Autologous stem cell transplantation in first complete remission may not extend progression-free survival in patients with peripheral T cell lymphomas
Presented in abstract form at the 2015 American Society of Hematology Annual Meeting, Orlando, FL.
Conflict of interest: Anthony R. Mato has received research funding from Pharmacyclics, Gilead, TG therapeutics, Pronai Pharmaceuticals and AbbVie. He is also a consultant to Celgene Corporation, Pharmacyclics, Gilead, AbbVie and Janssen. Jakub Svoboda has received research funding from Seattle Genetics, Celgene, Immunomedics and Celldex. Noelle V. Frey has received research funding from Novartis. David L. Porter has received research funding from and has intellectual property interests with Novartis. Stephen J. Schuster has received research funding from Novartis, Gilead, Janssen, Hoffman-LaRoche, Celgene and Pharmacyclics. He is also a consultant to Celgene, Pharmacyclics and Genentech. He is a member of the advisory board for Nordic Nanovector. All other authors have no relevant affiliations to disclose.
Abstract
Patients with peripheral T cell lymphomas (PTCL) generally have a poor prognosis when treated with conventional chemotherapy. Consolidation with autologous stem cell transplantation (ASCT) has been reported to improve progression-free survival. However, these studies have not compared consolidative ASCT with active observation in patients with PTCL achieving first complete remission (CR1) following induction chemotherapy. We conducted a retrospective analysis of PTCL patients treated at the University of Pennsylvania between 1/1/2007 and 12/31/2014. Patients with cutaneous T cell lymphoma, concurrent B cell lymphomas, and anaplastic lymphoma kinase-positive anaplastic large cell lymphoma (ALK-positive ALCL) were excluded from the study. We compared progression-free survival for patients who underwent ASCT in CR1 following CHOP-like induction regimens and patients who underwent active observation during CR1. 48 patients met all inclusion and exclusion criteria and underwent either active observation (28 patients) or consolidative ASCT (20 patients) in CR1. The 1-year cumulative incidence of relapse in the observation and ASCT groups was 50% (95% confidence interval [CI]: 30–67%) and 46% (95% CI: 23–67%), respectively (P = 0.55). Median progression-free survival in the observation and ASCT groups was 15.8 and 12.8 months, respectively (log rank, P = 0.79). Estimated 3-year progression-free survival in the observation and ASCT groups was 37 and 41%, respectively. In conclusion, for PTCL patients achieving CR1 following CHOP-like induction chemotherapy, ASCT does not appear to improve progression-free survival compared to active observation. This finding should be confirmed in a larger, prospective study. Am. J. Hematol. 91:672–676, 2016. © 2016 Wiley Periodicals, Inc.
Introduction
Peripheral T cell lymphomas (PTCL) are a group of heterogeneous mature T cell neoplasms. For a variety of reasons, prognosis is poor when treated with conventional chemotherapeutic agents. There is currently no consensus on the optimal frontline treatment strategy for PTCL due in part to the lack of data from randomized controlled trials. Based on extrapolated data from studies in aggressive B cell non-Hodgkin lymphomas 1-3, the vast majority of patients with PTCL will receive CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone)-like induction chemotherapy. Although complete remission (CR) rates of 50–70% have been reported with CHOP-like chemotherapy 4-9, relapse is common. Notably, despite significantly higher rates of initial response, 5-year event-free survival rates as low as 33% have been reported 10.
Consolidative autologous stem cell transplantation (ASCT) is frequently offered to PTCL patients in first complete remission (CR1) based on data from prospective studies showing improved overall survival (OS) and progression-free survival (PFS) when compared with historical controls 11, 12. However, since most studies of ASCT in PTCL include patients who achieve a partial response (PR) to standard chemotherapy, it is currently unclear if consolidative ASCT will lead to improved outcomes compared to active observation for patients achieving CR1. In addition, such studies may be affected by selection bias secondary to the criteria used for determining eligibility for stem cell transplantation with a propensity to include younger and fitter patients while excluding patients who are frail and less likely to tolerate aggressive treatment regimens 13. As such, it may be difficult to draw conclusions by directly comparing outcomes from patients in these studies with historical controls since historical controls represent a largely unselected population of patients. Furthermore, variations in standards of medical care between different time periods may also contribute to differing outcomes between historical controls and patients included in such studies.
As there are limited data comparing upfront ASCT with active observation in PTCL patients who achieve CR1 following CHOP-like induction chemotherapy, we examined outcomes of PTCL patients treated with CHOP-like induction chemotherapy at the University of Pennsylvania who were managed with either consolidative ASCT or active observation in CR1.
