Exploring the amyloid proteome in immunoglobulin-derived lymph node amyloidosis using laser microdissection/tandem mass spectrometry
Conflicts of interests: All authors have no conflicts to disclose.
Abstract
Amyloidosis affecting lymph nodes (LN) may occur in the setting of systemic amyloidosis or as an entity localized to the site of production (peritumoral). Why some LN amyloid remains peritumoral is unknown. We speculated that the composition of amyloid in these two presentations differs. We analyzed the amyloid proteome in LN amyloid samples to identify differences between the systemic and peritumoral subtypes. In immunoglobulin-derived LN amyloidosis (N = 26), 70% had heavy chain amyloid (AH or mixed AH/AL). True localized LN amyloidosis was rare, with only 2 patients without a monoclonal protein component. Nineteen patients (73%) had typical amyloid syndromes (100% of AL vs 67% of AH/AL, P = 0.02). A trend to improved survival for the AH/AL group in comparison to AL (median 5-year survival 48 vs. 19 months, P = 0.06) was seen. Mass spectrometric amyloid analysis is a powerful tool for characterizing amyloid and may provide additional prognostic information. Am. J. Hematol. 88:577–580, 2013. © 2013 Wiley Periodicals, Inc.
Introduction
The amyloidoses are heterogenous diseases associated with the deposition of insoluble protein or peptides extracellularly, with a characteristic cross-beta diffraction pattern 1. Occasionally, amyloid deposition has been identified in lymph nodes (LN). These patients may have a lymphoma-associated amyloidosis typically characterized by an IgM monoclonal protein 2-4 and low grade lymphoproliferative disease, specifically lymphoplasmacytic lymphoma and extranodal marginal zone lymphoma 5-7. The patterns of lymphoma-associated amyloidosis may be localized to the site of amyloid production (peritumoral), or may disseminate to distant organs causing specific amyloid syndromes and systemic amyloidosis 8. Patients with the former tend to have less life-threatening disease as opposed to the latter 8, and therefore this distinction is important. Why some amyloid remains localized while others evolve into systemic disease is yet unknown. We speculated that the composition of the amyloid may differ between the peritumoral and systemic forms. We therefore performed a mass spectrometry (MS)-based proteomic analysis of cases of LN amyloidosis to identify differences in the amyloid proteome between the two forms.
Material and Methods
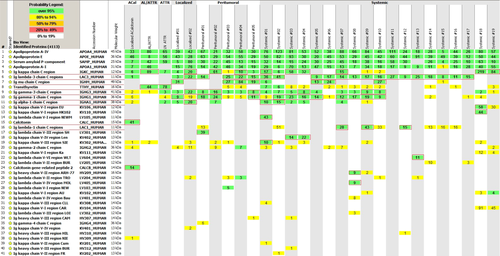
This study was approved by the Mayo Foundation Institutional Review Board, and conducted in accordance with Minnesota state regulations. We queried our Dysproteinemia database to identify patients with amyloidosis detected in LN at the Mayo Clinic, Rochester. Between May 1969 and October 2011, 44 patients were identified with amyloidosis involving one or more LN. We were able to obtain LMD/MS analysis in 30 cases, where the LN tissue block was available. Clinical data, including pathologic diagnosis was abstracted from patient charts. Patients without a circulating monoclonal protein (detected by immunofixation of serum and urine and serum free light chain assay) were classified as “localized.” We then defined amyloid involvement restricted to the sites of detectable lymphoma (e.g., LN, bone marrow) as “peritumoral” 8. Patients with involvement of distant organs or typical systemic amyloid syndromes as seen with involvement of distant organs such as heart, kidneys, gastrointestinal tract, nerves, fat aspirate were defined as “systemic” amyloidosis. The methods for laser microdissection (LMD) and MS have been published previously 9. In brief, LMD was performed in Congo red positive areas of formalin-fixed paraffin-embedded LN blocks. The dissected tissue was digested into tryptic peptides and analyzed by liquid chromatography electrospray tandem MS. The raw data was interrogated using the Swiss-Protein database and subsequently assigned protein and peptide probability scores in Scaffold (Proteome Software, Portland, OR). Peptide identifications were accepted if they could be established with a >95% probability, at a 90% confidence interval. A spectral counting approach was utilized and a count ≥ 8 was considered to be clinically significant with higher counts indicative of greater abundance. A higher mass spectrum value was also considered indicative of a greater confidence in protein identification.
