The differences and correlations of BCR-ABL transcripts between peripheral blood and bone marrow assays are associated with the molecular responses in the bone marrow for chronic myelogenous leukemia†
Conflict of interest: All authors declare no competing financial interests.
Abstract
Previous studies concerning BCR-ABL mRNA levels by quantitative real-time RT-PCR (Q-PCR) for chronic myelogenous leukemia (CML) have shown a significant concordance between peripheral blood (PB) and bone marrow (BM) assays. The objective of this study was to determine whether molecular monitoring using PB was comparable to using BM for CML. A comparative study was performed that analyzed the Q-PCR results of 712 simultaneous PB and BM samples from 330 patients before and during imatinib therapy. For the 78 paired pretreatment samples, the level of BCR-ABL mRNA in PB was lower than that in BM (P = 0.007). Although the overall amounts of BCR-ABL mRNA in the PB and BM were comparable (P= 0.072) and there was a strong correlation (r = 0.839, P < 0.001) with the 634 paired on-treatment samples, the depth of the molecular response in PB was lower than that in BM (P < 0.001). The level of BCR-ABL mRNA in PB was lower than that in BM where the BM BCR-ABL mRNA < 1 log reduction (P < 0.001) or ≥ 1–< 2 log-reductions (P = 0.008) from the baseline, and higher than that where the BM BCR-ABL mRNA ≥ 2 log-reductions (P < 0.001). A strong correlation (r = 0.811, P < 0.001) was only found where the BM BCR-ABL mRNA < 1 log reduction. We conclude that the differences and correlations of BCR-ABL mRNA between PB and BM assays depend on the depth of the molecular response in BM for CML during imatinib therapy. Am. J. Hematol., 2012. © 2012 Wiley Periodicals, Inc.
Introduction
The treatment paradigm for chronic myelogenous leukemia (CML) has changed with the introduction of imatinib [1, 2] and other tyrosine kinase inhibitors (TKIs) as the frontline therapy [3, 4]. More than 80% of the newly diagnosed CML patients in the chronic phase (CP) have achieved a complete cytogenetic response (CCR), BCR-ABL transcripts are often monitored thereafter by real-time quantitative polymerase chain reaction (Q-PCR) which allows us to identify CML at the stage of minimal residual disease (MRD) when it is not yet detectable by cytogenetic analysis. Recently, efforts have been made to define molecular milestones at the early phase on imatinib therapy that predict the long-term outcomes more reliably than cytogenetic analysis. The accurate evaluation of BCR-ABL transcripts by Q-PCR provides clinicians with important diagnostic and prognostic information [1, 5, 6].
Because the frequent monitoring of bone marrow (BM) is inconvenient, painful and costly, the ability to detect and quantify MRD in peripheral blood (PB) has several distinct advantages. There is little or no discomfort for the patient and the test can be easily repeated, thereby improving compliance and adherence to follow-up. Nowadays, cytogenetics, performed on BM cell metaphases, is required in the early phase until a CCR has been achieved and confirmed, then every 12 or 18 months if regular molecular monitoring cannot be assured during the long-term TKI therapy. Q-PCR assessment of BCR-ABL transcript levels, performed on whole buffy-colt blood cells, is recommended every 3 or 6 months [7-9]. Previous studies concerning BCR-ABL mRNA monitoring by Q-PCR have shown a significant concordance between BM and PB assays [10-15], suggesting that it is not necessary to obtain BM aspirants for accurate molecular monitoring. However, Stock et al. questioned the validity of the concordance between PB and BM values for BCR-ABL mRNA detection [16]. The question of whether BM Q-PCR test can be replaced by PB assay has not been answered. In addition, some doctors prefer to perform Q-PCR test on BM sample at the same time when cytogenetic was analyzed.
To determine whether molecular monitoring of BCR-ABL mRNA using PB is comparable to monitoring using BM, we performed a prospective study by Q-PCR of 712 simultaneous paired PB and BM samples from 330 CML patients prior to and during imatinib therapy, and found that the differences and correlations of BCR-ABL mRNA between PB and BM assays were associated with the molecular responses in BM.
