Variable bleeding phenotype in an Amish pedigree with von Willebrand disease
Abstract
Through a cross-sectional study design, the bleeding phenotype in the Amish in Indiana (IN) and Wisconsin (WI) was described using two different bleeding scores. von Willebrand factor (VWF) testing was performed and bleeding questionnaires from Centers for Disease Control and Prevention (CDC) and European MCMDM-1 (Tosetto bleeding score (BS)) were administered to the IN and WI cohort respectively. Seven hundred and seventy nine subjects were recruited, 17% were diagnosed with VWD based on Ristocetin cofactor, VWF:RCo < 30 IU/dl. Majority of the affected (AF), 67%, were tested and had a common mutation c.4120 C > T. The WI AF were much younger at a mean age 15 years vs 26 years in IN AF cohort. The AF subjects had a median VWF:RCo of 13IU/dl with a statistically significant higher median BS 1 versus 0 in the WI AF vs WI Unaffected (UA), 2 vs 1 in the IN AF vs IN UA, P < 0.01. Adults had a higher median BS compared to children in the WI and IN cohort, 2 vs 1 and 3 vs 1 respectively (P < 0.05) but there was no statistically significant difference in the BS between males and females in either cohort. The common symptoms reported were epistaxis and gingival oozing. BS ≥ 3 and BS ≥ 4 were observed in 46% of AF IN and 16.6% of AF WI, respectively. There was significant variability in the bleeding phenotype, with an overall low BS in the affected Amish with VWD, despite a unifying mutation. Am. J. Hematol. 91:E431–E435, 2016. © 2016 Wiley Periodicals, Inc.
Introduction
von Willebrand disease (VWD) is the most common inherited bleeding disorder with a prevalence of 0.5–1% with a variable bleeding phenotype dependent on several factors including the subtype, von Willebrand Factor (VWF) levels, age and sex 1-3.Type 2M von Willebrand disease is a functional disorder of VWF characterized by low or normal VWF antigen (VWF:Ag), low VWF Ristocetin (VWF:RCo) with a ratio of VWF:RCo/Antigen <0.5 and normal multimer distribution 4. The prevalence of this rare subtype is estimated to be approximately 8% of individuals affected with VWD 5 and is often mis-categorized as VWD type 1.
The Amish are known for simple living, reluctance to adopt many conveniences of modern living and tending to prefer an insular lifestyle. Indiana (IN) has the third largest Amish population in the US. The Old Order Amish (OOA) in IN and Wisconsin (WI) are affected with VWD, the vast majority carry the same missense mutation at position 4120 in exon 28 of the VWF gene represented by a single base substitution C→T that results in an arginine to cysteine change at position 1,374 in the A1 domain, R1374C 6 resulting in defective VWF binding to platelet GP-Iba (glycoprotein Ib). This mutation, c.4120 C > T, has been reported in the Sheffield database as associated with VWD types 1, 2A 7, 2M and unclassified. In our cohort, the index case exhibited a laboratory phenotype consistent with VWD 2M with low VWF: RCo/VWF:Ag ratio, normal multimers, muco-cutaneous bleeding symptoms and hence, the whole pedigree was initially categorized as having VWD type 2M. Eleven patients of this cohort underwent desmopressin (DDAVP) testing with variable response and only one patient achieved a level of VWF:RCo >40 IU/dl after 2 hrs 8. This article highlights the cohort's heterogeneous bleeding phenotype despite their unifying mutation using two different bleeding scores and analyzes the pitfalls of the standardized bleeding questionnaires in a homogenous population which is culturally distinct.
Methods
Study design and subjects
A cross-sectional study was completed in four separate clinics at two sites, IN and WI, in the OOA community from 2004 to 2008. Although several Amish communities have endogamous practices, no consanguinity (defined by marriage between second cousins or closer) was identified in this pedigree. These sites were comprised of two communities descended from a common founder population known to have VWD inherited in an autosomal dominant pattern. Subjects of all age groups were recruited without any exclusion criteria. The main objective of the initial investigation was to identify genetic modifiers of VWD that impact the bleeding phenotype using linkage analysis and to discover novel quantitative trait loci of hematologic traits 9, 10. The study presented here represents a sub analysis of the original study population including data on 779 individuals who underwent laboratory testing and responded to bleeding questionnaires.
