A biomimetic microfluidic chip to study the circulation and mechanical retention of red blood cells in the spleen
Conflict of interest: Nothing to report
P.A.N. was supported by the laboratory of Excellence GR-Ex.
Abstract
Red blood cells (RBCs) are deformable and flow through vessels narrower than their own size. Their deformability is most stringently challenged when they cross micrometer-wide slits in the spleen. In several inherited or acquired RBC disorders, blockade of small vessels by stiff RBCs can trigger organ damage, but a functional spleen is expected to clear these abnormal RBCs from the circulation before they induce such complications. We analyzed flow behavior of RBCs in a microfluidic chip that replicates the mechanical constraints imposed on RBCs as they cross the human spleen. Polymer microchannels obtained by soft lithography with a hydraulic diameter of 25 μm drove flow into mechanical filtering units where RBCs flew either slowly through 5- to 2-μm-wide slits or rapidly along 10-μm-wide channels, these parallel paths mimicking the splenic microcirculation. Stiff heated RBCs accumulated in narrow slits seven times more frequently than normal RBCs infused simultaneously. Stage-dependent retention of Plasmodium falciparum-infected RBCs was also observed in these slits. We also analyzed RBCs from patients with hereditary spherocytosis and observed retention for those having the most altered mechanical properties as determined by ektacytometry. Thus, in keeping with previous observations in vivo and ex vivo, the chip successfully discriminated poorly deformable RBCs based on their distinct mechanical properties and on the intensity of the cell alteration. Applications to the exploration of the pathogenesis of malaria, hereditary spherocytosis, sickle cell disease and other RBC disorders are envisioned.Am. J. Hematol. 90:339–345, 2015. © 2015 Wiley Periodicals, Inc.
Introduction
Mature red blood cells (RBCs) are non-nucleated, biconcave disc-shaped cells of 7–8 μm in diameter, 2-μm-thick with an average volume of 80–100 μm3 1. Normal circulating RBCs are highly elastic and deformable and flow through capillaries narrower than their own size. When RBC deformability is altered there is a risk of RBC blockade in small capillaries 2. However, the most stringent mechanical challenge takes place in the spleen when RBCs cross narrow inter-endothelial slits in the wall of red pulp sinuses 3, 4. In theory, a functional spleen clears the blood from mechanically altered RBCs before they are trapped in microvessels, where they would create tissue damage. Altered mechanical properties can be originated by multiple inherited RBC disorders, such as spherocytosis or sickle cell disease 5, or by infectious agents such as Plasmodium falciparum 6. Although these diseases result from very different molecular or cellular alterations they share the generic mechanism of poorly deformable RBCs triggering complications, contributing for example to coma during severe Plasmodium falciparum malaria and anemia in hereditary spherocytosis (HS) 7.
An important technical gap to study the pathophysiological impact of impaired RBC circulation is a device mimicking the dimensions of key microcirculatory structures 8. A physiologically relevant device should mimic 4- to 10-μm-wide small vessels (capillaries and small venules), and 0.5- to 3-μm-wide microcirculatory beds in the spleen 3, 9. Inter-endothelial slits in human spleens are typically in the 1-μm-wide range, as measured by transmission electron microscopy 3. To mimic the RBC retention rates encountered in the human spleen a device should thus contain narrow spaces of ∼2 μm. To replicate the specific shape deformation of RBCs as they cross narrow splenic slits, in vitro obstacles should also be <10-μm long. With recent advances in microtechnology and in particular in microfluidics 10-12, it is now possible to envision “in vitro” experiments mimicking the microvascular flow in vital organs 13. Microchannels with dimensions of several micrometers molded in PDMS have explored how RBC deformability may impact the pathogenesis of capillary obstruction 14, and malaria 15. Microfluidic devices with wider microchannels (>30 μm) have opened the way to the real-time analysis of vaso-occlusion in sickle cell disease 16, 17. Most microfluidic approaches have quantified RBC deformability based on velocity through devices that mimic microcirculatory structures 15. An experimental system that quantifies retention rather than velocity may better reflect pathophysiological processes occurring in malaria or other RBC diseases that are associated with marked clearance of altered RBCs in the spleen, a process that contributes to the enlargement of the spleen.
