Bortezomib-based therapy combined with high cut-off hemodialysis is highly effective in newly diagnosed multiple myeloma patients with severe renal impairment
Group authorship information: BAZ performed the research, collected, analyzed and interpreted data, and wrote the manuscript. MC and AS designed the study, analyzed and interpreted the data, and critically revised the manuscript. EZ, MS, LBD, PT, EM and LP were involved in data analysis, data interpretation, and reviewed the report. AB, RR, KM, SR, EB, CT and GM were involved in patient recruitment. AP performed statistical analyses.
Conflict of interest: MC has received honoraria and has been a member of the advisory board for Celgene and Jansen. All other authors declare to have no relevant financial interests in competing. The work had no specific funding.
Abstract
Multiple myeloma (MM) is often associated with renal insufficiency (RI) which adversely influences the prognosis. Several studies demonstrated that bortezomib can improve both renal function and outcome. We prospectively evaluated 21 newly diagnosed MM patients with severe renal impairment secondary to tubular-interstitial damage, most of them due to myeloma kidney, who were primarily treated with bortezomib-based therapy combined with high cut-off hemodialysis (HCOD). The median serum creatinine level at baseline was 6.44 mg dL−1 and calculated median estimated glomerular filtration rate (eGFR), according to Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation, was 8 mL/min/1.73 m2. Serum free light chain (sFLC) median concentration was 6,040 mg L−1. Post induction and best stringent complete response rates were 19 and 38%, respectively. Responses were fast, occurring within a median of 1.4 months. The combination of bortezomib and HCOD led to a prompt and remarkable (>90%) decrease in sFLC levels. Sixteen patients (76%) became dialysis independent within a median of 32 days. With a median follow up of 17.2 months, the 3-year PFS and OS were 76 and 67%, respectively. No early deaths were observed. This study demonstrates that incorporation of bortezomib into induction therapy combined with HCOD is a highly effective strategy in rescuing renal function and improving outcomes in patients with MM and RI. Am. J. Hematol. 90:647–652, 2015. © 2015 Wiley Periodicals, Inc.
Introduction
Renal impairment (RI) is a common complication of multiple myeloma (MM). At diagnosis, ∼20% of patients have serum creatinine levels higher than 2 mg dL−1, and up to 50% of patients can develop RI at various times during the course of the disease 1, 2. Severe renal failure requiring dialysis is present in 1–13% of cases 3.
RI in MM is mainly caused by increased monoclonal light chain (LC) excretion which induces a tubular-interstitial damage leading to the typical “myeloma kidney” (MK), or cast nephropathy 4. Contributing factors include dehydration, hypercalcemia, hyperuricemia and use of nephrotoxic drugs such as non steroid anti-inflammatory drugs 1.
Patients with RI have been historically considered to display a poor prognosis and have an increased risk of early death 5, 6. Conventional chemotherapies used in the past had limited efficacy and were associated with increased toxicity. More recently, the introduction of novel agents, such as the immunomodulators (IMiDs) and bortezomib, has led to improved outcomes of patients with RI. This finding is related to the ability of novel drugs to enhance the frequency and depth of response, ultimately reversing RI 7, 8. In particular, several studies have reported that the first-in-class proteasome inhibitor bortezomib is highly effective, fast acting and can improve renal function 9-11.
Based on the pathophysiology of RI in MM, recent studies have investigated the role of mechanical removal of free light chains (FLC), aimed at quickly decreasing the tumor load which is toxic to the kidney. High cut-off hemodialysis (HCOD), using large pore (45kD) dialysis membranes, allows the redistribution of extra-vascular FLC into the intra-vascular compartment, resulting in their prompt and sustained reduction 12. This new LC clearing dialyzer, combined with effective therapy, has shown encouraging, albeit preliminary, results 13, 14.
The aim of the present study was to prospectively evaluate the efficacy of an upfront treatment strategy including bortezomib-based induction therapy combined with HCOD in 21 newly diagnosed MM patients with severe RI, mostly due to biopsy-proven MK.