Statistical analysis was performed using JMP version 9.0 (SAS Institute, North Carolina). Overall survival was analyzed using the Kaplan-Meier method. A P-value < 0.05 was considered statistically significant.
Results
Of the 30 patients, 28 had immunoglobulin-derived amyloidosis (AL, AH, or AH/AL), 1 had localized LN transthyretin (ATTR), and 1 had ACalcitonin (Fig. 1). Two patients were excluded from the analysis (see below). Table 1 shows the clinical, laboratory characteristics of the 26 patients with immunoglobulin-derived amyloidosis (AID).
| AL amyloidosis, N 9 | AH or AH/AL amyloidosis, N 17 | P-value | |
|---|---|---|---|
| Age at diagnosis (years) | 62 | 62 | |
| Gender, (M:F) | 2:7 | 12:5 | 0.02 |
| Localized: Peritumoral: Systemic | 0:0:9 | 2:5:10 | 0.03 |
| Immunofixation electrophoresis positive | 7 (N = 9) | 10 (N = 14) | 0.7 |
| Free light chain assay abnormal | 6 (N = 6) | 6 (N = 12) | 0.01 |
| M-protein (g/dl) | 0.3 (N = 9) | 0.6 (N = 14) | 0.1 |
| Bone marrow amyloid involvement | 6 (N = 8) | 8 (N = 14) | 0.4 |
| Light chain deposits on LMD/MS | |||
| Kappa (K) | 2 | 3 | NS |
| Lambda (L) | 7 | 9 | |
| Mixed (KL) | 0 | 4 | |
| Heavy chain deposits on LMD/MS | |||
| Alpha (A) | 0 | 1 | |
| Gamma (G) | 0 | 7 | |
| mu (M) | 0 | 3 | |
| Mixeda | 0 | 6 | |
| Treatment | NS | ||
| None | 2 (22%) | 6 (36%) | |
| Chemotherapy | 6 (67%) | 9 (53%) | |
| Autologous Stem cell transplantation | 1 (11%) | 2 (11%) | |
| Median follow up of surviving patients (months) | 12 | 23 | 0.1 |
| Median OS (months) | 19 | 48 | 0.06 |
- a −2 (A/G), 4 (G/M).
Of these 26 patients with LN AID, only 2 were truly localized, that is, without a circulating monoclonal protein measured by both serum and urine immunofixation and serum free light chain analysis: one had AH; the other had AH/AL. Neither of these patients had evidence of organ involvement by organ biopsy or clinical criteria.
There were 24 patients with systemic LN AID; 5 (21%) patients had a peritumoral presentation albeit with a serum monoclonal protein, and 19 (79%) patients had evidence of systemic immunoglobulin derived amyloidosis (Tables 1 and 2). Overall, 9 of these patients had AL, 1 had AH, and 16 had AH/AL (Table 1). All of the patients with AL had a typical systemic amyloid syndrome as opposed to 59% of the mixed AH/AL (P = 0.02). While this study is restricted by the small number of patients, the presence of AH amyloid (i.e., AH or mixed AH/AL) in LN showed a trend to improved median overall survival, AH/AL 48 months versus AL 19 months (P = 0.06).