A microfiltration device has been recently developed that uses microspheres to mimic the mechanical sensing of RBCs by the human spleen 18. This device provides relevant retention rates for poorly deformable RBCs 19, 20 but does not allow a direct observation of RBCs as they cross narrow slits. By contrast, most microfluidic devices allow a direct morphological and dynamic exploration of RBCs as they navigate in the chip. RBC deformability is a complex function involving membrane stiffness, cytoplasmic viscosity and surface-to-volume ratio 21. The deformability of P. falciparum-infected RBCs is even more complex 22. Fine analysis of the event as it occurs is likely the most powerful way to decipher the complex cellular processes with a filtering capacity large enough to allow robust quantification of subpopulation enrichment. We present here the design, set-up and validation of a new biomimetic microfluidic device where the retention of poorly deformable human RBCs can be robustly quantified and observed.
Methods
Patients
Small (0.5–1 ml) venous blood samples from 4 patients with hereditary spherocytosis were retrieved with patient approval from EDTA tubes within 2 hr of collection in the context of normal medical care. The « Ile de France VI » Institutional Review Board has approved this approach as a non-biomedical research process (Article L1121-1 of the French Code for Public Health) that does not affect the medical care. Venous blood samples from three controls were also obtained using a similar approach.
Microfluidic device fabrication
The spleen-like chip includes polymer microchannels with an average hydraulic diameter of 25 μm, including local mechanical filtering units with an equivalent behavior of apertures from 5 to 2 μm (Fig. 1A). Side flow, which is observable in the spleen, is rendered possible in the device, with side channels added in the filter zone (Fig. 1A). The microfluidic shape that defines the filtering functions of the device is casted in polydimethylsiloxane (PDMS, Sylgard), a silicone elastomer 23. The procedure to design the device is based on standard microfabrication techniques associated to molding techniques 24. We first fabricated a master using SU-8 photoresist (Microchem, Newton, MA) to obtain a negative mold on a 4 in. Silicon substrate (Fig. 1B). A two-step phase of photolithography with UV exposure had been developed. The first photolithography defines the microchannels with a uniform thickness of 5 μm and a geometry corresponding to the repeated mechanical solicitation and filter design. The second photolithography gives the fluidic accesses with a uniform thickness of 25 μm in order to reduce the access fluidic resistance. From the SU-8 mold, the casting of PDMS (ratio 10:1) channels is realized. The chip obtained is then irreversibly bounded to a microscope cover slide using plasma 02 activation (30 W, 300 mT, 20 s) on both surfaces. Before sealing, access holes were done in the PDMS with a homemade punch giving female Luer (TM) connection compatibility.
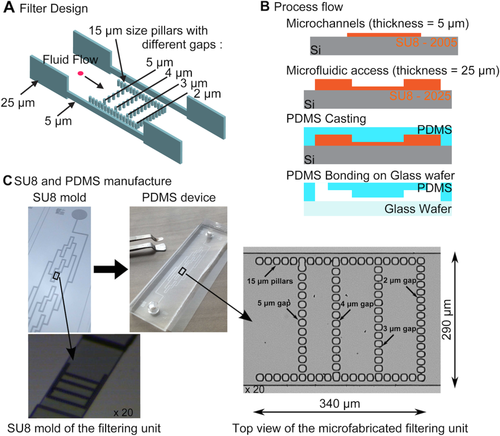
Design (A), process flow (B), manufacture (C) of the spleen-like chip. Each filtering unit comprises of fifty-three 2-µm-wide slits between the 15-µm pillars that form the boundaries of the lattice. The inner pillar lines comprise 12, 13, and 14 slits, the width of which is 5, 4, and 3 µm, from left to right. The U-shaped filter is 275-µm-wide (including pillars) included a single ×20 microscopic field captured by the camera sensor (18.6 × 6.7 mm2) Side channels are 10-µm-wide. The filtering unit is 5-µm-high. Microfluidic channels connecting the eight filtering units are 25-µm-high to minimize hydraulic resistance between filtering units. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The design is based on eight mechanical filtering units associated in parallel and connected together with a microchannel network as seen on the mold (Fig. 1C). To reduce the hydraulic resistance of the full design, the microchannel network is 25-μm-high compared to the 5-μm height of each filtering unit. The obtained microfluidic device is fully transparent which makes possible the use of conventional inverted microscopes.
Red blood cell preparation
Cell suspensions: Packed RBCs from the French blood bank (Etablissement Français du Sang, Rungis, France) were washed twice with 4 volumes of RMPI 1640 (Life technologies, France) and used for further preparation.