Bleeding assessment tools
The bleeding phenotype in the IN Amish community was assessed using a modified bleeding questionnaire that was originally designed and validated by the Epidemiology Branch of the Centers for Disease Control and Prevention (CDC) to screen women with VWD (referred to as the IN BS in the manuscript) 11. The questionnaire consists of 10 questions selected from a more extensive set on the basis of their positive predictive value. Questions include muco-cutaneous bleeding, bleeding after surgical challenges, and transfusion requirements. Questionnaires were graded on a Likert scale with the lowest (no bleeding phenotype) possible score being zero and the highest (most severe) score being seven. A score of ≥3 was considered significant. This questionnaire did not score for female reproductive bleeding symptoms including menorrhagia and post-partum hemorrhage.
The WI Amish community members were assessed using the European MCMDM-1 bleeding score 1 (referred to as Tosetto BS in the manuscript). Per bleeding symptom, the score ranges from −1 for hemostatic challenge without significant bleeding to a maximum score of +4 for bleeding requiring blood transfusions or need for surgical hemostasis. A score of ≥4 was considered significant. Comparison of the two bleeding scores is shown in Supporting Information Table 1.
Laboratory tests
Sample processing, aliquoting, and freezing were performed immediately on site at each clinic. Testing for VWF levels and FVIII levels was performed at the Blood Center of Wisconsin (Milwaukee, WI). Levels were measured for all recruited pedigree members who provided a blood sample. VWF:Ag was measured on standard enzyme-linked immunosorbent assay (ELISA) plates coated with a combination of two monoclonal antibodies. Plasma samples were plated into duplicate wells for three different dilutions per sample. Captured VWF was detected with polyclonal rabbit antibody with an enzyme conjugate reaction. VWF:RCo was measured by automated analysis of formalin-fixed platelet agglutination on the Dade-Behring BCS analyzer (Dade Behring Inc. Newark, DE). Data were analyzed using the manufacturer's end-point based software package. For patients with VWF:RCo <10, the value of 10 was used which could have led to overestimating this value and VWF:RCo/VWF:Ag ratio in some patients.
VWF mutation analysis
VWF:Ag and VWF:RCo levels in several pedigree members suggested a diagnosis of VWD type 2 inherited in an autosomal dominant pattern. Exon 28 of VWF is the largest exon and harbors the majority of qualitative VWF mutations. Exon 28 was analyzed in all pedigree members who provided blood samples. PCR and sequencing primers were designed for selective amplification of VWF to avoid amplification of the VWF pseudogene located on chromosome 22 12. Targeted sequence analysis identified a C > T mutation at position 4120 that segregated with VWF:RCo < 25 IU/dL. To rule out additional mutations, the entire VWF sequence, including introns, was analyzed in two pedigree members with the c.4120C > T mutation. No additional mutations were found. A 5′ allelic discrimination high-throughput fluorescent genotyping assay (TaqMan™) 13 was designed to determine the population frequency of the c. 4120C > T mutation in a group of individuals with European ancestry (n = 358) from different populations included in the Centre d'Etude du Polymorphisme Humain (CEPH) diversity panel 14. The c. 4120C > T mutation was not identified in the CEPH population. Finally the c.4120C > T mutation was not found in the human genetic variation databases 1000 genomes 15 or dbSNP 16.
Statistical methods
Population demographic characteristics were documented. Distributions (median, 25th and 75th percentile) were calculated for VWF levels (VWF:RCo, VWF:Ag, VWF:RCo/VWF:Ag), FVIII:C, and IN and WI bleeding scores in affected and non-affected cohorts. Independent samples t-tests were used to evaluate differences in means. Mann–Whitney U tests were employed to evaluate differences in scores and VWF or FVIII:C levels based on age, gender, and bleeding score magnitude. Bleeding symptom frequency was computed and graphed for affected VWD subjects. Pearson's product moment correlation coefficient was calculated to estimate a correlation between VWF levels and BS in each cohort.
