Bone marrow necrosis in acute leukemia: Clinical characteristic and outcome
Conflict of interest: Nothing to report.
Abstract
Bone marrow necrosis (BMN) is characterized by infarction of the medullary stroma, leading to marrow necrosis with preserved cortical bone. In reported small series, BMN in hematological malignancies is associated with poor prognosis. We sought to find the impact of BMN on clinical outcome in a relatively larger cohort of patients with acute leukemias. Overall we evaluated 1,691 patients; 1,051 with acute myeloid leukemia (AML) and 640 with acute lymphocytic leukemia referred to our institution between 2002 and 2013. Patients with AML and acute lymphoblastic leukemia (ALL) were evaluated separately to determine the incidence of BMN, associated clinical features and its prognostic significance. At initial diagnosis, BMN was observed in 25 (2.4%) patients with AML and 20 (3.2%) patients with ALL. In AML, BMN was significantly associated with French–American–British AML M5 morphology (32% vs. 10%, P = 0.002). The complete remission (CR) rate in AML with and without BMN was 32% and 59% respectively (P = 0.008). Likewise, CR rate in ALL with BMN was also inferior, 70% vs. 92% (P = 0.005). The median overall survival (OS) in AML with BMN was significantly poorer, 3.7 months compared to 14 months without BMN (P = 0.003). Similarly, the median OS in ALL with and without BMN was 61.7 and 72 months respectively (P = 0.33). BMN is not a rare entity in AML and ALL, but is infrequent. BMN in AML and in ALL is suggestive of inferior response and poor prognosis. Am. J. Hematol. 90:769–773, 2015. © 2015 Wiley Periodicals, Inc.
Introduction
Bone marrow necrosis (BMN) is a distinct clinicopathological entity characterized by infarction of the medullary stroma leaving an amorphous eosinophilic background, ill-defined necrotic cells, and preserved cortical bone 1. Definition excludes BMN without hematopoietic dysfunction or BMN extending to cortical bone 2. Histopathological biopsy specimens show loss of normal architecture as well as loss of medullary fat spaces 1, 3. BMN has been a rare reported finding at presentation, rather more commonly reported at autopsy 4.
In one series, BMN was classified into Grade I (mild necrosis) defined as focal necrosis occupying less than 20% of marrow, Grade II (moderate necrosis) involving 20–50%, and Grade III (severe necrosis) occupying more than 50% of bone marrow biopsy 5. Wide varieties of conditions are associated with BMN, ranging from infections and sepsis to sickle cell disease, metastatic carcinoma, and hematological malignancies. However, more extensive BMN is more commonly associated with hematological malignancies 2, 3, 6-18. BMN has been reported specifically in both acute myeloid (AML) and acute lymphoblastic leukemias (ALL) 11, 13-16, 18. In the published literature, the incidence of BMN ranges from 0.5% to 33%, however higher frequency usually been reported in patients after severe infections and typically involve focal areas of bone marrow (Grade I) 5. On the other hand, in leukemia it is relatively less frequent (≤ 0.5%), and typically has large foci of marrow necrosis (Grade III).
Few case reports and small series have suggested that the presence of BMN in acute leukemia is associated with lower remission rate and dismal prognosis. Moreover, in several instances it has been reported as after effects of intensive leukemia therapy 19, 20. The clinical characteristics and outcomes of patients with BMN in leukemia remain largely unknown. The purpose of this study was to determine the incidence, identify the associated features, and ascertain the clinical impact of BMN at initial diagnosis in larger group of patients with ALL and AML.
Patients and Methods
Clinical data for patients with newly diagnosed AML and ALL presenting to the Department of Leukemia at the University of Texas—MD Anderson Cancer Center (MDACC) between January 1, 2002 and December 31, 2013 were reviewed. Overall 1,691 patients; 1,051 AML and 640 patients with acute lymphocytic were evaluated for incidence of BMN, associated clinical feature, and impact on clinical outcome. The majority of the patients were treated in clinical trials approved by the Institutional Review Board (IRB) and conducted in accordance with the Declaration of Helsinki. All patients signed an informed consent document approved by the IRB before enrolling on the trials. IRB approval was also obtained for reviewing the records of patients treated outside clinical trials.