Heated RBCs: Washed RBCs were suspended in PBS 1% AlbuMAX II (Life Technologies, France) and then heated for 15 min at 50 °C in a water bath followed by two washes with PBS 1% AlbuMAX II (Life technologies, France).
PKH labeling: RBCs were stained with PKH 67 fluorescent Cell Linker Kit (heated or HS RBCs) or PKH 26 fluorescent Cell Linker Kit (control non-heated RBCs) according to the manufacturer's instructions (Sigma–Aldrich, France).
Infected RBCs: FUP line Plasmodium falciparum parasites (FCR3) was used for all experiments. Parasites were cultured following Worldwide Antimalarial Resistance Network (WARN) procedures in 5% hematocrit, at 37 °C, with gas mixture: 5% CO2, 10% O2 and 85% N2, and RPMI 1640 media containing Hepes 25 mmol l−1, NaHCO3 25 mmol l−1, Glutamine 0.3 g l−1, Gentamycin 10 mg l−1, and Serum 10%. Cultures were synchronized by sorbitol method (Lambros C, Vanderberg JP. 1979) and then by VarioMACS Separator (Miltenyi Biotec, France) to obtain different, tightly synchronized stages of maturation.
Parasites staining: Synchronized ring-infected RBCs (8–16 hr) were stained with Hoechst dye while trophozoites (26-34h) were stained with SYBR Green (Hoechst and SYBR Green stain the nucleic acid of DNA, Life technologies, France). RBC suspensions were prepared with the following composition: 5% of labeled ring-infected RBCs, 5% of labeled trophozoite-infected RBCs and 90% of control unlabeled RBCs. A 0.1% hematocrit RBC suspension in PBS 1% AlbuMAX II was prepared with this cell mixture before being perfused in the microfluidic device.
The microfluidic system setup
The microfluidic system consists of the microfluidic device, tubing, fluid connectors, syringe and syringe pump (Syramed SP6000, Arcomed'Ag, Regensdorf, Switzerland) or MFCS™-EZ microfluidic flow control system (Fluigent, France). Tubes and fluid connectors were used to connect the syringe to the chip. The syringe was filled with RBC suspensions and RBCs were perfused through the microchannel network at a specified flow rate (0.2 ml h−1 and a mean of 100 mBar). The device was placed in an inverted Zeiss AxioObserver Z1 microscope for monitoring RBC movements. Pictures of the eight filtering units were taken sequentially and at regular time intervals with a high resolution AxioCam MRm Rev.3 camera and AxioVision 4 analysis software (Carl Zeiss). Pictures of the whole filtering unit were taken using the 20×/0.45 Plan-Apochromate objective; smaller zones are imaged at higher magnification using the 63×/1.40-Oil Plan-Apochromate objective (Carl Zeiss). Blue, green and red fluorescent cells were visualized using the Colibri LED 365, 470, and 555 nm modules (Carl Zeiss), respectively.
Electronic microscopy of a human spleen
For the sizing of the microfluidic chip, samples of human spleen were processed as described 25 then examined and photographed with a JEOL JSM 6700F field emission scanning electron microscope operating at 5 or 7 kV (images were acquired with the upper SE detector on the lower secondary sector), or with a JEOL 1200 EX electron microscope operating at 80 kV.
Ektacytometry analysis
The ektacytometer 26 (LoRRca MaxSis, Mechatronics®, Hoorn, Netherlands) is a laser diffraction viscometer, in which the deformation of red cells suspended in a viscous PVP solution at defined values of applied shear stress of 30 Pa and at a constant temperature measurement of 37 °C are monitored as a continuous function of suspending medium osmolarity. Blood samples from 4 HS patients and three healthy controls were exposed to increasing shear stress and an osmotic gradient. Three distinct features of the osmotic gradient ektacytometry profiles are the Omin, the DImax and the O'hyper points. The Omin point corresponds to the osmolarity at the minimal deformability in hypoosmolar area or at the osmolarity when 50% of the red cells hemolyzed during the regular osmotic resistance test. It reflects the surface to area ratio. DImax corresponds to the maximal deformability index or elongation index (EI). The hyper point or O' corresponds to the osmolarity at half of the DImax and reflects the hydration state of the cells. In case of HS 27, 28, the constant and characteristic features are a decrease in the DImax, in conjunction with a shift of the Omin point to the right (reduced surface to volume ratio) and a shift of the O'hyper point to left (increased dehydration of the red cells). The severity is correlated to the level of the DImax.