Patients and Methods
Between May 2006 and July 2011, 330 patients who were Philadelphia chromosome (Ph) positive and were diagnosed with p210 BCR-ABL-positive CML were enrolled in our study. The patients were categorized according to World Health Organization criteria [17] as either in CP (n = 319) or accelerated phase (AP, n = 11). They were given imatinib at an initial dose of 400 mg (CP) or 600 mg (AP) daily. The dose was then adjusted according to the patient's response and/or toxicity. The hematological, cytogenetic and molecular responses were evaluated at frequent intervals. The cytogenetic responses in BM were analyzed every 3 or 6 months until a CCR was achieved, and subsequently analyzed every 12 or 18 months after CCR. The molecular responses in PB were analyzed every 3 or 6 months. Ethical approval was obtained from the ethics committee of the Peking University People's Hospital, and all patients provided written informed consent. The study protocol was registered in the Chinese Clinical Trial Registry (Registration Number: ChiCTR-ONC-11001697).
Cytogenetic studies
The classical Giemsa banding technique was used to evaluate the cytogenetic response in BM samples. CCR and partial cytogenetic response (PCR) were referred to as 0% and as 1–35% of the Ph-positive metaphase, respectively. Major cytogenetic response (MCR) was defined as CCR plus PCR, and no MCR was defined as 36–100% of the Ph-positive metaphase.
Molecular studies
-
Forward primer 5′-CCGCTGACCATCAATAAGGAA-3′
-
Reverse primer 5′-CTCAGACCCTGAGGCTCAAA GT-3′
-
Probe 5′-FAM-AGCCCTTCAGCGGCCAGTAGCATCT- TAMRA-3′
ABL primers and probe were referred to the report of the Europe Against Cancer Program [20]. ABL copy numbers of all the samples were more than 3×104. The reproducible sensitivity of Q-PCR was 5 copies. All experiments were performed in duplicate. If BCR-ABL mRNA was detected, the sample was considered positive and the number of transcripts was calculated as BCR-ABL/ABL %. If BCR-ABL mRNA was undetected, the sample was regarded as negative and BCR-ABL/ABL% was equal to zero. The molecular responses in PB and BM samples were defined as the log-reductions of BCR-ABL mRNA level from the baseline value of PB and BM, respectively, which were the median levels from newly diagnosed CP CML patients. Major molecular response (MMR) in PB and BM samples were defined as ≥ 3 log-reductions of BCR-ABL mRNA level from the baseline value of PB and BM, respectively.
Statistical analyses
The Wilcoxon Signed Ranks test was used to compare the difference of the values of BCR-ABL/ABL % between PB and BM assays. The Spearman rank order correlation coefficient was used to assess the correlation between paired PB and BM BCR-ABL values (with positive signals in both PB and BM samples) after logarithmic transformation (base 10). The Bland-Altman plot was used to assess the agreement if simultaneous PB and BM BCR-ABL mRNA levels showed a strong correlation. The Pearson Chi-Squared test was used to compare the categorical variables. These calculations, excluding the Bland-Altman plot, were performed using SPSS 13.0 software. The Bland-Altman plot was performed using Analyse-it software.
Results
All patients and samples
Seven hundred and twelve simultaneous paired PB and BM samples obtained from 330 patients were analyzed. Seventy-eight paired samples were collected from 78 newly diagnosed (pre-treatment) CP CML patients, and 634 paired samples, including 605 pairs from CP patients and 29 pairs from AP patients, were collected from 262 on-treatment patients at a median time of 18 months (range 3–120 months) from the initiation of imatinib therapy. Ten patients were diagnosed and followed from before treatment and to during the treatment. Two hundred and sixty-two on-treatment patients had sequential studies at the rate of one time (n = 87), two times (n = 68), three times (n = 48), four times (n = 37), five times (n = 14), six times (n = 7), and seven times (n = 1), with a median of two consecutive determinations (range 1–7 times) carried out for each patient. Simultaneous BM cytogenetic analysis showed that 78 samples from newly diagnosed patients were 100% Ph positive karyotype, and 525 of 634 samples (82.8%) showed that on-treatment patients had achieved CCR.
The median values of BCR-ABL mRNA in PB and BM samples from 78 newly diagnosed patients were 22.6% (range, 1.4–114.4%) and 35.7% (range, 8.2–110.4%), respectively, which were the baseline values of BCR-ABL mRNA in PB and BM samples, respectively, with a significant difference (P = 0.007).
