Spleen enlargement is a risk factor for thrombosis in essential thrombocythemia: Evaluation on 1,297 patients
Conflict of Interest: The authors declare no competing financial interests.
Abstract
Spleen enlargement, present in 10–20% of Essential Thrombocythemia (ET) patients at diagnosis, is a feature clinically easy to assess, confirmable by echography with a very low chance of misinterpretation. Nonetheless, the clinical and prognostic role of splenomegaly has been seldom evaluated. From 1979 to 2013, 1297 ET patients retrospectively collected in the database of the Lazio Cooperative Group and Bologna University Hospital were evaluable for spleen enlargement at diagnosis and included in the analysis. On the whole, spleen was enlarged in 172/1297 (13.0%) patients; in most cases (94.8%) splenomegaly was mild (≤5 cm). Patients with splenomegaly were younger, predominantly male, presented higher platelet count and JAK2V617F allele burden and had a lower incidence of concomitant cardiovascular risk factors. At least one thrombotic event during follow-up occurred in 97/1,125 (8.6%) patients without spleen enlargement compared to 27/172 (15.7%) patients with spleen enlargement (P = 0.003). Despite comparable use of cytoreductive/antiplatelet therapies in the two groups, the cumulative risk of thrombosis at 5 years was significantly higher in patients with baseline splenomegaly (9.8% versus 4.4% in patients without splenomegaly, P = 0.012). In multivariate analysis exploring risk factors for thrombosis, splenomegaly retained its negative prognostic role, together with previous thrombosis, leucocyte count and male gender. Baseline splenomegaly seems to be an independent additional risk factor for thrombosis in nonstrictly WHO-defined ET patients. This data could be useful in the real-life clinical management of these patients. Am. J. Hematol. 91:318–321, 2016. © 2016 Wiley Periodicals, Inc.
Introduction
Essential thrombocythemia (ET) is a chronic myeloproliferative neoplasm (MPN) characterized by persistently high platelet count and overall favorable prognosis, with a life expectancy similar to the general population, particularly in the first 10–15 years 1, 2. An increased risk of vascular complications over time is the main clinical feature of ET; consequently, the evaluation of the thrombotic risk at diagnosis is the crucial point for treatment decision 3, 4.
Multiple factors are believed to contribute to the pathogenesis of thrombosis in ET. Currently, advanced age (≥60 years) and history of previous thrombosis are the two major risk factors that identify high-risk patients requiring a platelet-lowering chemotherapy 5-7. To this regard, older thrombotic events (>24 months before ET diagnosis) seem to have a more prominent prognostic role compared to thrombosis occurring closer to diagnosis 8. Many other clinical, laboratory, and biological features, such as cardiovascular risk factors (CVRF), leucocytosis, JAK2V617F mutation and allele burden have been proposed as additional risk factors for thrombosis, with conflicting results 8-14.
Despite the relatively large number of the studies investigating potential risk factors for thrombosis, none has specifically addressed the role of spleen enlargement on thrombotic tendency. A mild to moderate spleen enlargement is present in about 5–20% of ET patients at diagnosis. Notwithstanding the relatively common occurrence of this feature, the prognosis of patients with spleen enlargement is still unclear. To address this issue, 1,297 ET patients followed by the Lazio Cooperative Group and the Bologna University Hospital between 1979 and 2013 were retrospectively analyzed to correlate spleen enlargement with baseline disease characteristics and outcome, with particular focus on thrombotic risk.
Patients and Methods
Study population
All consecutive patients with ET diagnosed from January 1979 to December 2013 in 11 Hematologic Centers of the Lazio region (5 University Institutes and 6 Community-based/3rd level teaching Hospitals) and in the Institute of Hematology “L. and A. Seràgnoli”, Bologna were considered eligible for the present retrospective analysis if evaluable for spleen size at diagnosis, JAK2 mutational status and CVRF assessment.
Diagnostic criteria of ET were according to Polycythemia Vera Study Group (PVSG) 15 before 2001 and according to World Health Organization (WHO) 2001 16 and 2008 17 after the application of these new definitions. Spleen enlargement was defined as a palpable spleen under costal margin 18. Cardiovascular risk factors included smoking, hypertension, diabetes, dyslipidemia, and overweight. The study was approved by the institutional review board of each institution and was conducted in accordance with the Declaration of Helsinki.
Definition of thrombotic events
For the purposes of this study, only major vaso-occlusive events were considered, specifically: (1) Arterial thrombosis: acute myocardial infarction (AMI), angina pectoris, ischemic stroke, transient ischemic attack (TIA), peripheral arterial occlusions; (2) Venous thrombosis: pulmonary embolism, peripheral or splancnic venous thrombosis, thrombosis of cerebral venous sinus.
