Monoclonal anti-transferrin antibody: A paradigm for better understanding of iron metabolism
Conflict of interest: Nothing to report.
An 82 years old patient was admitted for persisting abnormalities of iron metabolism parameters in the context of IgG kappa monoclonal gammopathy, discovered 12 years before, and classified as monoclonal gammopathy of undetermined significance (MGUS). Serum iron and transferrin concentration were constantly extremely high (96.9 µmol/L and 5.0 g/L, respectively), transferrin saturation was elevated (77.5%; N<45%), and serum ferritin moderately increased (608 µg/L; N<300 µg/L). Hemochromatosis was ruled out 1: no tissue iron overload was present and genetic studies searching for mutations in HFE and non-HFE genes were negative (p.Cys282Tyr mutation, HJV (HFE2), HAMP, TFR2, BMP6, SLC40A1, and FTL). The diagnosis of monoclonal anti-transferrin antibody was therefore highly suspected. A more in-depth study of iron metabolism was performed to get a better understanding of the disease.
According to Alyanakian et al. 2, we observed (Fig. 1A) Tf in the serum and in the IgG eluate fraction after affinity precipitation, confirming the presence of IgG directed against transferrin. Furthermore, the addition of purified transferrin in the serum prior to immunoglobulin affinity purification increased the amount of purified immunoglobulin transferrin complexes, suggesting the presence, in the serum, of unbound anti-transferrin IgG. To further characterize transferrin, its sialylation was studied. The basic electrophoretic profile (Fig. 1B) did not provide interpretable information. However, after immunoprecipitation of transferrin–IgG complexes, the remaining free transferrin glycoforms were, as expected, clearly found (Fig. 1C). The very high levels of serum iron and transferrin concentration suggest that the transferrin–IgG interaction could modify the ability of transferrin to link iron. Therefore, the increased transferrin concentration could be a compensatory mechanism aiming to maintain a normal affinity of the immunoglobulin-free transferrin for iron. Little is known on the mechanisms which determine transferrin synthesis, in contrast with the molecular regulation of transferrin receptor 1 synthesis. It could be proposed that the IgG–transferrin complex generates a form of acquired functional hypotransferrinemia triggering, in turn, transferrin oversynthesis by the hepatocytes. Another mechanism could be the development of an iron-deficiency like status. Indeed, in agreement with Forni et al. who found decreased urinary or serum hepcidin levels in two of their three patients 3, the hepcidin/ferritin ratio was strongly decreased (<0.1–N:4–30). These results closely remind the HFE genetic hemochromatosis profile, a condition related to a decoupling between iron status (including transferrin saturation) and hepcidin expression 4. In the case of circulating anti-transferrin monoclonal antibody, a disturbed interaction between transferrin iron and transferrin receptor 1 could impede iron delivery to cells, thus creating cellular iron deprivation, a condition leading to an increase of transferrin synthesis. We, therefore, explored whether the transferrin–IgG interaction disturbed the stability of the transferrin–iron complex. This was approached by quantifying abnormal forms of iron in serum. On the one hand, normal non-transferrin bound iron (NTBI) 5 concentration (<0.5 µmol/L), despite high transferrin saturation, suggested that the serum transferrin increase ensured the control of all the iron present in the serum. On the other hand, high labile plasma iron (LPI) 6 concentration (0.92 µmol/L–N < 0.5 µmol/L) despite normal NTBI suggested that unstable iron species, that can be engaged in oxidative stress, was present in the serum. This could be related to a weakened interaction between iron and transferrin, favored by transferrin–IgG interaction. This observation provides strong support to the fact that NTBI and LPI do not cover similar biochemical forms of iron. In this patient, no evidence of visceral iron overload or iron-related toxicity was observed. It should be noted that iron overload is inconstant in this syndrome.
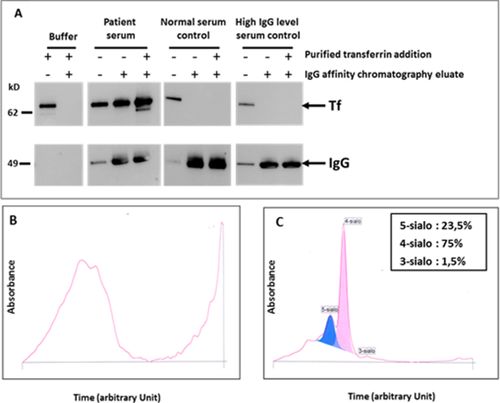
Characterization of the transferrin (Tf)/anti-transferrin immunoglobulins complex in the patient serum. Patient serum was first analyzed by Western blot using anti-transferrin and anti-IgG antibodies (A) prior or after affinity chromatography for immunoglobulin IgG (control sera were used for comparison) and secondly analyzed by capillary zone electrophoresis system before (B) and after (C) immunoprecipitation with specific anti- IgG, IgA, and IgM in order to determine transferrin glycoform pattern. (see methods in Supporting Information). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In conclusion, the observed abnormalities of iron metabolism in this further case of anti-transferrin monoclonal antibody raise important questions on the way this type of monoclonal immunoglobulin impacts iron status, especially in altering transferrin–iron delivery to cells and in favoring the production of reactive plasma iron.