Results
A total of 779 Amish individuals were evaluated, 313 from WI and 466 from IN, of which 133 (17%) were diagnosed with VWD, 91 from IN and 42 from WI. In the study, 89/133 (67%) were tested and found positive for the c.4120C > T VWF mutation, the remaining 44/133 (33%) subjects with VWD in the pedigree were not tested for the mutation due to lack of DNA availability.
All 779 subjects were administered the bleeding score questionnaires.
All patients were diagnosed with VWD for the first time during the study and had not been exposed to any hemostatic product like DDAVP/VWF containing concentrate or antifibrinolytic agents for any procedure as prophylaxis.
Affected versus Unaffected (Table I)
Affected were defined as individuals with a VWF:RCo of < 30 IU/dl. There was no significant difference in the age of the affected (AF) vs. unaffected (UA) individuals in the IN and WI cohorts. The only difference between the WI AF and the IN AF was that the former were much younger at a mean age of 15 years vs 26 years in the IN AF cohort. Among the AF, although the VWF:RCo was < 30 IU/dl in all subjects, the VWF:Ag ranged from 14 to 33 IU/dl (25th–75th percentile) accounting for the variability in the VWF:RCo/VWF:Ag ratio from 0.4 to ∼0.7. The AF subjects had a median VWF:RCo of 13IU/dl with a statistically significant higher median BS 1 vs 0 in the WI AF vs WI Unaffected (UA), 2 versus1 in the IN AF versus IN UA, P < 0.01 (Table 1). P value not shown in Table.
Comparison of the affected population by age and sex (Table 2)
Adults had a statistically significant higher median bleeding score as compared to children at P < 0.05, in both cohorts. Females had a higher score as compared to adult males in the WI cohort (Tosetto BS), although this difference was not statistically significant (P = 0.567). The scores were the same for both sexes in the IN cohort as female reproductive bleeding symptoms, menorrhagia and PPH, were not queried for in the bleeding assessment tool used in this population.
Bleeding symptoms among affected population
The most common bleeding symptom reported in both affected cohorts were epistaxis and oral/gingival oozing. Females did not report menorrhagia and only 2/5, 40% of affected females in the WI cohort reported PPH. Among pediatric patients, there was also a higher frequency of bruising and bleeding with cuts and wounds (Figs. 1 and 2).
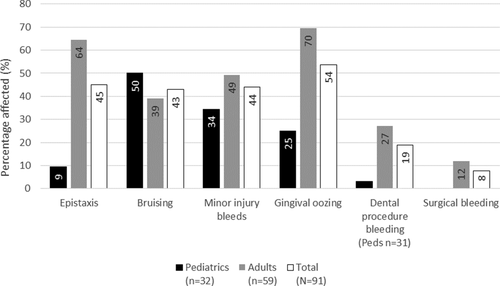
Bleeding symptoms in the AF IN Cohort. Menorrhagia and PPH were not a part of the questionnaire.
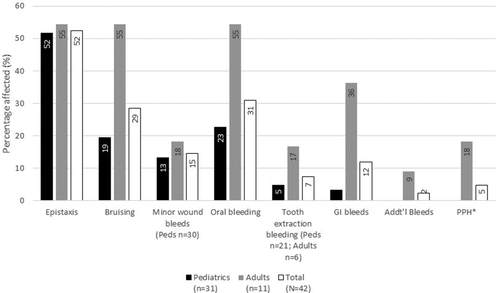
Bleeding symptoms in the AF WI Cohort. No patients reported bleeding with surgery or menstrual history.
*PPH = post-partum hemorrhage; Addt'l = Additional bleeds.