Methods
Patient cohort and study design
We conducted a retrospective analysis of patients with PTCL who were treated at the University of Pennsylvania between 1/1/2007 and 12/31/2014. Patients with cutaneous T cell lymphoma (CTCL), concurrent B cell lymphomas and ALK-positive anaplastic large cell lymphoma (ALK-positive ALCL) were excluded. Patients with ALK-positive ALCL were excluded because such patients tend to have better prognosis with induction chemotherapy alone 9, 14-17 and current National Comprehensive Cancer Network (NCCN) guidelines do not recommend consolidative ASCT in ALK-positive ALCL patients who achieve CR1 following induction chemotherapy 18.
We then selected patients who underwent either active observation or up-front ASCT during CR1 following CHOP-like induction chemotherapy. The decision to proceed with active observation or consolidative ASCT was made by the treating physician at the time of CR1. Patients who did not achieve CR1 or received other forms of consolidative therapy were excluded from further analyses.
Baseline clinical information and follow up data were collected through 10/24/2015 via a retrospective review of electronic medical records. This study was approved by the University of Pennsylvania Institutional Review Board, with a waiver of informed consent for the retrospective review of patient records (IRB protocol #820741).
Study variables
Histological subtype was determined through a review of biopsy reports. The International Prognostic Index (IPI) score was defined according to the International Non-Hodgkin Lymphoma Prognostic Factors Project 19 and determined through a review of clinical records as well as relevant laboratory and radiological reports. The stage at diagnosis was defined according to the Lugano Classification 20 and determined through a review of clinical records and relevant radiological reports. Lactate dehydrogenase (LDH) levels were determined through a review of laboratory reports. The Eastern Cooperative Oncology Group (ECOG) Performance Status 21, presence of B symptoms at diagnosis and choice of primary therapy were determined through a review of clinical records.
We also assessed the impact of data from the study of upfront autologous stem cell transplantation from the Nordic Lymphoma Group 11 on clinical practice by comparing the rates of utilization of ASCT in CR1 at our institution in patients diagnosed before and after 12/31/2012.
Response assessment and time-to-event end points
Treatment response was confirmed by review of positron emission tomography-computed tomography (PET-CT) or computed tomography (CT) scan reports, clinical records, and when relevant, biopsy reports.
Relapse was defined as clinical or radiological evidence of disease demonstrating pretreatment characteristics and time to relapse was defined as the time from first documented CR to relapse. Progression-free survival (PFS) was defined as the time from diagnosis to disease progression or death from any cause, whichever came first. Overall survival (OS) was defined as the time from diagnosis to death from any cause. Time-to-event outcomes were censored at the time of last contact.
Fisher's exact test was used to compare binary and categorical variables. Continuous and ordinal variables were compared using the Wilcoxon's rank-sum test. The estimated cumulative incidence of relapse was compared using Gray's test with death in remission accounted for as a competing risk. Estimates of median PFS and OS were calculated using the Kaplan Meier method and time-to-event distributions were compared using the log-rank test.
A two-sided P value of less than 0.05 was considered statistically significant. All data were analyzed using R Statistical Software (version 3.2.3; R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
We identified 105 patients with PTCL who received CHOP-like chemotherapy, of which 52% (55/105) achieved CR1. 87% (48/55) of these patients then underwent either active observation or consolidative ASCT and were included in our study. Of the remaining seven patients, three underwent consolidative radiation, three were lost to follow up shortly after achieving CR1, and one received weekly methotrexate as consolidative therapy. Of the 48 patients included in our study, response was assessed with a PET-CT and a CT scan with intravenous contrast in 88% (42/48) and 13% (6/48) of patients, respectively.
Table 1 summarizes the baseline characteristics and primary systemic therapy received by patients stratified by consolidative strategy (active observation or ASCT). There were no statistically significant differences in median age at diagnosis (P = 0.066), IPI scores (P = 0.88), stage (P = 0.31), presence of B symptoms (P = 1), presence of an elevated lactate dehydrogenase (LDH) (P = 0.46), Eastern Cooperative Oncology Group (ECOG) performance status at diagnosis (P = 0.26), or distribution of histological subtypes (P = 0.41) between the two groups.