JAK2V617F molecular evaluation
Qualitative assessment of JAK2V617F mutation was performed as described elsewhere 19; JAK2V617F allele burden was assessed in granulocyte DNA by quantitative polymerase chain reaction–based allelic discrimination assay (Ipsogen JAK2 MutaQuant Kit) on 7900 HT Fast Real Time PCR System (Applied Biosystem).
Statistical analysis
Data were expressed as mean ± standard deviation (SD) (normally distributed data), median and interquartile range (IQR) (non-normally distributed data), or as percentage frequencies, and within-patient comparisons were made by paired t test and χ2 test, as appropriate. The Kaplan-Meier product-limit method was used to estimate univariate survival curves, and the log-rank test was adopted to compare the survival curves. Thrombosis-free Survival (TFS) was calculated from the date of diagnosis to the occurrence of any event above defined as thrombotic complication.Cox proportional hazards regression was used to carry out multivariate survival analyses. The significance level was of P < 0.05 in all analyses. All analyses were performed using Statistical 7.1 (StatsoftInc, Tulsa, OK), Stata 9 (StataCorp LP, College Station, TX) software, and Microsoft Excel.
Results
Patient characteristics of the whole cohort and according to spleen enlargement
On the whole, 1,297 patients [M/F 490/807, median age 59 years (IQR 43.5–73.9)] were evaluable, 699 from the Lazio cooperative group database and 598 from the Bologna database. Peripheral blood median values at diagnosis were as follows: Hb 14.1 g/dl (IQR 13.1–15.1), WBC 8.7 × 109/l (IQR 7.2–10.6), PLTs 771 × 109/l (IQR 648–964). At least one CVRF was present at diagnosis in 779 patients (60.1%). The JAK2V617F mutation was present in 798 patients (61.5%); allele burden was available in 254 (31.8%) cases and showed a median value of 20% (IQR 8.5–40.0). Thrombotic events before or at the time of ET diagnosis were reported in 231 (17.8%) out of 1,297 patients. Previous thromboses were arterial in 182/231 patients (78.8%) and venous in 49/231 (21.2%). According to IPSET score for thrombosis (20), 326 patients (25.1%) were low-risk, 274 (21.1%) were intermediate-risk and 697 (53.7%) were high-risk. Median follow-up was 5.7 years.
Overall, 1196 patients (92.2%) required anti-platelet treatment and 997 patients (76.9%) cytoreductive therapy. The main causes for cytoreductive treatment start were: age ≥ 60 years (466 patients, 46.7%), thrombotic event (213 patients, 21.3%), extreme thrombocytosis (116 patients, 11.6%), microvessel symptoms resistant to anti-platelet therapy (202 patients, 20.4%). In no case treatment was started due to the presence or worsening of splenomegaly.
Among the evaluable 1297 patients, spleen was enlarged in 172 (13.3%). In 163 (94.7%) cases, spleen was palpable ≤ 5 cm below costal margin. The main baseline features of patients with and without spleen enlargement are reported and compared in Table 1. Patients with palpable splenomegaly were younger, predominantly male, presented higher platelet count and a lower incidence of concomitant CVRF. The proportion of patients carrying the JAK2V617F mutation was comparable, but the median mutation load was higher in patients with palpable splenomegaly. No difference in terms of previous thrombotic events and IPSET score risk classification was detected between patients with or without spleen enlargement. Use of antiplatelet treatment and cytoreductive therapy was comparable between the two groups of patients (Table 1). Ten-year overall survival was similar between patients with and without spleen enlargement (95.6% vs. 93.5%, P = 0.12).