Comparison of high and low BS among affected
In the AF IN cohort, 46% (42/91) had a high BS of ≥3; these patients were in the higher age bracket (mean age 34.2 years vs. 19.7 years) with no predilection for a specific gender (data for gender not shown) (see Supporting Information Table 2). In the AF WI cohort 16.6% (7/42) had a BS ≥4 without significant age or gender predilection. No difference was seen in the VWF and FVIII:C levels among the low vs. high score groups. Among the high score patients, commonly reported symptoms included gingival oozing, epistaxis and bleeding from minor wounds in 93%, 71% and 64% respectively of the IN cohort and 71% for each symptom in the AF WI cohort (Supporting Information Figs 3 and 4).
Correlation between VWF:RCo and BS
In the individual cohorts, there was a strong negative correlation between the VWF:RCo levels and BS; Pearson correlation coefficient was, r = −0.212 for WI cohort, and r = −0.193 for IN cohort, P < 0.01.
Discussion
Despite being the most common reported bleeding disorder, VWD is extremely challenging to diagnose and classify into subtypes. Patients with low VWF levels, VWF:RCo and/or VWF:Ag <30 IU/dl with bleeding symptoms have a definitive diagnosis of VWD 17. The ratio of VWF:RCo/VWF:Ag to define 2M has been controversial as 0.4 to 0.6 18. Although the bleeding phenotype is not always related to the observed ratio or the presence of a mutation, the probability of finding a mutation in the A1 domain is higher if VWF:RCo/VWF:Ag <0.4 [18]. Type 2M VWD is a heterogeneous category comprised of both collagen- and platelet-binding defects 19.
The c.4120C > T mutation was first described by Mancuso et al (1989) 20; the association of this mutation to VWD was first described in 1993 21. The c.4120C > T mutation is unique in the fact that it has been reported as different VWD subtypes in different parts of the world varying from unclassified, 2A or type 1 20 or 2M in the Sheffield database 22, 23 because the HWM are not totally absent but are present and some are even larger than normal. There is a large cohort in Spain (Galicia) with the c.4120C > T mutation where 2 unrelated families with 21 patients were classified as type 1 but based on their VWF:RCo/VWF:Ag ratio of <0.7 and showing relatively lower proportion of HMW multimers, they subsequently were reclassified as 2Ms. These patients exhibited heterogeneity in the multimer profile even among members of the same family, and similar to our cohort had a transient response to DDAVP 8, 24
Bleeding scores have been utilized since 2005, initially with the aim of quantifying bleeding symptoms in type 1 VWD to develop guidelines for optimal treatment. At present, they are being used more commonly as a screening tool to evaluate for potential bleeding disorders.
This study utilized two different bleeding assessment tools, Tosetto and IN bleeding score, in a related Amish cohort. In several studies, it has been shown that VWF levels and bleeding scores have an inverse relationship 1, 18. The negative correlation between VWF:RCo and BS in our study is similar; although this correlation is lost at levels of VWF: RCo < 30 IU/dl, it does assist us in differentiation of affected subjects from the unaffected in our study population.
In our study group, adults had a higher bleeding score as compared to children. The higher score can be explained by increased gingival oozing with progressive periodontitis due to poor dental hygiene with advancing age and an increase in dental extractions, adults involved in manual labor sustaining more cuts and wounds and increase in surgical challenges with age as seen in the IN cohort. Epistaxis was more frequent in adults as they spend more time working outdoors, exposed to dry air and they are less likely to seek medical attention for themselves. Females did not have a higher score in the IN cohort as questions on menorrhagia and PPH unfortunately were not a part of the questionnaire used in this population. The WI cohort females had the same BS as the males which can be explained by the fact that Amish women uncommonly seek medical attention/consultation which escalates the score to 1 and above in the categories of menstrual bleeding and PPH in the Tosetto scoring system The WI cohort had a smaller number of subjects which could be another explanation for the lack of statistical significance between age groups and genders.