| Active observation (n = 28) | ASCT (n = 20) | P value | |
|---|---|---|---|
| Median age—years (range) | 61.9 (32.8–78.3) | 56.9 (21.4–71.9) | 0.066 |
| Histological subtype | |||
| PTCL NOSa—n (%) | 16 (57%) | 6 (30%) | |
| AITLb—n (%)b | 5 (18%) | 6 (30%) | |
| ALK-negative ALCLc—n (%) | 5 (18%) | 3 (15%) | |
| EATLd—n (%) | 0 | 1 (5.0%) | 0.41 |
| ATLLe—n (%) | 1 (3.6%) | 0 | |
| HSTLf—n (%) | 1 (3.6%) | 1 (5.0%) | |
| Unknown—n (%) | 0 | 3 (15%) | |
| IPIg score at diagnosis | |||
| 0–1—n (%) | 5 (18%) | 3 (15%) | |
| 2–3—n (%) | 13 (46%) | 10 (50%) | 0.88 |
| 4–5—no.(%) | 3 (11%) | 2 (10%) | |
| Unknown—n (%) | 7 (25%) | 5 (25%) | |
| Stage at diagnosis | |||
| I—n (%) | 4 (14%) | 0 | |
| II—n (%) | 3 (11%) | 4 (20%) | |
| III—n (%) | 8 (29%) | 5 (25%) | 0.31 |
| IV—n (%) | 11 (39%) | 11 (55%) | |
| Unknown—n (%) | 2 (7.1%) | 0 | |
| B symptoms at diagnosis | |||
| Present—n (%) | 17 (61%) | 13 (65%) | |
| Absent—n (%) | 9 (32%) | 7 (35%) | 1 |
| Unknown—n (%) | 2 (7.1%) | 0 | |
| LDHh at diagnosis | |||
| Elevated—n (%) | 12 (43%) | 11 (55%) | 0.46 |
| Not elevated—n (%) | 7 (25%) | 3 (15%) | |
| Unknown—n (%) | 9 (32%) | 6 (30%) | |
| ECOGi performance status at diagnosis | |||
| 0–1—n (%) | 17 (61%) | 17 (85%) | 0.26 |
| ≥2—n (%) | 7 (25%) | 2 (10%) | |
| Unknown—n (%) | 4 (14%) | 1 (5.0%) | |
| Primary therapy | |||
| CHOP—n (%) | 18 (64%) | 13 (65%) | |
| CHOP/etoposide—n (%) | 7 (25%) | 6 (30%) | 0.82 |
| Other—n (%) | 3 (11%)j | 1 (5.0%)k |
- a Peripheral T cell lymphoma, not otherwise specified.
- b Angioimmunoblastic T cell lymphoma.
- c ALK negative anaplastic large cell lymphoma.
- d Enteropathy associated T cell lymphoma.
- e Adult T cell leukemia/lymphoma.
- f Hepatosplenic T cell lymphoma.
- g International prognostic index.
- h Lactate dehydrogenase.
- i Eastern Cooperative Oncology Group.
- j Two patients received HyperCVAD and one patient received HyperCVED.
- k patient received CHOP alternating with DHAP.
Among the patients in the observation group, 64% (18/28) received CHOP without etoposide 22, with or without intrathecal chemotherapy. Of these 18 patients, one received CHOP plus zidovudine and another received CHOP plus bevacizumab as part of a clinical trial. 25% (7/28) received etoposide in addition to CHOP (as CHOEP or EPOCH) 9, 23-26, with or without intrathecal chemotherapy, 7.1% (2/28) received hyperCVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone) 27 and 3.6% (1/28) received hyperCVED (cyclophosphamide, vincristine, etoposide, dexamethasone), a regimen based on the hyperCVAD regimen with substitution of doxorubicin by etoposide 50 mg/M2 on days 1, 2, and 3.
In the ASCT group, 65% (13/20) received CHOP without etoposide 22, with or without intrathecal chemotherapy. Of these 13 patients, one received ABVD (adriamycin, bleomycin, vincristine, dexamethasone) prior to CHOP due to an initial diagnosis of Hodgkin's disease and one patient may have received brentuximab vedotin in addition to CHOP as part of a blinded clinical trial. 30% (6/20) received etoposide in addition to CHOP (as CHOEP or EPOCH) 9, 23-26, with or without intrathecal chemotherapy. 5.0% (1/20) received CHOP alternating with DHAP (dexamethasone, high dose cytarabine, cisplatin) 28, 29. There were no significant differences in primary systemic therapies received between the two groups (P = 0.82, Table 1).