Methods
Patients
Between November 2009 and January 2014, all consecutive patients who received at the Units of Hematology and Nephrology in Bologna a diagnosis of MM and RI secondary to tubular-interstitial damage were enrolled in a prospective study of bortezomib-based induction therapy combined with HCOD. Patients could be enrolled provided that their involved serum FLC (sFLC) concentration at baseline was above 1,000 mg L−1. Additional inclusion criteria were an upper age limit below 85 years and an Eastern Cooperative Oncology Group (ECOG) performance status ≤2 15. Informed consent was obtained from all patients.
Kidney biopsy
A kidney biopsy was planned in every patient after the diagnosis of MM and RI was established. The samples were examined on light and electronic microscopy and by immunofluorescence, using anti-kappa and anti-lambda light chain antibodies. Patients at high risk of developing kidney biopsy-related complications were excluded.
Assessment of hematological response to therapy
Hematological response was assessed on the first day of every induction cycle and within 30 days after the last cycle of induction therapy. Response criteria for patients with measurable M protein concentration (20 patients) or sFLC level (1 patient) were those established by the International Myeloma Working Group 16.
Assessment of renal function and renal response to therapy
Renal function was assessed by estimated glomerular filtration rate (eGFR), calculated using CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) creatinine equation 17. The degree of RI, based on values of eGFR measured as mL/min/1.73 m2, was graded as follows: G1 (normal renal function) ≥90; G2 (mild RI) 60-89; G3a (mild to moderate RI) 45–59; G3b (moderate to severe RI) 30–44; G4 (severe RI) 15–29; G5 (renal failure) <15 or on dialysis. Renal response was defined as complete (CRrenal), partial (PRrenal), or minor (MRrenal), according to the criteria formulated by the IMWG. In particular, CRrenal was defined as an increase in eGFR from < 50 to 60 mL min−1 or better; PRrenal was an increase from <15 to 30–59 mL min−1 and MRrenal was an increase from <15 to 15–29 mL min−1, or from 15–29 to 30–59 mL min−1 1.
HCOD procedure
All patients received three 4-hr-sessions a week of intermittent HCOD until a sFLC reduction >60% was achieved. A single cycle of sFLC removal lasted 2 weeks and included a total of 6 HCOD sessions. A double lumen jugular vein catheter was inserted as vascular access. The filter used was a poly-aryl-ether-sulfone membrane (Gambro HCO 1100 dialyzer) with a 1.1 sm surface area and high molecular cut-off (45 kDa). sFLC levels were determined before, after and the day after each HCOD session to evaluate the sFLC rebound. sFLC load was calculated as deltaFLC, expressed as the difference between involved and uninvolved sFLC.
Toxicity and adverse events
Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 3.
Statistical analysis
Frequencies variables were compared by use of the χ2 test or Fisher's Exact test (as appropriate). Trend test among groups of patients were performed by Cuzick's trend test.
Time to event data was estimated by use of the Kaplan–Meier method and were compared with the log-rank test.
The Kaplan–Meier method was used to estimate time to progression (TTP), progression-free survival (PFS) and overall survival (OS); as usually defined.
Results
Patients characteristics
A total of 21 patients who met the inclusion criteria were treated upfront with a bortezomib-based induction regimen combined with HCOD. Full details of the study population are listed in Table 1. The most frequent M protein isotype was Bence–Jones kappa (38%); 13 patients (62%) had kappa sFLC. The median concentration of involved sFLC was 6,040 (range, 1,076–36,420) mg L−1.
| No. patients | 21 |
| Age, median (range) (years) | 62 (33–82) |
| - Age > 65 years (%) | 9 (43) |
| Male/female | 12/9 |
| M protein isotype: | |
| BJ lambda | 2 |
| BJ kappa | 8 |
| IgG/kappa | 5 |
| IgG/lambda | 3 |
| IgA/lambda | 1 |
| IgD/lambda | 1 |
| sFLC lambda only | 1 |
| Involved sFLC (%): | |
| -lambda | 8 (38) |
| -kappa | 13 (62) |
| sFLC concentration, median (range) (mg L−1) | 6,040 (1,076–36,420) |
| ISS: | |
| - I/II | 2 |
| -III | 19 |
| Serum creatinine, median (range) (mg dL−1) | 6.44 (1.88–17.20) |
| eGFR (CKD-EPI), median (range) (mL/min/l.73 m2) | 8 (3–35) |
| Stage of RI (%): | |
| - 3 (moderate) | 3 (14) |
| - 4 (severe) | 4 (19) |
| - 5 (failure) | 14 (67) |
| Hemoglobin median (range) (g dL−1) | 9.3 (7.5–11) |
| Serum calcium, median (range) (mg dL−1) | 9.3 (7.2–15) |
| Proteinuria, median (range) (mg/24 h) | 2,500 (50–20,520) |
| Lactic dehydrogenase, median (range) (U L−1) | 250 (97–704) |
- sFLC. serum free light chain; ISS, international staging system; eGFR,estimated glomerular filtration rate;.