In general, the median values of BCR-ABL mRNA in PB and BM samples from 634 paired on-treatment patients were 0.0890% (range, 0–87.1%) and 0.0695% (range, 0–124.2%), respectively, which were comparable (P = 0.072). Five hundred and seven paired samples (80.0%) were BCR-ABL mRNA positive for both PB and BM, 88 paired samples (13.9%) were BCR-ABL negative for both PB and BM, 22 samples (3.5%) were PB BCR-ABL positive while the simultaneous paired BM samples were BCR-ABL negative and 17 samples (2.7%) were BM BCR-ABL positive while the simultaneous paired PB samples were BCR-ABL negative.
The differences and correlations of BCR-ABL transcripts in PB vs. BM according to the cytogenetic responses
Based on the cytogenetic responses, the values of BCR-ABL mRNA from 634 paired on-treatment samples were grouped for the comparison of differences. Those from 507 paired samples that were BCR-ABL positive in both PB and BM were grouped for comparison of the correlations. These different groupings arise from the fact that 127 paired samples were BCR-ABL negative in both PB and BM (n = 88) or in either PB or BM (n = 39).
The levels of BCR-ABL mRNA in PB samples were found to be lower than the levels in BM samples in the groups with no MCR (P < 0.001) and PCR (P < 0.001), however, the levels of BCR-ABL mRNA were higher than those in the BM samples for the patients in CCR (P = 0.004), as shown in Table I.
| The proportion of Ph positive cell in BM % | Difference | Correlation | |||||
|---|---|---|---|---|---|---|---|
| No. pairs | BM BCR-ABL/ABL, % median (range) | PB BCR-ABL/ABL, % median (range) | P-value | No.a pairs | r | P value | |
| 36–100 | 40 | 29.7 (0.49–108.9) | 21.8 (0.49–87.1) | < 0.001 | 40 | 0.768 | < 0.001 |
| 1–35 | 69 | 3.1 (0–124.2) | 1.2 (0–36.3) | < 0.001 | 65 | 0.758 | < 0.001 |
| 0 | 525 | 0.045 (0–24.5) | 0.054 (0–9.1) | 0.004 | 402 | 0.732 | < 0.001 |
| Total | 634 | 0.0695 (0–124.2) | 0.089 (0–87.1) | 0.072 | 507 | 0.839 | < 0.001 |
- a BCR-ABL transcripts can be detected in both PB and BM.
Although the levels of BCR-ABL mRNA in the PB and BM samples strongly correlated overall (r = 0.839, P < 0.001), those in any cytogenetic response showed only modest correlations (r < 0.8), as shown in Table I.
Among the 634 paired samples, the median log-reduction value of BCR-ABL mRNA from the baseline in PB was comparable to those in BM in the no MCR samples (0.004 vs. 0.084, P = 0.619) and PCR samples (1.263 vs. 1.065, P = 0.185), however, the median log-reduction value was lower than which in the BM samples where a CCR was achieved (2.610 vs. 2.903, P < 0.001), as shown in Fig. 1.
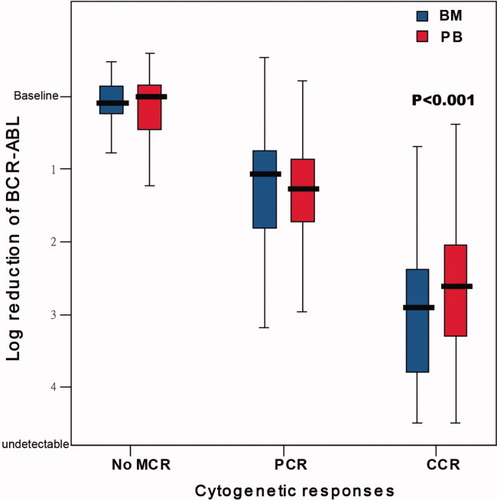
The molecular responses in PB vs. BM associated with the cytogenetic responses. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The differences, correlations, and agreement of BCR-ABL transcripts in PB vs. BM associated with the molecular responses in BM
Further comparative study was performed associated with the molecular responses in BM because of the more than 80% contemporaneously obtained BM samples from patients who had achieved a CCR.