| Presentation | Positive tissue biopsy (in addition to LN) | MS (heavy chain/light chain) | IFE (heavy chain/light chain) | FLC | Systemic involvement (biopsy and/or clinical) |
|---|---|---|---|---|---|
| Localized #1 | – | AH (MG) | – | – | – |
| Localized #2 | ND | AH/AL (AG/KL) | – | – | – |
| Peritumoral #1 | BM (with NHL) | AH/AL (G/L) | GL | – | – |
| Peritumoral #2 | No | AH/AL (G/K) | – | K | – |
| Peritumoral #3 | ND | AH/AL (M/L) | ML | – | – |
| Peritumoral #4 | BM (with NHL) | AH/AL (M/KL) | ML | – | – |
| Peritumoral #5 | BM (with NHL) | AH/AL (MG/L) | ML | L | – |
| Systemic #1 | Fat | AH/AL (A/KL) | ML | – | Kidney |
| Systemic #2 | GI | AH/AL (AG/L) | – | – | stomach |
| Systemic #3 | GI | AH/AL (G/K) | GK | K | Heart, kidney, liver |
| Systemic #4 | Lung (diffuse) | AH/AL (G/K) | MK | K | Lung |
| Systemic #5 | Liver, spleen, kidney | AH/AL (MG/L) | ND | ND | Heart, liver, kidney, spleen |
| Systemic #6 | Fat, BM | AH/AL (M/L) | ML | ND | Fat |
| Systemic #7 | GI | AH/AL (G/KL) | AK | – | Duodenum |
| Systemic #8 | Rectum | AH/AL (G/L) | ND | ND | Heart, kidney, |
| Systemic #9 | BM, Fat, GI | AH/AL (G/L) | GL | L | Heart |
| Systemic #10 | BM, pleura | AH/AL (MG/L) | ND | ND | Pleura |
| Systemic #11 | Fat | AL (L) | GL | L | Kidney |
| Systemic #12 | BM, Fat, GI | AL (L) | ML | L | Heart, kidney, liver, stomach |
| Systemic #13 | BM | AL (L) | GL | L | Heart, kidney, intestine |
| Systemic #14 | Liver, BM, Fat | AL (L) | – (SPE) | ND | Kidney, liver |
| Systemic #15 | Liver, spleen | AL (L) | – | L | Heart, liver, spleen |
| Systemic #16 | Fat, BM | AL (L) | GL | ND | Heart |
| Systemic #17 | Fat, BM | AL (L) | ML | L | Gall bladder |
| Systemic #18 | BM, Kidney, Liver | AL (K) | K | K | Heart, kidney, liver |
| Systemic #19 | Carpal tunnel, GI, Ovaries, Skin | AL (K) | K | ND | Heart, nerve, intestine, ovary, skin, ligaments |
- LN: lymph node; IFE: immunofixation electrophoresis; SPE: serum protein electrophoresis; FLC: free light chain (monoclonal or skewed ratio); BM: bone marrow; NHL: Non Hodgkin lymphoma; GI: gastrointestinal; A: alpha; G: gamma; M: mu; K: kappa; L: lambda; ND: not done; −: absent.
- All MS results were performed on LN amyloid tissue.
We also observed that 5/16 AH/AL and the AH case had more than 1 heavy chain and 4/16 AH/AL had both light chains detected on mass spectroscopy; none of the AL cases had both light chains in deposits (Tables 1 and II). Additionally, 5 of these 10 patients had systemic amyloidosis and thus this finding was not restricted to the localized or peritumoral types alone. No other discernible differences were found in the amyloid proteomes of between the groups who had more than one heavy chain or both light chains versus those that did not.
Among the AID patients, a diagnosis of a non-Hodgkin lymphoma (NHL) was made in seven based on morphology, either on LN or bone marrow histology. The associated NHL in six patients was a lymphoplasmacytic lymphoma and all of these patients had AH/AL amyloidosis. One patient with a NHL had AL amyloidosis with a coexistent grade 1 follicular lymphoma in LN in addition to a 40% involvement of the bone marrow with plasmacytosis.
Two patients were excluded from analysis. One of the AL patients (AL/ATTR in Fig. 1) was noted to have abundant AL kappa and transthyretin deposits. He was a 60-year-old male with Waldenstrom macroglobulinemia/lymphoplasmacytic lymphoma. He was treated with rituximab and cladribine with a partial response, but was not a candidate for autologous hematopoietic stem cell transplantation due to inability to mobilize sufficient number of stem cells. He is currently on observant management 18 months after chemotherapy without progression of lymphoma or amyloidosis. The ATTR sequence in his amyloid was deduced by MS and found to be normal. Presumably, this patient has age-related systemic amyloidosis and was excluded from the clinical and survival analysis. Another case had small amounts of AL and AH but did not meet the prespecified spectral count cut-off for protein abundance, and was excluded from analysis (not shown in Fig. 1).
Non-AID LN amyloidosis
The two patients with non-AID included an 84-year-old male with urothelial cancer of the bladder who had a radical cystoprostatectomy and pelvic lymphadenectomy, with incidentally detected ATTR deposition in iliac LN and a 30-year-old female with multiple endocrine neoplasia Type II, with medullary thyroid carcinoma (MTC) and ACalcitonin found a cervical LN affected by the MTC metastasis. The ATTR patient is alive and well, 2 years later at the time of this analysis, without any symptoms of systemic amyloidosis. The patient with ACalcitonin underwent a thyroidectomy and neck dissection, and she is alive and well 20 years later.