- CKD-EPI. Chronic Kidney Disease Epidemiology Collaboration; RI, renal impairment.
| VTD | VD+ VMP | |
|---|---|---|
| Patients eligible to ASCT (%) | 13 (100) | – |
| Patients not eligible to ASCT (%) | – | 8 (100) |
| Median number of cycles | 3 (2–3) | 8 (5–9) |
| TW | OW | |
| Patients eligible to ASCT (%) | 13 (100) | – |
| Patients not eligible to ASCT (%) | – | 8 (100)a |
- a With the exception of the first cycle that consisted of tw VD.
- ASCT, autologous stem cell transplantation; VTD: bortezomib-thalidomide-dexamethasone; VMP, bortezomib-melphalan-prednisone; VD, bortezomib-dexamethasone; TW, twice weekly; OW, once weekly.
The majority of patients (67%) had G5 degree of CKD-EPI classification, which was equivalent to either an eGFR <15 mL/min/1.73 m2 or dialysis requirement; three patients presented oligo-anuria. The median creatinine serum level was 6.44 mg dL−1 (range, 1.88–17.20) and the median eGFR was 8 mL/min/1.73 m2 (range, 3–35).
A kidney biopsy was performed in 15 patients and revealed in all of them a typical MK. In three patients MK coexisted with light chain deposition disease (two patients) or Fanconi's syndrome (one patient). An additional patient who was affected by diabetes mellitus had superimposed diabetic nephropathy. In the remaining six patients the kidney biopsy was not performed because of the elevated bleeding risk related to uremia and/or thrombocytopenia and/or age >80 years; all these patients had a selective proteinuria consisting mainly of monoclonal FLC. Overall, the rate of kidney biopsy complications was low and a clinically not relevant sub-capsular hematoma was detected in only two patients.
Treatment received
All patients were homogeneously treated with a triplet bortezomib-based induction regimen which included either thalidomide or melphalan according patients’ eligibility to receive or not an autologous stem cell transplantation (ASCT). Thirteen patients who were eligible for high-dose therapy (HDT) and ASCT were treated with up to three 3-week cycles of twice weekly (tw), standard dose, bortezomib plus thalidomide and dexamethasone (VTD), followed by a single ASCT to support reduced-dose intravenous (iv) melphalan (100-140 mg/m2) and 2 subsequent cycles of VTD consolidation treatment. Details of VTD induction and consolidation therapy were reported elsewhere 18, 19. Transplant-ineligible patients were treated with up to eight 6-week cycles of the VMP regimen, comprising bortezomib 1.3 mg sm−1 on days 1, 8, 15, 22, plus melphalan 0.13 mg kg−1 on days 1–4, and prednisone 2 mg kg−1 on days 1–4. VMP induction therapy was preceded by a single cycle of tw bortezomib and dexamethasone (VD) aimed at reducing tumor burden and improving RI before melphalan was introduced as part of front-line therapy. Details of treatment regimens are reported in Table II. The median number of induction cycles was 6 (range, 2–9) in the overall patient population, 3 in the VTD- and 8 in the VMP-treated subgroups. Subcutaneous (sc) bortezomib was available at our centers since October 2012 and was offered to the last 9 patients who were enrolled, while the remaining 12 patients received iv bortezomib. Bortezomib-based induction treatment was started within a median of 4 (range, 2–8) days from kidney biopsy and of 5 (range, 3–11) days from the first dialytic session.
All patients received HCOD for a median of 1.4 months, corresponding to a median of 17.25 sessions each patient.