Bone marrow evaluation and cytogenetics and molecular markers
Bone marrow aspirate smears with core biopsies were performed on all patients at the time of presentation. Bone marrow aspirate smears were assessed by Wright–Giemsa stain followed by cytochemical analysis for myeloperoxidase and α-naphthyl butyrate esterase, as previously described 21. BMN was defined as necrosis of the myeloid tissues and stroma without involvement of the cortical bone, verified by hematopathologist at MD Anderson Cancer Center. Patients with BMN grade III 5 occupying ≥ 50% of marrow space were included in the analysis. Patients with focal BMN (occupying one high power field/or combined area of involvement occupying less than 20% of bone marrow specimen), moderate BMN (occupying more than one high power but less than 10× field/or combined area of involvement occupying between 20% and 50% of bone marrow specimen), necrosis extending to cortical part of bone, and BMN after chemotherapy were excluded from the analysis. Patients who were initially treated outside and later referred to MD Anderson Cancer Center (MDACC), their initial bone marrow specimen were reviewed by hematopathologist at MDACC for the presence or absence of BMN.
Cytogenetic analysis was assessed by G-banding with at least 20 metaphases counted, as previously described 22. Cytogenetics risk stratification was based on Medical Research Council (MRC) adult and children leukemia parties; MRC AML 10 trial 23. FLT3-ITD and codon 835 point mutations in the activating loop of the tyrosine kinase domain of FLT3 were analyzed using genomic DNA extracted from bone marrow aspirate specimens by polymerase chain reaction assays followed by capillary electrophoresis. Mutations in NRAS and KRAS were assessed by polymerase chain reaction analysis followed by pyrosequencing, as described previously 24.
Response criteria
Response to therapy was classified according to the International Working Group (IWG) criteria 2003 25. A complete remission (CR) was defined by the presence of <5% blasts in the bone marrow with recovery of peripheral counts with absolute neutrophil count >1,000/µL and platelet count >100,000/µL. Relapse from CR was defined as the reappearance of peripheral blasts or greater than 5% blasts in the bone marrow, or the occurrence of extramedullary disease. Early mortality was defined as death within 4 weeks of diagnosis. An antecedent hematologic disorder (AHD) was defined as an abnormality in blood counts for at least 1 month before presentation to our institution.
Statistical analysis
Patient characteristics were tabulated by status of BMN. Differences between categorical covariates were tested using Fisher's exact tests, and differences between continuous covariates were compared by the Wilcoxon rank-sum test. Overall survival (OS) was defined as the time interval between treatment start date and death date, and was censored at last follow-up date for patients who were alive. Event-free survival (EFS) was defined as the time interval between treatment initiation date and the date of resistance, relapse, or death, whichever came first, and was censored at last follow-up date for patients who were alive without relapse. Relapse-free survival (RFS) among CR patients was defined as the time interval between response dates and date of relapse or death, whichever came first, and was censored at last follow-up for patients who were alive without relapse. Survival curves were estimated using the Kaplan–Meier methods 26 and compared using two-sided log-rank tests. Univariate and multivariate Cox proportional hazards regression models were used to assess the association between patient characteristics and OS, EFS, or RFS 27. Univariate and multivariate logistic regressions were applied to evaluate the association between patient characteristics and CR or early induction death. A stepwise variable selection was applied with P-value of less than 0.05 for entry and more than 0.1 for removal. BMN were added to the reduced model if it was not selected in the stepwise variable selection. Predictive variables were transformed as appropriate. Statistical analyses were performed using SAS 9.3, and graphics were created using Stata 13.1.
Results
Baseline characteristics
BMN was found in 20 (3.2%) and 25 (2.4%) patients with ALL and AML respectively, at initial diagnosis. The clinical characteristics of patients with AML and ALL with BMN at diagnosis are presented in Tables 1 and 2.