Results
Size of human spleen microcirculation
The dimensions of an interendothelial slit were determined from a transmission electron microscopy picture (Supporting Information Fig. S1A). The length and width of this slit were graphically estimated as 1.8–2 μm and 4.5 μm (Supporting Information Fig. S1B), respectively, i.e., similar to previous measurements 3, 4. We used these values for the width and length of the narrowest slits in the filtering part of the chip.
From in silico to in vitro validation of physiological flow through the chip
The whole dimensions of the microfluidic biomimetic filter were optimized using 3D numerical calculation based on finite element analysis (Comsol©), with the goal to obtain 80% of the cell flow passing in the slit-shaped central zone that mimics the slow open microcirculation of the human spleen; the 10-µm-wide side channels mimic the closed fast microcirculatory pathways (Supporting Information Fig. S2A).
The microfilter hydraulic resistance, calculated from the numerical simulation, was 6.13 × 1014 Pa m−3 s−1. Such a high resistance value affects considerably the fluidic properties within the device. Indeed, a cell solution flowing at 1 μl mn−1 within the device will induce 100 mbar pressure drop, while the average cell velocity will be 1.1 cm s−1. The complete microfluidic structure, including eight filtering units set in parallel and access channel, presents 2.4 × 1014 Pa m−3 s−1 as hydraulic resistance value 29.
On Supporting Information Fig. S2B, the velocity flow behavior inside the chip is represented, close to the filter. It had been obtained with an applied pressure of 100 Pa. The fluid velocity inside the filtering unit is different in the inner slit-shaped zone and in the side channels.
The velocity profile (perpendicular to the flow, Supporting Information Fig. S2C) obtained in the filter highlights the difference of velocity value between the side channels and the inner zone of the filtering unit. The average ratio between these velocities was calculated to be 4.5 with the chosen geometry, which was further confirmed by following experiments. Such velocity ratio between fast and slow circulations is reminiscent of observations in isolated-perfused spleens 3, 4.
Mechanical retention of rigid heated red blood cells
The capacity of the microfluidic chip to retain poorly deformable RBCs was tested. RBC rigidity was induced by heating the cells at 50 °C for 15 min. This reduces their size, induces their sphericity and increases their irreversible stiffness 30. Heated and control RBCs were then fluorescently labeled with PKH67 (green) or PKH26 (red), respectively. A RBC suspension at 0.1% hematocrit, containing equal amounts of heated and control RBCs (Fig. 2A), was perfused through the device. RBC retention was monitored over time in the eight filtering units. Pictures were taken at different pre-set time points during the experiment. For each filtering unit images were captured in the green, red and brightfield channels and merged. A picture was also taken before perfusing the RBC suspension in order to confirm that the filtering units where not blocked by debris (Fig. 2B-T0). Early pictures showed that stiff heated RBCs (green-labeled) but not normal (red-labeled) RBCs were retained preferentially in the 2 μm-wide slits (Fig. 2B-T1; T2; T3; T4 and C). The number of stiff heated RBCs retained in slits increased over perfusion time, with the vast majority of the 2-μm-wide slits being clogged by these RBCs at the end of the assay (Fig. 2B-T4).
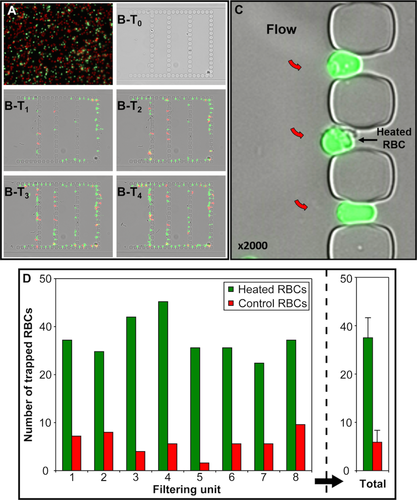
Preferential retention of poorly deformable heated RBCs in slits of the filtering unit: (A) Equal concentrations of PKH26-stained normal (red) and PKH67-stained poorly deformable heated RBCs (green) in the RBC suspension perfused through the chip. (B) T1→T4 progressive accumulation of poorly deformable heated RBCs in slits: 4 (T1), 8 (T2), 12 (T3), 16 (T4) minutes after initiation of RBC circulation through the filtering unit (flow is from left to right). (C) Ellipsoid or quasi spherical aspect of heated RBCs retained in 2-µm-wide slits (flow is from left to right, red arrows). (D) Number of poorly deformable heated RBCs (green) and normal RBCs (red) retained in narrow slits of each of the eight filtering units in a chip, and mean values (right panel). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
At the experiment endpoint, retained RBCs present in each zone were counted and sorted depending on their fluorescence (Fig. 2D). At the end of the assay, poorly deformable heated RBCs were seven times more abundant in the filtering unit than control RBCs (Fig. 2D, right panel) and often had a quasi-spherical aspect (Fig. 2C). Filtering units could thus discriminate between deformable and non-deformable RBCs, and could reveal the presence of subpopulations of rigid RBCs in a suspension.