|
No spleen enlargement (no. 1125) |
Spleen enlargement (no. 172) | P value | |
|---|---|---|---|
| Median age, years (IQR) | 60.2 (44.4–71.4) | 52.8 (38.0–67.1) | <0.001 |
| Gender male/female,(male%) | 397/728 (35.2) | 93/79 (54.0) | <0.001 |
| Median hemoglobin, g/dL (IQR) | 14.1 (13.1–15.0) | 14.4 (13.2 - 15.3) | 0.12 |
| Median leukocyte, × 109/L(IQR) | 8.7 (7.1–10.6) | 8.6 (7.4–10.7) | 0.49 |
| Median platelet count, × 109/L,(IQR) | 757 (646–951) | 840 (676–1000) | 0.005 |
| Mutated JAK2V617F, n° of patients (%) | 687 (61.0) | 111 (64.5) | 0.38 |
| Median JAK2V617F allele burden (%) (IQR) | 19.3 (8.0–37.4) | 25.3 (13.8–44.2) | 0.024 |
| One or more cardiovascular risk factors, n° of patients (%) | 693 (61.6) | 86 (50.0) | 0.004 |
| Previous thrombosis, n° of patients (%) | 192 (17.0) | 39 (22.6) | 0.075 |
|
IPSET score, n° of patients (%): Low-risk Intermediate-risk High-risk |
287 (25.5) 242 (21.5) 596 (53.0) |
39 (22.7) 32 (18.6) 101 (58.7) |
0.37 |
| Antiplatelet treatment, Y/N (%) | 1042/83 (92.6/7.4) | 154/18 (89.5/10.5) | 0.76 |
| Cytoreductive therapy, Y/N (%) | 879/246 (78.1/21.9) | 123/49 (71.5/28.5) | 0.063 |
- The bold values are statistically significant (<0.05) while the italic ones are not significant (>0.05).
Spleen enlargement and thrombotic events
Out of the whole cohort of 1,297 patients, 124 (9.6%) presented one or more thrombotic events during follow-up, that were mainly arterial (76 patients, 61.2%). The overall rate of thrombosis was 1.32% patients/year, with a cumulative incidence of 11% at 10 years.
The frequency and type of thrombotic events during follow-up according to spleen enlargement are shown in Table 2. Overall rate of thrombosis was significantly higher in patients with palpable splenomegaly (15.7% versus 8.6%, P = 0.003). Accordingly, the cumulative risk of thrombosis at 5 years was significantly higher in patients with spleen enlargement (9.8% compared with 4.4% in patients without spleen enlargement, P = 0.012) (Fig. 1). The overall rate of thrombosis was 1.79% patients/year in patients with spleen enlargement and 1.15% patients/year in patients without spleen enlargement. Notably, the increased thrombotic risk was due to increased frequency of both arterial and venous events.
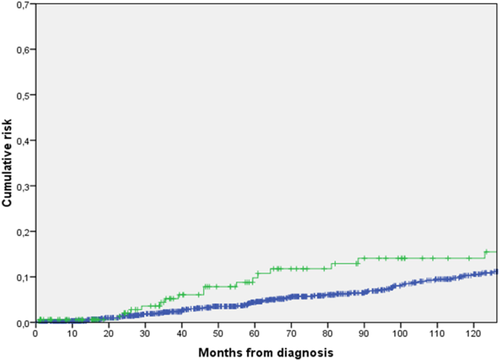
Five-year cumulative risk of thrombosis according to spleen enlargement at diagnosis. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
|
No spleen enlargement (n° 1,125) |
Spleen enlargement (n° 172) |
P value | |
|---|---|---|---|
| All thrombosis, n° of patients (%) | 97 (8.6) | 27 (15.7) | 0.003 |
| Arterial thrombosis, n° of patients with the event (%) | 61 (5.4) | 16 (9.3) | 0.033 |
| Cerebro-vascular (%) | 29 (2.5) | 12 (6.9) | 0.002 |
| Cardiac (%) | 27 (2.4) | 3 (1.7) | 0.59 |
| Peripheral (%) | 5 (0.5) | 1 (0.7) | 0.80 |
| Venous thrombosis, n° of patients with the event (%) | 36 (3.2) | 11 (6.4) | 0.028 |
| Cerebro-vascular (%) | 2 (0.2) | 2 (1.1) | 0.08 |
| Peripheral (%) | 28 (2.5) | 7 (3.7) | 0.23 |
| Splancnic (%) | 5 (0.4) | 2 (1.1) | 0.23 |
| Pulmonar embolism (%) | 1 (0.1) | 1 (0.5) | 0.12 |
- The bold values are statistically significant (<0.05) while the italic ones are not significant (>0.05).
Prognostic factors for thrombosis
Baseline clinical, laboratory and molecular data were evaluated for correlation with the incidence of thrombosis during the follow-up. In univariate analysis, baseline features that significantly correlated with higher thrombotic risk were: spleen enlargement (P = 0.003), male gender (P = 0.006), leucocyte count above the median value of 8.7 × 109/l (P = 0.019) and previous thrombotic episodes (P = 0.001). Conversely, IPSET score (P = 0.051), presence of at least one CVRF (P = 0.066) and age ≥ 60 years (P = 0.072) showed only a trend for association. No significant correlation was found between thrombosis and JAK2V617F mutational status, JAK2V617F allele burden, leucocytosis ≥11 × 109/l, hemoglobin levels or platelet count, both in the entire cohort and in patients with or without spleen enlargement. The JAK2V617F mutational status was also evaluated in the conventionally defined low-risk group (age < 60 years and no previous thrombotic event), but even in this subset of patients it did not show any influence (P = 0.538).