| Median [range], number (%) | |||
|---|---|---|---|
| Variables | Without BMN (n = 1,026) | With BMN (n = 25) | P value |
| Age, years | 62 [14–88] | 68 [25–83] | 0.19 |
| WBC × 109/L | 3.8 [0.2–433] | 12.4 [0.7–269.4] | 0.07 |
| BM Blast % | 42 [0–99]a | 59 [1–95]a | 0.08 |
| Antecedent hematological disorder | 178 (17) | 3 (12) | 0.60 |
| Cytogenetics | |||
| Poor risk | 321 (31) | 11 (44) | 0.19 |
| Diploid | 452 (44) | 7 (28) | 0.15 |
| Intermediate risk | 206 (20) | 5 (20) | > 0.99 |
| Unknown | 47 (5) | 2(8) | 0.33 |
| Molecular mutation | |||
| FLT3 ITD (n = 957; without BMN [948], with BMN [9])b | 148 (16) | 0 (0) | 0.37 |
| NPM1 (n = 598; without BMN [591], with BMN [7])b | 135 (23) | 2 (29) | 0.66 |
| RAS (n = 878; without BMN [869], with BMN [9])b | 92 (11) | 0 (0) | 0.61 |
| FAB | |||
| M0 | 56 (5) | 2 (8) | 0.64 |
| M1 | 127 (12) | 2 (8) | 0.76 |
| M2 | 185 (18) | 2 (8) | 0.29 |
| M3 | 0 (0) | 0 (0) | |
| M4 | 151 (15) | 5 (20) | 0.40 |
| M5 | 101 (10) | 8 (32) | 0.002 |
| M6 | 54 (5) | 0 (0) | 0.63 |
| M7 | 9 (1) | 0 (0) | > 0.99 |
| RAEB—Type 1 | 140 (14) | 1 (4) | 0.24 |
| Unknown | 203 (20) | 5 (20) | > 0.99 |
| Induction chemotherapy | |||
| HDAC-based | 542 (53) | 9 (36) | 0.11 |
| HMA-based | 162 (16) | 6 (24) | 0.27 |
| LDAC based | 218 (21) | 3 (12) | 0.33 |
| Investigational agents | 104 (10) | 3 (12) | 0.73 |
| Not treated | 0 (0) | 4(16) | <0.0001 |
- No; number of patients, WBC; white blood cell count, BMN; bone marrow necrosis, AHD; antecedent hematologic disorder, Chemo; chemotherapy, XRT; radiation therapy, HDAC; high dose ara-C, LDAC; low dose ara-C, HMA; hypomethylating agent, FAB; French American British classification, RAEB; refractory anemia with excess blast.
- a Some patients receive treatment outside and achieve response, which represent lower BM blast at the time of referral.
- b Number of patients evaluated for mutations.
| Median [range], No. (%) | |||
|---|---|---|---|
| Variables | Without BMN (n = 620) | With BMN (n = 20) | P value |
| Age, years | 42 (13–84) | 51.5 (17–69) | 0.58 |
| WBC × 109/L | 5.45 (0–602.4) | 5.05 (0.0–11.5) | 0.38 |
| BM blast % | 80 (0–100)a | 38.5 (0–91)a | 0.004 |
| ALL Lineagec | |||
| B-cell | 509 (84) | 15 (100) | 0.15 |
| T-cell | 99 (16) | 0 (0) | |
| Cytogenetics | |||
| Diploid | 194 (31) | 4 (20) | 0.34 |
| MLL | 7 (1) | 0 (0) | >0.99 |
| Ph+ | 160 (26) | 2 (10) | 0.12 |
| Else | 259 (42) | 14 (70) | 0.02 |
| Treatment | |||
| Hyper-CVAD 28 +/− monoclonal Abs or TKIb | 509 (82) | 15 (75) | 0.38 |
| Augmented BFM 29 | 111 (18) | 3 (15) | >0.99 |
| Not treatedd | 0 (0) | 2 (10) | 0.001 |
- No; number of patients, WBC; white blood cell count, BMN; bone marrow necrosis, BM; bone marrow, MLL; mixed lineage leukemia, Ph+; Philadelphia chromosome positive.
- a Some patients receive treatment outside and achieve response, which represent lower BM blast at the time of referral.
- b Patients received HyperCVAD with monoclonal antibodies (ofatumumab or rituximab) based on CD20 expression or TKI (tyrosine kinase inhibitor) if Ph+.
- c Overall; 17 (2.6%) patients were non-evaluable for immunophenotyping.
- d 1 died without receiving treatment and 1 received treatment outside MD Anderson Cancer Center.
Among AML patients, median age at diagnosis was 68 and 62 years with and without BMN respectively (P = 0.19). Three (12%) patients with BMN and 178 (17%) patients without BMN had a history of antecedent hematological disorder (P = 0.60). Poor risk cytogenetics (CG) was observed in 11 (44%) and 321 (31%) of patients with and without BMN respectively (P = 0.19). The most common morphological classification according to French–American–British (FAB) in patients with BMN was AML M5 32% and AML M4 20% compared to 10% and 15% in patients without BMN (P = 0.002 and 0.40) respectively.