Retention of red blood cells parasited by plasmodium falciparum
The ability of the chips to retain poorly deformable RBCs was further tested using RBCs infected with Plasmodium falciparum. P. falciparum proteins (RESA, KAHRP, PfEMP3) exported at the membrane of infected RBCs contribute, among other factors 22, 25 to their stage-dependent decrease in deformability 31, 32. To test the differential retention of infected RBCs, we perfused the biochip with a cell suspension at 0.1% hematocrit containing 3 different RBC populations: 5% of blue-labeled ring-infected RBCs, 5% of green-labeled trophozoite-infected RBCs and 90% of control non-infected unlabeled RBCs (Fig. 3A).
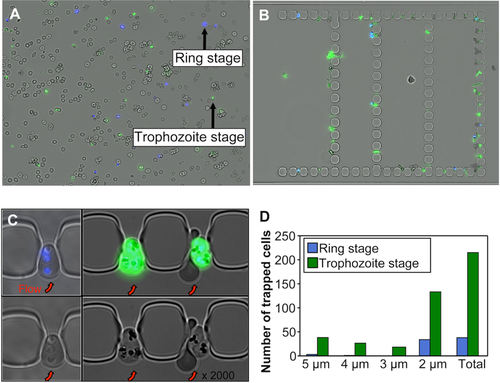
Stage-dependent retention of Plasmodium falciparum-infected RBCs in narrow slits. (A) Image capture of RBC suspension at 0.1% hematocrit containing subpopulations of young ring-infected RBCs (rings) and late trophozoite-infected RBC, each at 5% parasitemia. Ring-infected RBCs were stained with Hoechst dye (blue) and triphozoites were stained with SYBR Green (green). (B) Retention pattern of infected RBCs in the chip at the end of the experiment. (C) High magnification of infected RBCs trapped in 2-µm-slits (flow in the chip is from bottom to top, red arrows). (D) Number of rings- or trophozoite-infected RBC trapped according to the size of the slits at the end of experiment. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
RBC retention was monitored every 4 min in the eight filtering units of the chip. For each filtering unit images were captured in the blue, green and brightfield channels and merged. They showed that infected RBCs (blue- and green-labeled RBCs) were preferentially retained in the slits as compared to non-infected control RBCs (unlabeled) (Fig. 3B,C). We analyzed the retained infected RBC sub-populations and found that the mature trophozoites (26–34 hr of maturation) accounted for a higher proportion of retained RBCs than young ring-infected RBCs (8–16 hr of maturation after reinvasion). Indeed, the retained RBCs were composed of 85% (215/253) of green-labeled trophozoites and 15% (38/253) of blue-labeled ring-infected RBCs (Fig. 3D). Furthermore, during the first minutes of perfusion, the ring-infected RBCs were preferentially retained in the 2-µm slits while trophozoites were retained in slits of all sizes (from 2 to 5 µm), consistent with the differences in the RBC mechanical properties between these two infection stages.
Thus, despite the relatively low proportion of infected RBCs in the initial RBC suspension, the spleen-like microfluidic device successfully discriminated infected RBCs from non-infected RBCs, as well as young-infected from the late-infected RBCs, based on their distinct mechanical properties and on the intensity of the mechanical alteration.
Retention of red blood cells from patients with hereditary spherocytosis
We next explored another pathophysiological model using RBCs from patients with hereditary spherocytosis (HS). In HS, RBCs are poorly deformable because of mutations in RBC membrane proteins that affect the vertical interactions between the lipid bilayer and the membrane skeleton, negatively impacting membrane cohesion and leading to surface loss.