Among the 634 paired samples, the level of BCR-ABL mRNA in PB was lower than that in BM where the BM BCR-ABL mRNA < 1 log-reduction (P < 0.001) or ≥ 1–< 2 log-reductions (P = 0.008) from the baseline, and higher than those in BM samples where the BM BCR-ABL mRNA ≥ 2 log-reductions (P < 0.001). Although the levels of BM BCR-ABL mRNA were undetectable, 22 of 110 (20.0%) simultaneous PB samples signaled positive with the highest BCR-ABL mRNA value of 0.094%, as shown in Table II.
| BCR-ABL transcript reductions from the baseline in BM | Difference | Correlation | |||||
|---|---|---|---|---|---|---|---|
| No. of pairs | BM BCR-ABL/ABL, % median (range) | PB BCR-ABL/ABL, % median (range) | P value | No. pairsb | r | P value | |
| < 1 log | 78 | 19.6 (3.6–124.2) | 7.45 (0.13–87.1) | < 0.001 | 78 | 0.811 | < 0.001 |
| ≥ 1 log–< 2 log | 99 | 0.86 (0.36–3.5) | 0.64 (0–5.8) | 0.008 | 98 | 0.494 | < 0.001 |
| ≥ 2 log–< 3 log | 214 | 0.091 (0.037–0.34) | 0.13 (0–1.5) | < 0.001 | 212 | 0.270 | < 0.001 |
| ≥ 3 loga | 133 | 0.014 (0.0014–0.035) | 0.022 (0–0.25) | < 0.001 | 119 | 0.227 | 0.013 |
| Undetectable | 110 | 0 | 0 (0–0.094) | < 0.001 | |||
| Total | 634 | 0.0695 (0–124.2) | 0.089 (0–87.1) | 0.072 | 507 | 0.839 | < 0.001 |
- a BCR-ABL transcripts can be detected in BM.
- b BCR-ABL transcripts can be detected in both PB and BM.
Among the 507 paired samples that were BCR-ABL positive in both PB and BM, there was a strong correlation (r = 0.811, P < 0.001) only for the pairs with the BM BCR-ABL mRNA < 1 log-reduction from the baseline. However, a weak correlation (r < 0.5) was found in samples with the BM BCR-ABL mRNA ≥ 1 log-reduction. These results are shown in Table II.
The agreement analyses of the BCR-ABL mRNA values in PB and BM, performed with the Bland-Altman plot, showed an agreement between the 507 paired PB and BM samples (mean difference = 0.5118, P = 0.6013). However, the 95% limits of the agreement range were broad (–1.0150 to 0.9913), and the 78 paired PB and BM samples with the BM BCR-ABL mRNA < 1 log-reduction showed a lack of an agreement (mean difference = 0.3645, P < 0.0001).
The discordance of BCR-ABL transcripts in PB vs. BM
The discordances in our study included minimal, modest, and major discordances, which referred to the differences of the BCR-ABL values between PB and BM assays after logarithmic transformation of less than 0.5 log, 0.5 to 1 log and more than 1 log, respectively, among the 507 paired on-treatment samples that were BCR-ABL-positive in both PB and BM samples. There were 360 pairs (71.0%) with minimal discordance, 127 pairs (25.0%) with modest discordance, and 20 pairs (3.9%) with major discordance.
To analyze the proportion of minimal discordance, we grouped the 507 pairs based on their molecular responses in BM. The lowest proportion of minimal discordance was found in the group with BM BCR-ABL mRNA ≥ 3 log-reductions, which was significantly lower than those in groups with BM BCR-ABL mRNA ≥ 1–< 2 log-reductions (58.8% vs. 80.6%, P = 0.001) and ≥ 2–< 3 log-reductions (58.8% vs. 74.1%, P = 0.004). There was no significant difference of proportion of the minimal discordance between the other two groups (data not shown).
The molecular responses in PB vs. BM
Among the 634 paired on-treatment samples, the overall log-reduction value of the BCR-ABL mRNA from the baseline of PB was lower than that of BM, with a median of 2.39 vs. 2.71 (P < 0.001). The proportions of BCR-ABL mRNA < 1 log-reduction from the baseline in PB and BM were 11.8% vs. 12.8% (P = 0.796), those with ≥ 1–<2 log-reductions were 21.8% vs. 15.6% (P = 0.005), those with ≥ 2–<3 log-reductions were 37.1% vs. 33.8% (P = 0.218), those with ≥ 3–<4 log-reductions were 12.8% vs. 19.9% (P = 0.001), and those with ≥ 4 log-reductions were 16.6% vs. 18.5% (P = 0.375), respectively. In total, the proportion of MMR in PB and BM was 29.3% vs. 38.3% (P = 0.001), respectively.