Hematologic response
The overall rate of at least partial response (PR) to induction therapy was 81%, including 19% stringent complete response (sCR) and 48% very good partial response (VGPR) (Table 3). The best response rate was 95%, including 38% sCR and 86% ≥ VGPR. Median time to response was 1.4 (range, 0.7–1.7) months.
| sCR | VGPR | PR | SD | PD | |
|---|---|---|---|---|---|
| After induction therapy | |||||
| All patients (%) | 4 (19) | 10 (48) | 3 (14) | 3 (14) | 1 (5) |
| ASCT eligible (%) | 2 (15) | 6 (46) | 3 (23) | 2 (15) | 0 |
| ASCT not eligible (%) | 2 (25) | 4 (50) | 0 | 1 (12.5) | 1 (12.5) |
- ASCT, autologous stem cell transplantation; sCR, stringent complete response; VGPR, very good partial response; PR, partial response; SD, stable disease; PD, progressive disease.
Renal response
The overall renal (≥MRrenal) response rate was 57%, including 3 patients (14%) who achieved a CRrenal, 4 (19%) a PRrenal and 5 (24%) a MRrenal; 9 patients (43%) had stable eGFR. Ten patients (48%) had a decrease in their creatinine levels below the threshold of 2 mg dL−1 while on study treatment. In 11 patients (52%) the post-treatment eGFR improved by ∼50%. Sixteen patients (76%) became dialysis independent within a median of 32 (range, 14–78) days from start of induction therapy. Of these, 12 patients obtained at least a MRrenal (P = 0.006) while < MRrenal was observed in the remaining 4 patients. The combination of a bortezomib-based induction regimen and HCOD yielded a fast and progressive decrease in both serum creatinine (Fig. 1) and deltaFLC levels (Fig. 2). More specifically, a 27% deltaFLC reduction was observed after the first session of HCOD and before bortezomib-based induction treatment was started. A more remarkable decrease in deltaFLC levels was seen after cycle 1 (93% reduction), cycle 2 (96% reduction) and cycle 3 (98% reduction) of induction therapy. After the last cycle of induction therapy, the overall deltaFLC reduction was 99%. Overall, 16 patients (76%) obtained > 90% deltaFLC reduction, 9 of them after cycle 1, 5 after cycle 2 and 2 after cycle 3. DeltaFLC reduction progressively increased with progressive improvement in the depth of hematologic response (Cuzick's trend test, P = 0.003).
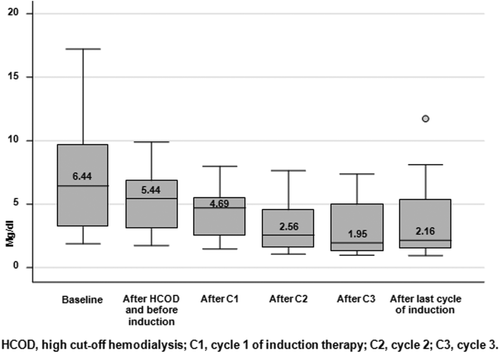
Serum creatinine reduction throughout cycles of induction treatment
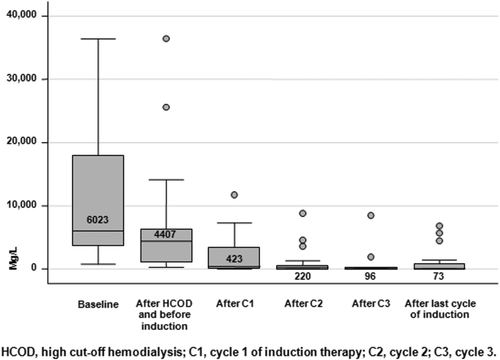
DeltaFLC reduction throughout cycles of induction treatment
Toxicity
Bortezomib-based induction treatment was well tolerated. The most frequent adverse event was peripheral neuropathy (PN), grade 1–2 in 57% of patients, grade 3 in 2 patients (9%). Three patients required bortezomib dose reductions from 1.3 to 1 mg sm−1 due to PN and in 1 of them a further dose reduction from 1 to 0.7 mg sm−1 was needed.