31% (10/32) of patients diagnosed prior to 12/31/2012 underwent consolidative ASCT in CR1. In contrast, 63% (10/16) of patients diagnosed after 12/31/2012 underwent consolidative ASCT in CR1. Although the percentage of patients diagnosed after 12/31/2012 who underwent consolidative ASCT in CR1 was slightly higher, this difference was not statistically significant (P = 0.062).
Relapse and progression
The median duration of follow up in the entire patient cohort was 26.4 months (range 6.6–128.6 months). The median duration of follow-up was 27.4 months (range 8.8–128.6 months) in the observation group and 23.1 months (range 6.6–52.5 months) in the ASCT group (P = 0.13). The 1-year cumulative incidence of relapse in the observation and ASCT groups was 50% (95% confidence interval [CI]: 30–67%) and 46.3% (95% CI: 23–67%), respectively (P = 0.55).
The median PFS was 15.8 months for the patients who underwent active observation and 12.8 months for patients who underwent consolidative ASCT (log rank, P = 0.79, Fig. 1). The estimated 1, 2, and 3-year PFS was 57, 41, 37, and 54, 48, 41% for the observation and ASCT groups, respectively.
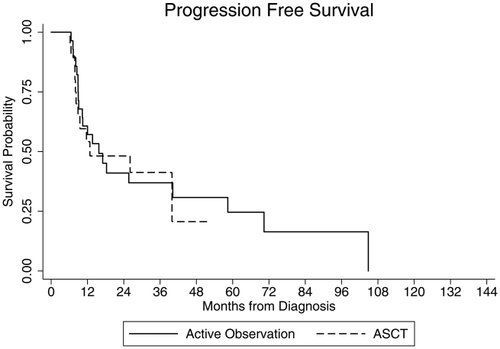
Progression-free survival (PFS) stratified by consolidative strategy. Kaplan–Meier PFS plots for patients who underwent a consolidative autologous stem cell transplant (ASCT, dashed line) versus patients who underwent active observation (solid line) in CR1 following CHOP-like induction chemotherapy (log rank, P = 0.79).
The median OS was 76.6 months for the patients who underwent active observation. The median OS for patients who underwent consolidative ASCT could not be computed as the survival probability was more than 50% at the end of follow-up. Despite that, there was no difference in OS between the two groups based on the corresponding survival curves (log rank, P = 0.94, Fig. 2). The estimated 1, 2, and 3-year OS for the observation and ASCT groups was 93, 72, 66, and 89, 72, 72%, respectively.
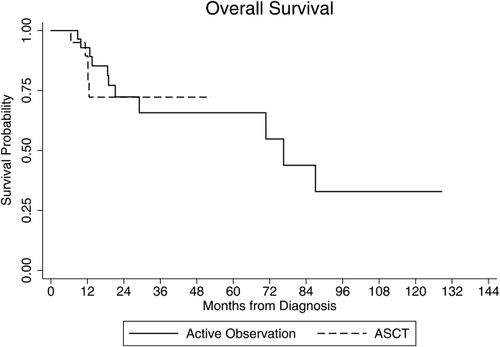
Overall survival (OS) stratified by consolidative strategy. Kaplan–Meier OS plots for patients who underwent a consolidative autologous stem cell transplant (ASCT, dashed line) versus patients who underwent active observation (solid line) in CR1 following CHOP-like induction chemotherapy (log rank, P = 0.94).
Discussion
In this retrospective analysis of PTCL patients who achieved CR1 following CHOP-like induction chemotherapy, we found that patients who underwent consolidative ASCT did not have a reduced risk of relapse or experience a statistically significant improvement in PFS or OS as compared to patients who underwent active observation. Interestingly, our study also found that at our institution, patients who were diagnosed after 12/31/2012, approximately 6 months after the study from the Nordic Lymphoma Group was published 11, seemed more likely to receive a consolidative ASCT in CR1. However, this difference was not statistically significant.
In this study, we found a CR rate of 52%, similar to what has previously been reported 4-9. However, with the exception of patients with ALK-positive ALCL, patients treated with combination chemotherapy alone have poor long-term outcomes and high relapse rates even after successful induction therapy. Upfront ASCT has emerged as a consolidative strategy aimed at extending PFS based on data from several retrospective and prospective phase II studies. However, most of these studies included patients who may or may not have achieved CR1 following induction chemotherapy, making it difficult to apply the results directly to the subset of patients achieving CR1 following induction chemotherapy.