We analyzed RBC samples from 4 HS patients. HS and control RBCs were fluorescently labeled with PKH67 (green) and PKH26 (red), respectively, and perfused in the microfluidic chip as described above for heated RBCs. RBCs of two patients (P1 and P2) were retained in the chip, preferentially in 2-µm slits (Fig. 4A), while those from the 2 other patients (P3 and P4) were not. We hypothesized that this difference resulted from the known heterogeneity among HS patients. As a matter of fact, HS patients exhibit heterogeneous phenotypes depending on their mutated protein(s), the disease severity being primarily dependent on the extent of membrane surface area loss 33, 34. We evaluated the deformability and the surface area loss of the 4 HS RBC samples by osmotic gradient ektacytometry profiles, measuring three parameters: 1 the maximal deformability index (DImax) value, which represents the maximal cellular deformability of the RBC population and 2 the Omin point, which corresponds to the osmolarity at which 50% of the RBCs are lysed in the classical osmotic fragility test and reflects the reduction in surface area to volume ratio of the RBC population being analyzed (a shift to the right of the Omin corresponding to a reduced surface-to-volume ratio), and 3 the O' or the hyper point, which reflects the hydration state of the red cells. As expected, in conjunction with the presence of spherocytes on the blood smears, all HS RBC samples exhibited the classical features of HS when compared with control RBCs (Fig. 4B). In addition, the two RBC samples from P1 and P2, that were retained in the microfluidic chip, showed lower DImax and higher Omin than those from P3 and P4 (Fig. 4B), indicating that these RBCs were less deformable and more spherical (reduced surface-to-volume ratio) than the two others and that the microfluidic chip only retained HS RBCs displaying the greatest phenotypic alterations.
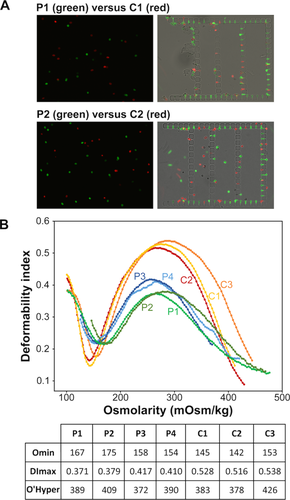
Microfluidic and ektacytometry analyses of RBCs from hereditary sperocytosis patients. (A) RBC suspensions with equal concentrations of PKH26-stained control (red) and PKH67-stained HS RBCs (green, left panels) were perfused through the chip. HSRBCs from patients P1 and P2 were preferentially retained in the filtering units (right panel). (B) Ektacytometry curves of four HS patients (P1, P2, P3, and P4) and three control (C1, C2, and C3) and a table showing the values of Omin, Dlmax, and O'Hyper for each RBC sample. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Discussion
We developed a simple microfluidic device to study and quantify the mechanical ability of RBCs to cross the human spleen. We favored a simple chip design that would reproduce key microcirculatory structures of the human spleen and display a biomimetic behavior. These include unique, narrow filtering slits localized at the exit of a slow circulatory pathway, and a wider fast circulatory pathway into which RBCs are derived when slits are saturated. This fluidic architecture was initially designed with numerical simulations using 3D finite element analysis. Soft-lithography molding techniques were precise enough to generate PDMS pillars delineating slits as narrow as 2 µm, the channel height being 5 µm. Relatively large slits (3- to 5-μm wide) are predicted to exert mechanical constraints on RBCs similar to those generated by the tortuous structures of the cords in the splenic red pulp (also called reticular meshwork 35). The narrowest 2 µm-wide slits mimic the last and most stringent mechanical obstacle that RBCs must cross on their way from the cords back to the venous circulation 18. The width of side channels mimicking the fast circulation were set at 10 µm to allow RBC circulation with minimal mechanical constraints. A biomimetic spleen-like device has been recently reported, beautifully mimicking the closed-fast and the open-slow microcirculations, the latter ending by a row of a dozen of parallel 2-μm constrictions resembling the inter-endothelial slits. Dynamic crossing could be observed but the number of slits was too limited to robustly quantify enrichment of poorly deformable subpopulations 36. Our device includes eight filtering units per chip, each containing more than fifty 2-μm-wide slits. This offers 400 slits into which mechanical retention can be quantified with statistical robustness. Wider slits upstream from these slits allow an estimate of the intensity of RBC stiffness; mildly altered RBCs are retained only in 2-μm slits while markedly altered RBCs accumulate in slits of all sizes. Using hydrogel micro-particles that mimic the mechanical behavior of RBCs 37 a more precise determination of retention thresholds should be achievable.