On the other hand, patients with ALL were relatively younger, with median age at diagnosis 51.5 and 42 years in patients with and without BMN respectively (P = 0.58). Interestingly, patients with BMN had a median bone marrow (BM) blast percentage of 38.5% compared to 80% without BMN (P = 0.004). Among the 15 patients with BMN, evaluable for immunophyenotyping, all had B-cell lineage ALL, compared to 84% in patients without BMN (P = 0.15).
Treatment and outcome
AML patients with BMN
Patients received different induction chemotherapy regimens based on available clinical trials, age, and performance status. For the purpose of analysis, induction chemotherapy regimens were grouped as follows: high-dose Ara C based (HDAC; ≥1,000 mg/m2/dose), low dose Ara C based (LDAC; 20–500 mg/m2/dose), hypomethylating agent (HMA; azacytidine or decitabine) based, and investigational agents on clinical trial. (Only a few patients received highly investigational agents as frontline therapy because treating physician felt that due to age, co-morbidities, and performance status patients were not fit for intensive therapy). As shown in Table 1, no significant differences were observed in the use of each strategy. Four (16%) patients with BMN did not receive induction therapy; 2 died before initiating therapy due to sepsis/multiorgan failure and 1 was lost to follow-up.
The AML patients with BMN had significantly lower rate of complete remission of 32% compared to 59% in patients without BMN (P = 0.008). Three (12%) and 37 (3.6%) patients had early death (< 4 weeks of diagnosis), with and without BMN respectively (P = 0.07) (Supporting Information Table I).
ALL patients with BMN
Almost all patient with ALL received hyper-CVAD (cyclophosphamide, vincristine, adriamycin and dexamethasone) 28 based induction chemotherapy regimens or the augmented Berlin–Frankfurt–Munster (BFM) regimen 29. Majority of patients treated with hyper-CVAD also received either an anti-CD20 monoclonal antibody such as Rituximab or Ofatumumab based on leukemic cell CD20 expression or a tyrosine kinase inhibitor (imatinib or dasatinib) if Philadelphia chromosome positive. As shown in Table 2, no significant difference was observed based on induction regimens patients received in each group. Two patients with BMN did not received induction chemotherapy; one died before initiating treatment due to sepsis and the other preferred to be treated at an outside facility.
The complete remission rate in ALL group was also significantly inferior in those who had BMN (70%) compared to 92% who did not (P = 0.01). Seventeen (3%) patients without BMN and 1 (5%) patient with BMN had early death (in < 4 weeks) (P = 0.44) (Supporting Information Table I).
Survival
AML patients with BMN
The median RFS was 15 months in patients with BMN, whereas 11 months for patients without BMN (P = 0.58) (Supporting Information Table I, Fig. 1A).The overall survival (OS) was severely compromised in patients with BMN, with median of 3.7 months compared to 14 months without BMN (P = 0.003) (Fig. 1B). Similarly, patients with BMN had a median event-free survival (EFS) of 3.4 months compared to 9 months in AML patients without BMN (P = 0.04) (Fig. 1C).
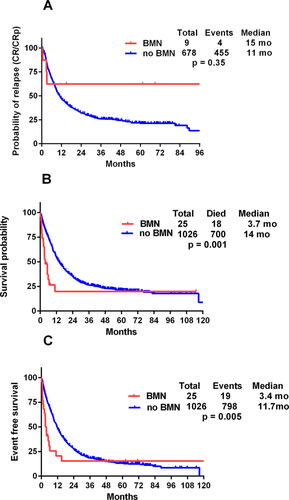
Kaplan–Meier curves using log rank test for (A) relapse-free survival, (B) overall survival, and (C) event-free survival in AML patients with and without BMN. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
ALL patients with BMN
Among patients with ALL, the median RFS was 59.5 months in patients with BMN compared to 55 months without BMN (P = 0.71) (Supporting Information Table I, Fig. 2A). The median EFS was not significantly inferior in patients with BMN, 49.2 months compared to 48.2 months without BMN (P = 0.47) (Fig. 2B). The median OS was inferior in patients with BMN; 61.7 months compared to 72 months without BMN, but not significant (P = 0.33) (Fig. 2C).