Hematological toxicity was uncommon; only a single patient who received VMP showed neutropenia grade 4 and thrombocytopenia grade 3, and needed a reduction in melphalan dose from 0.13 to 0.09 mg kg−1.
Progression-free and overall survival
With a median follow up of 17.2 (range, 11.3–44) months, the 3-year progression free survival (PFS) was 76% and the 3-year overall survival (OS) was 67%. No early deaths (e.g., within 60 days from diagnosis) were observed. Three patients died due to disease progression, after 26, 25, and 12 months, respectively, from the beginning of induction therapy. A correlation emerged between response to induction therapy (≥PR) and PFS (P = 0.03). Because of the small sample size, no possible correlation between baseline prognostic characteristics and hematologic response, renal response and clinical outcomes could be explored.
Discussion
RI is a frequent complication of MM which results primarily from a tubular-interstitial damage induced by FLC excretion. The excess of FLC causes direct injury to proximal tubular cells through the induction of pro-inflammatory cytokines (IL-6, IL-8, TNF) and the activation of the NF-kB pathway, leading to tubular cell death 20, 21.
The presence of RI in patients with MM is associated with poorer prognosis. In a large study of ∼3.000 patients enrolled in clinical trials between 1980 and 2002, RI and infection were the major causes of death occurring within the first 2 months after the diagnosis of MM. Therefore, all patients with MM and RI should be considered at high risk of early mortality during the first months of induction therapy 6, 22.
The cornerstone in the management of RI in MM is the achievement of a prompt and depth reduction in FLC load. A recent analysis of 1,773 newly diagnosed MM patients who were treated over different time periods, showed that the frequency of RI was unchanged from 1,990 to ≥2,005, while both hematological response and OS for patients with severe RI significantly improved over the past decade 8. This gain was due to the recent introduction into the therapeutic armamentarium for MM of novel, highly active, agents which have changed the natural history of MM.
The role of the novel agents thalidomide, bortezomib and lenalidomide in the management of MM patients presenting with RI was recently evaluated 23. A significant improvement in renal function (≥PRrenal) was observed in 77, 55, and 43% of patients treated with bortezomib, thalidomide and lenalidomide, respectively. In a multivariate analysis, bortezomib-based therapies were independently associated with a higher probability of renal response, compared with thalidomide- or lenalidomide-containing treatments. In addition, for patients treated with bortezomib, renal response occurred faster than with other drugs, the median time to PRrenal being 1.3 months versus 2.7 months for thalidomide- and more than 6 months for lenalidomide-treated patients.
Based on the above mentioned results, a bortezomib-containing regimen is actually considered in many centers the treatment of choice for MM patients presenting with RI. Advantages of bortezomib include not only the remarkable and fast anti-MM activity, but also its ability to reduce inflammation in MK and protect tubular cells through the effect on the NF-kB pathway. Furthermore, bortezomib can be administered at the full approved dose in patients with RI, its safety profile being superimposable in patients with and without renal failure 24, 25. In a sub-study of the VISTA trial comparing VMP with melphalan-prednisone (MP), the CR rate and TTP were significantly better with VMP versus MP, and no differences in the VMP arm were observed across the renal cohorts with creatinine clearance values above and below 50 mL min−1 26. In the HOVON-65/GMMG-HD4 trial, patients were randomized to receive 3 cycles of either vincristine, adriamycin, and dexamethasone (VAD) or bortezomib, adriamycin and dexamethasone (PAD) before a single or double ASCT, followed by maintenance with either thalidomide in the VAD arm or bortezomib in the PAD arm. Patients with a baseline serum creatinine ≥2 mg dL−1 who were randomized in the VAD arm had a significantly shorter 3-year OS (34%) than those in the PAD arm (74%). OS for these latter patients was not influenced by the presence or absence of RI 27.
Based on the knowledge that tubular-interstitial damage in MM is secondary to elevated FLC excretion, recent studies have investigated the impact of direct FLC removal on renal recovery. However, whether direct extracorporeal removal of FLC can provide an additional clinical benefit compared to the current standard of care still remains an open issue. Evidence regarding the role of plasma exchange has been conflicting 28-30.
