-C) and FDG-PET (D) of the brain. Axial T2-FLAIR images of the brain show an edematous left frontal subcortical white matter lesion (A), and an additional lesion in the left perisylvian white matter (B). Multiple smaller foci of abnormal T2 hyperintensity are seen throughout the subcortical white matter bilateral on both images, and are non-specific in nature. Axial diffusion weighted imaging (DWI) shows mild reduced diffusivity (hyperintensity) at the periphery of the left frontal lesion (C). Axial FDG-PET overlaid on a CT image shows hypometabolism associated with the left frontal lesion (D). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The radiographic findings support a multifocal process with a tumefactive lesion in the left frontal lobe. The juxtacortical white matter hyperintensities on T2-weighted images are consistent with an infiltrative condition. The absence of prominent reduced diffusivity makes primary CNS lymphoma or abscess less likely. Furthermore, the absence of contrast enhancement and hypometabolism on PET scan are atypical for high-grade primary or metastatic brain neoplasms. The radiological differential includes PML or BNS. PML produces a pattern of demyelination that is predominantly localized to the frontal and parieto-occipital lobes. Lesions are generally hypointense and non-enhancing on T1 2. Another potential diagnosis is BNS. This entity is an extremely rare complication affecting about 1% of patients with WM, in which lymphoplasmacytic cells invade the leptomeninges and brain parenchyma to produce solitary or multifocal lesions in the cerebral white matter. The MRI commonly demonstrates areas of abnormal subcortical T2 hyperintensity without altered diffusivity. The PET scan shows a corresponding region of hypometabolism 1. Although there are no formal diagnostic criteria, cerebrospinal fluid (CSF) analysis is often essential to confirmation by providing molecular and cytological evidence of disease.
Examination of the CSF revealed 150 WBC/mm3 (77% lymphocytes) and 2,050 red blood cells/mm3. Protein was elevated at 142.9 mg/dL and glucose was normal at 70 mg/dL. CSF cultures for bacteria, fungal and mycobacterial infections were negative. Cytology demonstrated rare lymphoid cells with plasmacytoid features. Flow cytometry was negative for a B-cell lymphoproliferative disorder. CSF electrophoresis revealed monoclonal gammopathy with IgM Kappa paraprotein.
The CSF analysis is consistent with either a pauci-inflammatory condition such as PML or BNS. The CSF profile in BNS often demonstrates a lymphocytic pleocytosis, total protein >100 mg/dL, and IgM kappa or lambda light chain restriction. The cytology and flow cytometry may show small lymphoplasmacytoid cells that express CD19, CD20, CD22 and CD38, but not CD5 or CD10 1. However, given the poor sensitivity of cytological analysis and limited number of neoplastic cells, negative results, as in our case, do not exclude the diagnosis. Despite a monoclonal IgM band in the CSF, leakage of paraproteins across the blood-brain barrier could produce a false-positive result. The MYD88 L265P gene mutation is a molecular biomarker identified in the peripheral blood and bone marrow in up to 90% of patients with WM 3. The mutant protein encoded by the MYD88 L265P gene stimulates a number of signaling molecules, including Bruton's tyrosine kinase, to activate kappa light-chain enhancer of activated B cells (NF-kB). The latter transcription factor promotes malignant lymphocyte proliferation and survival. The MYD88 L265P mutation has been detected by PCR in the CSF of patients with BNS, although this finding has not been systematically confirmed in a large cohort 4, 5.
The CSF PCR for MYD88 L265P mutation was negative. Serum JC virus antibody and CSF PCR for the JC virus returned positive, suggesting PML. Given the possibility of concurrent BNS and PML causing the patient's symptoms, our patient underwent biopsy of the left frontal lobe lesion. The biopsy showed many enlarged glial nuclei with viral cytopathic changes, macrophage infiltrates, and sparse perivascular mononuclear cells (Fig. 2A). Immunohistochemistry for polyoma virus demonstrated strong uptake in glial cells (Fig. 2B). Stains for CD3 and B-cell specific activator protein (BSAP) revealed a sparse perivascular T-cell infiltrate and absence of B-cells (Fig. 2C,D). The patient was diagnosed with PML. His functional capacity rapidly declined, prompting his family to prioritize comfort measures. He expired twelve weeks after his initial neurological symptoms.
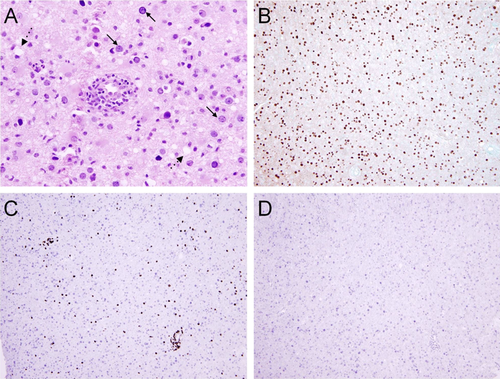
H&E (A) and Immunohistochemistry slides (B-D) from left frontal mass biopsy. The H&E features are classic for PML, and include many enlarged glial nuclei with viral cytopathic change (solid arrows), macrophage infiltrates (dashed arrows), and sparse perivascular mononuclear cell infiltrates without cytologic atypia (A). Immunohistochemistry for polyoma virus demonstrates strong and diffuse uptake in glial cells (B). Immunohistochemical stains for CD3 (C) and BSAP (D) demonstrate a sparse predominantly perivascular T cell infiltrate and an absence of BSAP-positive B cells. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Discussion
The positive CSF JC virus PCR along with the histopathological findings of demyelination, abnormal oligodendroglia nuclei and polyoma viral inclusions, confirms PML. In 1958, Astrom et al first described this disease in patients with B-cell lymphoproliferative disorders 6. Subsequently, the infection was observed in the context of HIV/AIDS and more recently as a consequence of exposure to immunomodulatory drugs (Table 1).