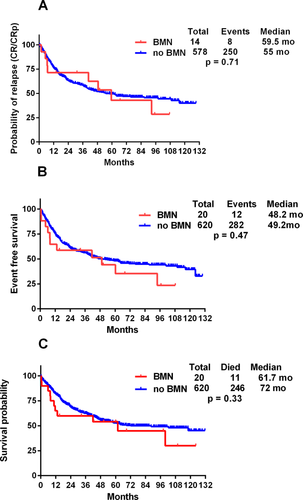
Kaplan–Meier curves using log rank test for (A) relapse-free survival, (B) event-free survival, and (C) overall survival in patients with acute lymphocytic leukemia (ALL) with and without BMN. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Univariate and multivariate analysis
Age, log (WBC), cytogenetics, and BMN were included in the univariate and multivariate models for both AML and ALL patients. Pre-chemo or radiotherapy was also included for AML patients. The Supporting Information Tables II–V list the results of univariate and multivariate analysis. Logistic regression analyses for CR rates and early induction deaths, and Cox regression analyses for RFS, OS, and EFS were used for evaluation. Multivariate analysis in AML with BMN showed a significant effect on OS (HR = 2.31; 95% CI; 1.40–3.81, P = 0.001) and EFS (HR = 1.97; 95% CI; 1.22–3.20, P = 0.01). Although, AML with BMN showed significant effects on CR and early induction death in the univariate model but did not show a significant effect in multivariate model. In ALL, BMN did show a significant effect on CR in univariate model (HR; 0.21, 95% CI; 0.08–0.56, P =0.002) but not in the multivariate model. For early induction death, RFS, OS, and EFS, BMN failed to show significance in ALL.
Discussion
In our series of 640 patients with ALL and 1,051 patients with AML, the incidence of BMN was 3.2% and 2.4%, respectively. BMN in AML was associated with poorer outcome with significantly inferior OS and EFS. However in ALL, BMN had lower CR rate but it did not had an impact on survival outcome.
BMN is a rare finding, to the best of our knowledge, there are no previous published reports regarding the incidence, clinical characteristics, and outcomes in a larger group of patients with acute leukemia. Overall incidence of BMN in hematological malignancies has been reported in a range of 0.15%–0.32%, in unselected ante mortem bone marrow biopsies 4. Our results, therefore, suggest that BMN at initial diagnosis is an underreported entity.
In our series, among the patients with AML and with BMN, 32% had the FAB-M5 histology, compared to 10% without BMN, signifying that it is more prevalent among patients with M5 histology 30. None of the patients in our series had the FAB-M7 histology suggesting that, despite its association with marrow fibrosis, the M7 histology is not associated with BMN. Furthermore, possible associations with specific cytogenetic abnormalities were evident. Among 23 evaluable AML patients with BMN, 70% had intermediate or adverse cytogenetics, while none had favorable cytogenetics. More importantly, 44% of the patients with BMN at diagnosis had a complex karyotype, significantly higher than the reported historical cohorts 31. None of nine patients with AML with BMN, evaluated for the presence of FLT3-ITD and RAS mutations, had the aberration, while only two of seven evaluable patients had a NPM1 mutation; this may indicate that BMN is solely a result of a proliferative disease associated with a proliferative genotype. In the present series, the presence of BMN was associated with a worse outcome among the patients with AML with a median OS of only 3.7 months 32. Primary resistant disease appeared to be the main reason for these poor outcomes, as BMN at initial diagnosis was significantly associated with lower CR rate compared to patients without BMN.
Among the evaluable patients with ALL and with BMN, all had B-cell lineage disease, and none had T-cell lineage. Only two patients had Philadelphia chromosome, despite the relative advanced median age of the patients in this series. Similar to AML patients, ALL patients with BMN had significantly inferior CR rate compared to patients without BMN. Though, the inferior response rate did not translated into significantly inferior RFS, EFS, or OS.
Several issues need to be addressed in future studies. Apart from one series, to date there is no standardized grading system to evaluate the extent of necrosis, and the clinical impact of the extent of BMN remains unknown. Furthermore, the underlying pathophysiology of BMN remains poorly understood. Although hypoxemia resulting from the failure of the microcirculation may be a critical event, the role of various toxins, cytokines, and vasoactive substances released from the malignant cells as well as the supporting cells in the microenvironment in the pathogenesis of BMN is not known 23. To date, no studies have assessed the potential role of cytokines in the underlying pathogenesis of BMN. Further studies are therefore needed to better elucidate these potential mechanisms. In summary, BMN is an infrequent, but not a rare finding in both AML and ALL. It occurs more commonly with the FAB-AML M5 histology. Furthermore, BMN is suggestive of lower CR rate and overall survival in patients with AML and ALL.