A new generation of hemodialysis membranes with larger pores and higher molecular weight cut-off, averaging the 65 kDa value which is closer to that of the native kidney, were recently created to allow a more effective removal of FLC. Several case reports and a small open-label study of chemotherapy combined with HCOD have been published so far 13. A prospective, randomized trial comparing FLC removal hemodialysis versus standard high flux hemodialysis, both combined with a modified PAD regimen, is currently ongoing 31. In a series of 19 patients with MK and severe acute RI requiring dialysis, recovery of renal function to dialysis independence was seen in 74% of cases after chemotherapy and HCOD 13. However, 75% of these patients were newly diagnosed and only 58% received bortezomib as part of therapy. More recently, the combination of HCOD with bortezomib-based therapy was evaluated in a limited series of 7 patients with newly diagnosed MM and acute RI 32. All but one of them became dialysis independent after therapy, suggesting that HCOD is highly effective in the management of MM and RI.
To the best of our knowledge, the present study is the first one in which a consecutive series of newly diagnosed MM patients with severe RI, mostly due to biopsy-proven MK, were prospectively treated with HCOD combined with either VTD or VMP which are a standard of care for ASCT-eligible or not eligible MM patients. All the patients enrolled in our study had acute kidney injury (AKI), a broad clinical syndrome for which there is an actual lack of consensus on the optimal classification and staging for severity. Because of this reason, and accordingly to other studies, we chose to assess RI by eGFR calculated using CKD-EPI creatinine equation. However it is worth noting that this system is the best tool to measure renal function in patients with chronic kidney disease (CKD) and stable creatinine concentration 17. Differently from the indications in CKD, hemodialysis in AKI is performed before the fall of eGFR to less than 15 mL/min/1.73 m2. Accordingly, in our series of patients, HCOD was started regardless of the degree of RI and was mainly aimed to remove the burden of sFLC, and correct severe metabolic imbalance and/or oligo-anuria.
In comparison to other studies, the present series was characterized by a more severe degree of RI, the frequency of grade 4–5 RI being as high as 86%. This finding makes more difficult a proper evaluation of the added value of HCOD combined with bortezomib-based induction therapy compared to previously reported studies not including HCOD.
Hematologic response occurred frequently and was of high quality in most of the patients, the ORR being 81%, including 19% sCR and 67% ≥VGPR. In addition, response was fast, occurring within a median of 1.4 months. Remarkably, the combined approach of bortezomib-based therapy and HCOD resulted in a fast improvement in renal function and a dramatic decrease in deltaFLC, up to 98% reduction after the third cycle of induction therapy. Sixteen patients (76%) became dialysis independent in a median time of 32 (range, 14–78) days, and 48% had a decrease in their creatinine levels below the threshold of 2 mg dL−1. Overall, the rate of CRrenal was 14%, a value lower than that reported in other studies, again reflecting the more severe degree of RI seen in our series of patients. Because of the small sample size and the great inter-individual variability, it was not possible to find a relationship between the number of HCOD sessions performed and renal response.
With a median follow up of 17.2 months (range, 11.3–44), the 3-year estimated PFS and OS were 76 and 67%, respectively. No early deaths were observed. Three patients died due to progressive disease after 26, 25, and 12 months from the beginning of induction therapy.
Overall, the combined approach of bortezomib-based therapy and HCOD was well tolerated, grade 3 PN being the most relevant toxicity, which was reported in 9% of patients.
The major limitation of this study is represented by the small sample size that precluded to find out clear correlations between hematologic response and renal response, or between renal response and clinical outcomes, as well as to identify the most relevant prognostic factors. By the opposite, major strengths were that all enrolled patients were rigorously assessed both before and after the start of therapy, the majority of them underwent a kidney biopsy to confirm the diagnosis of MK and all were homogeneously treated with bortezomib-based regimens according to their ability to receive or not ASCT. Extremely encouraging results herein reported support the design of further studies aimed at confirming the benefits of this combined approach in larger series of patients.
In conclusion, in MM patients presenting with severe RI the combination of a bortezomib-based induction therapy with HCOD is a remarkably effective strategy to quickly reduce tumor cell mass, including sFLC burden, and improve or rescue RI, ultimately leading to improved clinical outcomes.