| Monoclonal antibodies | Non-antibody immunotherapies |
|---|---|
| Alemtuzumab | Azathioprine |
| Belatecept | Dimethyl fumarate |
| Efalizumab | Fingolimod |
| Infliximab | Fludarabine |
| Natalizumab | Leflunomide |
| Rituximab | Mycophenolate mofetil |
HIV/AIDS remains the most common underlying cell-mediated immunodeficiency associated with PML in the United States, followed by bone marrow transplant, autoimmune vasculitis and lymphoproliferative disorders 7-9. WM-associated PML most often arises in the context of chemotherapy, but may also emerge de novo 10, 11. Exposure to rituximab, an anti-CD20 monoclonal antibody, is of clinical interest. The Research on Adverse Events and Reports project evaluated 57 patients who developed PML after exposure to rituximab 12. Although the majority of patients had a lymphoproliferative disorder, a few had autoimmune conditions. The median time to PML from last rituximab dose was 5 months with a median survival time after PML diagnosis of 2 months. The fatality rate was 90%.
The clinical presentation of PML depends on the brain region affected and is therefore quite varied 8. Common symptoms include limb weakness, cognitive disturbances, gait instability, and visual changes. Laboratory studies may show signs of cell-mediated immune dysfunction, such as lymphopenia and low CD4 counts. The presence of serum JC virus antibodies is an established risk factor for PML development, but high false-negative rates hinder its use as a reliable diagnostic criterion for active infection 13. Brain MRI is the preferred imaging modality due to its high resolution and sensitivity for detecting white matter lesions. PML affects the subcortical white matter and appears hypointense on T1-weighted sequences and hyperintense on T2-weighted sequences. Hypercellularity, hemorrhage, infarction, calcification, and significant mass effect are not typical features of PML. Contrast enhancement is uncommon, but may appear as a faint, peripheral pattern in up to 15% of patients with HIV and up to 40% of those receiving natalizumab 2, 14. No more than 12% of lesions exert mass effect 15. CSF examination is nonspecific and generally shows a normal or mildly increased cell count and an abnormally elevated protein 8.
With the advent of highly sensitive CSF PCR assays, the diagnosis of definite PML is transitioning from an approach using histopathology toward algorithms combining radiographic appearance with positive JC virus DNA in the CSF 16. Arguably, a brain biopsy would not be necessary to confirm PML in this case. However, the presence of rare lymphoid cells with plasmacytoid features in our patient's CSF raised concern for concomitant CNS involvement from lymphoplasmacytic cells, which, if present, would have altered treatment options.
The most prevalent neurological complication of WM is an IgM-mediated peripheral neuropathy. When WM affects the CNS in BNS, it often occurs as a late complication of disease, although a small proportion have symptoms at the time of diagnosis 5, 17. Lymphoplasmacytic cells infiltrate multiple CNS locations, including the leptomeninges, cortex, gray-white matter junction, and Virchow-Robin perivascular spaces. Motor deficits and changes in mental status are common manifestations, and MRI often demonstrates leptomeningeal enhancement and T2 hyperintense white matter lesions 1, 5.
There are no standardized criteria for diagnosing BNS. The diagnosis generally requires cytological (phenotypically malignant lymphoplasmacytoid cells on CSF cytology or clonal B-cells on CSF flow cytometry) or histopathological evidence of CNS involvement 5, 18. In our patient, there were rare lymphoplasmacytic cells in the CSF, but the flow cytometry was unrevealing. In up to 13% of cases, flow cytometry is negative and the diagnosis relies exclusively on cytology 5. Recent advances in genome sequencing using PCR assays or Sanger sequencing may provide a surrogate for histopathology 3. Detection of MYD88 L265P mutation in the CSF is a promising diagnostic tool, but larger studies are still required to validate its use in BNS 4, 5. Our patient did not have histopathological features of BNS, but our study was limited by lack of leptomeningeal sampling on biopsy. An international task force consensus document on the diagnosis and management of BNS is underway (8th International Workshop on Waldenström Macroglobulinemia, London, UK).
Both PML and BNS present therapeutic challenges. There is no standardized treatment approach to either disease entity. In the largest retrospective cohort of BNS, most patients received systemic high dose methotrexate and/or cytarabine accompanied by intrathecal chemotherapy. The response rate was approximately 70% with an overall survival of 71% after 5 years and 59% after 10 years 18. Nevertheless, the drug regimens varied widely and there was no consensus on first line treatment.
The prognosis in PML is bleak. Cidofovir, mefloquine, and mirtazapine have demonstrated minimal to no success in randomized control trials and case reports 19-22. In one case series of 19 individuals with non-AIDS PML, intravenous cytarabine led to neurological improvement or disease stabilization in 36% of treated patients 23. Immune reconstitution is still the most recognized approach and cessation of immunosuppressants is essential 8. Application of drugs that promote the adaptive immune response may prove an effective and novel strategy. Recombinant IL-7, a cytokine that stimulates T-cell proliferation, led to clinical improvement and JCV viral load reduction in recent case reports of PML 24, 25. Larger studies are still required to validate the drug's use.
Our patient's treatment options for PML were limited. His presentation illustrates the predisposition to PML based on his underlying WM, as well as prior exposure to chemoimmunotherapy. PML may confound the diagnosis of BNS in those patients with brain lesions. We anticipate that a growing understanding of immune dysregulation will help generate novel therapeutic agents for individuals with PML.
Conflict of Interest
JJC received honoraria from Alexion, Biogen Idec, Celgene, Janssen and Otsuka, and research funding from Abbvie, Gilead, Millennium and Pharmacyclics.