The Nordic Lymphoma Group (NLG) published the largest prospective series to date (NLG-T-01) 11, reporting a 3-year OS and PFS of 56 and 48%, respectively, in patients who underwent upfront ASCT after achieving at least a partial response (PR) to CHOP/CHOEP. Of the 160 patients included in the study, 52% (82/160) achieved a CR and 31% (49/160) achieved a PR following induction chemotherapy. However, as this was a single arm phase II study by design, outcomes were not compared to those of a contemporaneous control group. However, the results were certainly encouraging when compared with historical controls 22, 30. Similarly, a smaller prospective study by Reimer et al. 12 reported a 3-year PFS of 36% by intention to treat analysis for patients with PTCL undergoing ASCT. This study also reported a difference in 3-year OS in transplanted versus nontransplanted patients (71 versus 11%). However, these results should be interpreted with caution as 64% (18/28) of the nontransplanted patients were excluded from transplant because of progressive disease. Likewise, a recent registry based retrospective study from Sweden 31 reported a 5-year PFS of 41% in patients who were planned for upfront ASCT versus 20% in patients who were not. However, the patients who were planned for upfront ASCT were significantly younger when compared to the patients that were not. In addition, data for the subset of patients who achieved CR following induction chemotherapy with CHOP/CHOEP were not reported.
On the contrary, a recent retrospective study by Abramson et al 32 reported that after controlling for CR following initial chemotherapy, patients who underwent consolidative stem cell transplantation did not appear to have an improved PFS compared to patients who did not receive a stem cell transplant. However, that study included patients who underwent allogeneic stem cell transplantation in addition to patients who underwent an autologous transplant, making the results difficult to apply clinically.
In the absence of data from randomized controlled trials, the utility of upfront ASCT in patients who achieve CR1 following CHOP-like induction chemotherapy remains controversial. Interestingly, our study found that such patients did not appear to benefit from ASCT in CR1. Patients in the observation group had a median PFS of 15.8 months compared to 12.8 months in the ASCT group. This closely mirrors the recent study by Abramson et al. 32 which reported no improvement in PFS in patients undergoing stem cell transplantation after controlling for CR following initial chemotherapy.
Our study reported a 3-year PFS of 41% in transplanted patients which is lower than what was reported by the Nordic Lymphoma Group (48%) 11, but higher than what was reported by the Reimer et al (36%) 12. However, data from both of these studies were analyzed using an intention to transplant approach and calculations of PFS included patients who did not ultimately proceed with an ASCT. This is in contrast to our study where all patients in our ASCT group received a consolidative ASCT.
The lack of benefit of ASCT in CR1 in our patient population might be explained in part by the somewhat higher median age of our transplanted patients (56.9 years) compared to other studies of consolidative ASCT in PTCL which reported median ages ranging from 41 to 47 years 12, 33-36. However, the Nordic Lymphoma Group 11 reported a median age of 57 years which was similar to our study. Notably, in the study by the Nordic Lymphoma Group 11, increasing age was found to be negatively associated with PFS on multivariable analysis. In addition, 80% of our patients in the ASCT group had stages III or IV disease. This is somewhat higher than what was previously reported by Reimer et al. (75%) 12, but comparable to that reported by the Nordic Lymphoma Group (81%) 11.
There are a few limitations to our study. First, the retrospective nature of our study meant that the chosen consolidative strategy was determined largely by physician and patient preference. In many cases, it was not possible to determine retrospectively if the patients initiating active observation in CR1 would have been eligible for transplantation. This may have led to a selection bias resulting in patients with poorer performance status or older age being represented to a greater degree in the observation group. However, we did not observe any statistically significant differences in baseline characteristics between the two groups and patients in the observation group had a slightly longer median PFS compared to patients who underwent consolidative ASCT, which would be unexpected if the observation group had negative prognostic characteristics. Second, our small sample size may have limited our ability to detect treatment effects. Lastly, the generalizability of our results may be limited by the fact that this is a single center study of patients treated in a tertiary referral center.
In conclusion, our analysis suggests that upfront ASCT does not improve PFS when compared to active observation in PTCL patients who achieve CR1 with CHOP-like induction chemotherapy. Future prospective studies are needed to clearly define the role of upfront ASCT in PTCL and better identify patients who will benefit from this strategy. In addition, overall poor outcomes in this patient population suggest that newer approaches including the use of novel agents as part of first-line or consolidative therapy, should be considered in the context of clinical trials.
Acknowledgment
The authors would like to acknowledge Jacqueline Smith, CRNP; Patricia Mangan, CRNP; Danielle Land, CRNP; and Brenda Shelly, CRNP for providing exceptional patient care.