Contrary to the reported literature where patients with VWF:RCo < 15 IU/dl have significant bleeding symptoms and high bleeding scores, our cohort had a low median bleeding score of 1 to 2, irrespective of the type of scoring system used. One possibility is that the low score observed in several members of the families reported here is due to a real lack of bleeding symptoms perhaps owing to a variable multimer structure (multimer information and data not available on all patients) with higher score achievers having a shift to low molecular weight multimers (LMWM) or loss of high molecular weight multimers (HMWM) which are crucial to hemostasis. In a recent study 25 of a 24 member pedigree with VWD type 2 and variable multimer distribution and bleeding, the variability was partially explained on the basis of mutant monomer incorporation in the final multimer structure of plasma VWF. In this family, different individuals carrying the same mutation could be classified as type 1, type 2A, or type 2M. We hypothesize that this could be the explanation for our cohort as well. In our patient population, the median VWF:RCo/VWF:Ag ratio was 0.57. As the index case had normal multimers and all patients tested had the same mutation c. 4120 C > T, the pedigree was diagnosed as type 2M.
Finally, another explanation for the low bleeding score is based on the experience of our center with this population and reflects the inadequacy of the questionnaires to score bleeding symptoms accurately in a culturally distinct population that minimizes health related concerns, are hesitant to seek medical care and may be poor historians. The cultural difference is proven in another study done by our center where 40 Amish VWD patients were compared to 28 non Amish VWD (NAV) patients using the Tosetto BS. It was found that the although NAV had a higher mean VWF: RCo of 37 IU/dl vs 13 IU/dl for the Amish, they had a higher mean BS of 7.9 versus 2.8 (P < 0.01) for the Amish VWD patients 26. The most common bleeding symptom in our present study was gingival oozing, clearly impacted by poor oral hygiene that can artificially inflate the BS. Similarly, heavy menses are not commonly treated with OCPs or medications by the Amish as they uncommonly seek medical attention but rather use over the counter remedies; hemoglobin with iron studies are not routinely performed and hence the bleeding score for women is artificially depressed.
The IN BS did not include female reproductive bleeding symptoms; it is possible that no significant difference would have been found due to underreporting of these symptoms by the Amish women. Considering the lack of these symptoms as a drawback of the IN BS questionnaire, the WI cohort was queried with the Tosetto BS which had been validated in 2006 after the research started in 2004. Our center plans to follow the patients with BS ≥ 3/4 over time to complete their hemostatic work up to ensure absence of other coexisting bleeding disorders. Multimer distribution and collagen binding is critical information to explain the bleeding phenotype and accurately sub-classify patients which could not be performed. It would be helpful to identify co-existing thrombophilia traits such as Factor V Leiden and the Prothrombin gene mutation especially in the low bleeding score patients as a contributor for the difference in their hemostatic milieu compared to the high scoring subjects. There was also a possibility that the existence of other mutations in the VWF gene could cause the variability in the bleeding phenotype. Genomic studies are underway in an attempt to explain the variability in bleeding. Additionally, sequencing of VWF in 16 affected individuals with the c.4120C > T mutation, demonstrated several common variants but no other mutations (data not shown). Finally, the questionnaire was inept for the Amish population where medical care is infrequently sought and hence our center is now utilizing a modified questionnaire and scoring system where a score of 2 is given if home treatment is initiated for a bleeding symptom irrespective of whether medical consultation was sought.
Conclusions
VWD remains a significant diagnostic issue. At this time, the VWD Amish patients in Indiana are treated by our center based on the historical bleeding symptoms for surgical procedures with VWF –FVIII containing concentrate due to their variable response to DDAVP. To optimize therapy and use expensive resources judiciously, a tiered risk stratification and replacement therapy program was developed to manage women with VWD during labor, delivery and the puerperium. VWD clinics were held in in 2014 to diagnose new family members, mainly children (∼22 newly identified cases) by using a single laboratory value- VWF:RCo in conjunction with the modified bleeding questionnaire.