In addition, to minimize a possible confounding role of the different diagnostic criteria used in the different time-periods, a calendar variable dividing patients in 3 groups (PVSG, WHO 2001 and WHO 2008 diagnosed patients) was introduced: however, the 5-year TFS of these 3 groups did not show differences (96.7% for PVSG diagnosed patients vs 93.5% for WHO 2001 diagnosed patients vs 95.2% for WHO 2008 diagnosed patients, P = 0.348).
In multivariate Cox regression analysis, the occurrence of a previous thrombosis (HR 1.74, 95%CI 1.13–2.67; P = 0.011), spleen enlargement (HR 1.73, 95%CI 1,08–2.79; P = 0.023), leucocytes > 8.7 × 109/l (HR 1.52, 95%CI 1.03–2.22; P = 0.032) and male gender (HR 1.46, 95%CI 1.00–2.15; P = 0.048) maintained their unfavourable prognostic impact.
Discussion
Spleen enlargement is a quite common clinical feature at diagnosis in patients with ET, with a relatively broad spectrum of incidence in the different studies, ranging from 5% to about 20% 20-22. It is generally regarded as a sign of a hyper-proliferative disease and may in some cases be the ultimate cause of cytoreductive treatment start.
In the present retrospective analysis, we evaluated 1,297 ET patients diagnosed in the Lazio region and in the Bologna University Hospital for baseline spleen size, looking for correlation with thrombosis over the follow-up. At ET diagnosis, spleen enlargement was present in 13.3% of the patients, and was mild (<5 cm below costal margin) in most cases. Additionally, the presence of baseline spleen enlargement identified a subpopulation of ET patients characterized by younger age, higher platelet count and greater JAK2V617F mutation load.
Together with a history of thrombosis, leucocytosis and male sex, baseline splenomegaly resulted significantly and independently associated with thrombotic risk. Notably, neither older age nor the IPSET score were found to significantly correlate with subsequent thrombosis. The association between splenomegaly and thrombosis is particularly significant, since patients with splenomegaly were overall younger than patients without spleen enlargement.
The rate of thrombosis was not influenced by cytoreductive/antiplatelet therapy, that was equally administered in patients with and without spleen enlargement. It is plausible that spleen enlargement identifies a subset of patients with increased myeloproliferation, leading to higher thrombotic risk. A contributory role of JAK2V617F allele burden may not be excluded, since patients with spleen enlargement showed higher mutation load. It is worth of note, however, that the presence of a JAK2V617F mutation, which has been formally included in the International Prognostic Score for Essential Thrombocythemia (IPSET) among the risk factors for thrombosis 20, did not show a prognostic role in our cohort of patients. However, the IPSET score applies to a strictly WHO-defined population of ET patients, while the present cohort includes patients diagnosed also with the PVSG criteria, according to the date of diagnosis. As a result, some cases of early-primary myelofibrosis (early-PMF) could have been included in the analysis. However, the strict distinction between ET and early-PMF is not of pivotal importance when looking for thrombotic risk factors, since the incidence of thrombosis has been reported to be comparable in these two clinical entities 23, 24. Moreover, the histological discrimination between ET and early-PMF is not always easy to assess and reproducible, particularly in peripheral Centers without a specific expertise on MPNs histology; therefore, exploring additional risk factors for thrombosis in these patients is of utmost relevance for everyday clinical management.
As a matter of fact, the role of spleen enlargement on the pathogenesis of thrombosis and its prognostic relevance remains still unclear. Unfortunately, from a biological point of view, we were unable to give any explanation due to the lack of data in our retrospective multicentric database in which only clinical features were recorded: also from a clinical point of view, notwithstanding many efforts to correlate spleen enlargement with the increased thrombotic risk, no firm and meaningful explanation was found.
In conclusion, the present study demonstrates a significant independent association between spleen enlargement and thrombosis in a large cohort involving more than 1,200 ET patients followed for a long observation time. Spleen enlargement is a clinical feature very easy to assess in all patients, retains a very low chance of misinterpretation and if needed may be confirmed by echography, a non-invasive and low-cost exam. Thus, baseline spleen enlargement should be carefully evaluated for thrombosis prognostication and for treatment decision in ET patients.