The pathophysiological relevance of this spleen-like microfluidic device was established using several subpopulations of poorly deformable RBCs. A marked accumulation in very stiff heated RBCs was observed in slits, a result perfectly consistent with the previous observation of their clearance from the peripheral blood and accumulation in the spleen of patients 38. Retention of RBCs parasitized by early and late asexual stages of P. falciparum was then analyzed. A selective retention of both parasitized subpopulations was observed, albeit with a stage-dependent variation in intensity. While RBCs parasitized by mature trophozoite stages were, as expected, retained in slits of all sizes (2–5 µm), RBCs parasitized by ring stages were retained almost exclusively in the narrowest 2-μm-wide slits that mimic inter-endothelial splenic slits in human spleens. In full agreement with these results, complete and partial retention of trophozoite- and ring-infected RBCs (respectively) had been previously observed in an ex vivo human spleen perfusion 25. Interestingly, ring-infected RBCs—the deformability of which is mildly altered 39—were retained only partially in human spleens 29 and accumulated selectively upstream from interendothelial slits. Behavior of poorly deformable RBCs from different subpopulations in the chip thus corresponded to expectations from previous observations in vivo and ex vivo. This biomimetic behavior was further confirmed by the ability of the chip to accumulate poorly deformable RBCs from HS patients. In this genetic hereditary disease patient RBCs exhibit a wide spectrum of altered mechanical properties because of mutations in proteins that sustain the interactions between the lipid bilayer and the skeleton 33, 34. The direct consequence of such alterations is a surface area loss of variable extent with a direct impact on the RBC surface-to-volume ratio that was shown to play a critical role in RBC splenic entrapment. This had been demonstrated using a model of normal human spleens perfused ex vivo with RBCs displaying various degrees of surface area loss after treatment with increasing concentrations of lysophosphatidylcholine 40. The study showed splenic entrapment for RBCs with > 18% of surface area loss (>27% reduced surface area-to-volume ratio) and identified an adaptive volume regulation mechanism that allows spherocytic RBCs to prolong their life span in circulation by loosing volume thus escaping splenic retention. Partial retention of RBCs from HS patients had also been observed in this human spleen model 18. In agreement with these observations our chip discriminated between HS RBC samples by trapping those with the lowest surface-to-area ratio (corresponding to the highest Omin) confirming its spleen-like function and emphasizing its potential use as a sensor of poorly deformable RBCs in the blood circulation. Further technical improvement is envisioned whereby high-speed video will analyze the fate of the first few hundred RBCs entering the chip. This complementary approach will allow expression of results as retention rates, in a chip not yet saturated by retained poorly deformable RBCs.
The spleen-like microfluidic device mimicking the RBC filtering function is a promising tool for the exploration of RBC disorders associated with mechanical alterations. Moreover it provides precious real-time information of the RBC mechanical behavior in highly constrained microfluidic. Indeed, the RBC mechanical rigidity and membrane abnormalities contribute to occlusion of small vessels and splenic microcirculatory beds 41. In RBC disorders such as sickle cell disease, pathological interactions between blood cells, endothelial cells and proteins of the extracellular matrix lead to vaso-occlusion events and splenic sequestration crises. To date, very few reports have designed chip models to investigate microcirculatory behavior of RBCs in relevant geometrical contexts. Successful experiments in three complementary pathophysiological situations establish the relevance of the device and show the robustness of a quantified approach of RBC retention. This opens the way for future fruitful experiments and applications. Another advantage of the spleen-like chip resides in its ability to be functionalized with purified proteins or endothelial cells and adapted to fit with specific pathological disorders in which RBCs exhibit abnormal deformability.
Finally, in addition to applications in the RBC field our device opens new perspectives to investigate nuclear rheology and the trafficking of nucleated cells across microporous barriers. Nuclear viscoelasticity was shown to be dependent on the expression level and the ratio between lamins A and B and might account for the retention of hematopoietic cells, such as erythroblasts and megakaryocytes in the bone marrow 42. Furthermore, the entrapment of mesenchymal stem cells in the lungs following their intravenous administration 43 might partly result from mechanical constraints and this could be evaluated and measured using our microfluidic device.
Acknowledgments
The authors thank Selin Topçu for her help during the fabrication phase of the biodevices.