It is essential to recognize that the concept of “one size fits all” does not hold true in our cohort as evidenced by the heterogeneity in the bleeding phenotype, challenging our treatment assessments specifically in children <8 years of age with VWF:RCo <10 IU/dl where we lack the benefit of a substantial bleeding history or stressor events to unmask a bleeding tendency. It would be an important clinical advancement to have available a single reliable, reproducible VWF level which reflects the bleeding phenotype with high sensitivity and specificity allowing hematologists to guide rational therapeutic decision making.
ACKNOWLEDGEMENTS
The authors thank the Amish families from IN and WI who donated their time and blood samples and William Kronenberger Ph.D. for helping us with the statistical analysis. This work was supported by grants from the National Institutes of Health, National Heart, Lung and Blood Institute R01 HL084086 to J.D., Postle Family Chair of Pediatric Cancer and Blood Disorders (JD).
Author Contributions
Sweta Gupta did the data analyses and the interpretation. Meadow Heiman, Jesse Hinckley, Jorge Di Paola and Amy Shapiro helped in data collection and performing the research. Sweta Gupta and Natalie Duncan performed the statistical analysis. Jorge Di Paola, Amy Shapiro edited the manuscript and supervised the study. Sweta Gupta, Jesse Hinckley and Natalie Duncan wrote the manuscript.
Conflicts of Interest
The authors have no conflicts of interests to declare.
| WI* | WI | IN* | IN | P* value | |
|---|---|---|---|---|---|
| AF*, N = 42 | UA*, N = 271 | AF, N = 91 | UA, N = 375 | WI AF vs. IN AF | |
| Pediatric n (%) | 31 (73.8) | 137 (50.6) | 32 (35.2) | 180 (48.0) | NA |
| Adults n (%) | 11 (26.2) | 134 (49.4) | 59 (64.8) | 195 (52.0) | NA |
| Males n (%) | 24 (57.1) | 131 (48.3) | 46 (50.5) | 187 (49.9) | NA |
| Females n (%) | 18 (42.9) | 140 (51.7) | 45 (49.5) | 188 (50.1) | NA |
| Mean age, years (Range) | 15.2 (0-67) | 20.9 (0-106) | 26.4 (0-87) | 22.8 (0-81) | 0.002** |
| Median VWF:RCo (25th, 75th percentile) | 13.0 (9, 17) | 109.5 (84, 145) | 13.0 (9, 16) | 107.0 (87, 131) | 0.509 |
| Median VWF:Ag (25th, 75th percentile) | 23.0 (18, 32) | 117.5 (78, 127.5) | 24.0 (14, 33) | 99.0 (88, 155) | 0.509 |
| Median VWF:RCo/VWF:Ag (25th, 75th percentile) | 0.57 (0.42, 0.66) | 0.92 (1.00, 1.21) | 0.57 (0.47, 0.72) | 1.09 (0.82, 1.02) | 0.898 |
| Median FVIII:C (25th, 75th percentile) | 49.4 (35, 61) | 147.8 (94, 129) | 47.0 (38, 79) | 108.0 (118, 189) | 0.677 |
| Median BS* (25th, 75th percentile) | 1.0 (1, 3) | 0.0 (0, 2) | 2.0 (0, 2.25) | 1.0 (0, 1) | NA |
- *P < 0.05.
- **P < 0.01 *AF: Affected, *UA: Unaffected, *WI: Wisconsin, *IN: Indiana, *BS: Bleeding score.
| Pediatric | Adults | P* | Adult females | Adult males | P* | |
|---|---|---|---|---|---|---|
| Tosetto BS | N = 31 | N = 11 | 0.048* | N = 5 | N = 6 | 0.567 |
| Median, (25th, 75th percentile) | 1.0 (0, 2) | 2.0 (1, 7) | 7.0 (1, 7.5) | 2.0 (0.5, 3) | ||
| IN –BS | N = 32 | N = 59 | 0.044* | N = 30 | N = 29 | 0.910 |
| Median, (25th, 75th percentile) | 1.0 (0, 2) | 3.0 (2, 4) | 3.0 (1.75, 4) | 3.0 (2, 4) |
- *P < 0.05.
- **P < 0.01, *AF: affected, *IN BS: Indiana BS.