Discussion
LN amyloidosis is uncommon with only 1 case in a published single institution series of 20 patients, and none from a review of 290 additional cases from the literature 10. This is confirmed in our current series with a 1% incidence in a large database. Heavy chain amyloidosis has also been considered rare, and has been described mostly in renal and pulmonary amyloidosis 11-14. Miyazaki et al. reviewed AH amyloidosis and found that three of the nine cases reported in the literature had a B-cell lymphoproliferative disease associated with AH amyloid 13. In their review, only 2 patients had localized disease, whereas the remaining 10 patients had an amyloid syndrome, predominantly nephrotic syndrome. With the advent of LMD/MS, it has become apparent that AH and AH/AL historically have been under diagnosed. In our series, two thirds of LN amyloidosis (70%) had AH deposits. Of the localized and peritumoral LN amyloidosis, all were AH/AL. Recently, nodular pulmonary amyloidosis, another localized amyloidosis was also shown to be associated with mixed AH/AL type when evaluated by LMD/MS 11. This finding implies that immunoglobulin heavy chains, due to their larger molecular size are more pathogenic to the local area of production, that is, more amyloidogenic. Although these heavy chains can be found in circulation, they are less likely to affect distant organs, and form amyloid in situ. Additionally, only half of the AH/AL cases had an abnormal free light chain (Table 1), which are the most common cause of end organ damage.
The presence of a mixture of heavy and light chains in amyloid deposits may be a consequence of the unique pathogenesis of LN AH/AL amyloidosis. Normal LN contain abundant B cells and plasma cells, which produce IgM and IgG antibodies 15, of which IgG tend to predominate. Therefore, it is likely that the cases that contain a mixture of immunoglobulin heavy chains or polytypic light chains reflect an integration of localized non-neoplastic immunoglobulin into the monoclonal deposits. This is supported by the fact that the cases with an IgG paraprotein in the serum did not have an M or A signal upon LN MS, and conversely when two heavy chains were detected it was either A/G or M/G but never A/M. Similarly, cases with both K/L AL deposits of the AH/AL cases most likely represent a mixture of pathogenic amyloid with a background of locally produced polyclonal non-neoplastic immunoglobulins. Grogg et al. also observed this in two cases of Sjögren syndrome with pulmonary MALToma and nodular pulmonary amyloidosis with AH/AL and suggested these a result of chronic inflammatory response and polytypic AID 11. None of the AL cases had a mix of both K/L deposits. This feature appeared to be solely restricted to the AH and AH/AL proteomes. An important question that is not answered by our current study is whether these additional immunoglobulin proteins are constituent of the amyloid fibrils themselves participating in the amyloidogenic process or if they are mere background picked up because of the high sensitivity of MS analysis. Further studies are needed to clarify this issue.
An additional advantage of LMD/MS is that it identifies other proteins, which may be important precursor and/or chaperone proteins in the amyloidogenic process. In our series, several apolipoproteins were consistently seen, including apoA-I, apoA-IV, and apo E. Serum amyloid P was also detected in all but three cases, one localized and two systemic. These proteins have been described in AL, age-related and hereditary amyloidosis. Bergstrom et al. 16 suggested that the apolipoproteins A-I, A-IV, and E tend to have similar molecular structures, are intrinsically flexible molecules, and may be prone to amyloidogenicity.
Conclusions
In conclusion, we make several novel observations in this large series of LN amyloidosis. First, true localized LN amyloidosis is rare. Second, a majority of immunoglobulin-derived LN amyloid cases are AH or mixed AH/AL. Third, the presence of heavy chain in amyloid may be associated with either a localized, peritumoral or systemic amyloidosis, and it is important to make this distinction in order to prognosticate and treat patients appropriately. Differences in clinical features, organ involvement, prognosis of LN AID between AL, AH, and AH/AL subtypes need to be further explored. Finally, not all LN amyloidosis is lymphoma-associated or immunoglobulin-derived. Mass spectrometric analysis of amyloid is a sensitive technique not only in the accurate typing of amyloidosis, but also in defining amyloidogenic precursors and chaperone proteins.
Author Contributions
A D'Souza and A Dispenzieri designed the study, abstracted and analyzed the data and wrote the manuscript. JT, PQ, JV, and A Dogan performed the mass spectrometry testing, interpreted the analysis, and helped in manuscript preparation. RK, MG, and SZ helped in manuscript preparation. All authors reviewed the final draft of the manuscript.