The dynamics of the molecular responses in PB vs. BM during imatinib therapy
Our study analyzed 212 paired PB and BM samples taken from 55 CP patients who underwent sequential BCR-ABL mRNA detections every 3 or 6 months at least three times, at a median of four consecutive determinations (range, 3–7 times), from the onset of imatinib therapy at a median time of 12 months (range, 3–60 months). Fifty-three patients (96.4%) achieved a CCR at a median time of 6 months (range, 3–18 months) on imatinib therapy.
Although the median value of the BCR-ABL mRNA in PB for the 55 patients on imatinib treatment was comparable overall to that in BM (P = 0.606), it was lower than that in BM at 3 months (P < 0.001), comparable to that in BM at 6 months (P = 0.109) and 12 months (P = 0.114) and higher than that in BM at 18 months and later (all P values < 0.05), as shown in Table III.
| Months since the imatinib therapy | Difference | Correlation | |||||
|---|---|---|---|---|---|---|---|
| No. of pairs | BM BCR-ABL/ABL, % median (range) | PB BCR-ABL/ABL, % median (range) | P value | No.a, pairs | r | P value | |
| 3 | 37 | 1.8 (0–124.2) | 1.1 (0–43.4) | < 0.001 | 36 | 0.900 | < 0.001 |
| 6 | 52 | 0.255 (0–54.0) | 0.220 (0–35.6) | 0.109 | 49 | 0.856 | < 0.001 |
| 12 | 50 | 0.0735 (0–52.1) | 0.115 (0–65.2) | 0.114 | 43 | 0.713 | < 0.001 |
| 18 | 29 | 0.049 (0–2.2) | 0.079 (0–5.8) | 0.017 | 25 | 0.836 | < 0.001 |
| 24 | 19 | 0.029 (0–0.71) | 0.047 (0–0.86) | 0.039 | 12 | 0.629 | 0.028 |
| ≥36 | 25 | 0.014 (0–0.23) | 0.026 (0–0.49) | 0.028 | 17 | 0.712 | 0.001 |
| Total | 212 | 0.0695 (0–124.2) | 0.089 (0–87.1) | 0.606 | 182 | 0.842 | < 0.001 |
- a BCR-ABL transcripts can be detected in both PB and BM.
The correlations of the BCR-ABL mRNA values in 182 paired PB and BM samples with both positive signals were analyzed. There were strong correlations among the samples as a whole, at 3, 6, and 18 months (r > 0.8, P < 0.001), with except of those at 12, 24 months and later (all r values < 0.8), as shown in Table III.
Among the 212 paired samples, overall log-reduction value of PB BCR-ABL mRNA from the baseline was lower than that of BM, with a median of 2.14 vs. 2.56 (P < 0.001), which was similar to the results of the total of the study samples. Based on the time point of imatinib therapy, the depth of the molecular responses in PB and BM were comparable at 3 months (P = 0.975) and 6 months (P = 0.076). However, the log-reduction values of BCR-ABL mRNA from the baseline in PB were lower than those in BM at 12, 18, and 24 months (all P values < 0.05), as shown in Fig. 2.
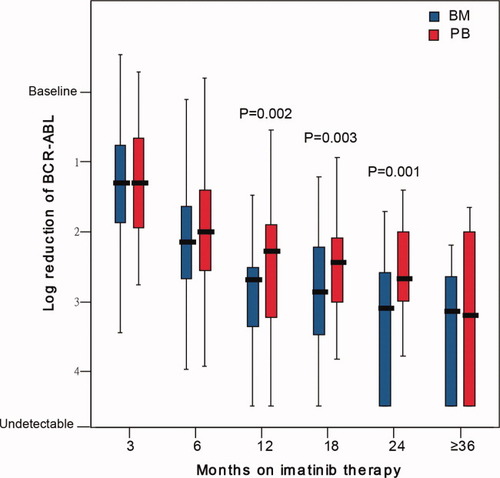
The dynamics of the molecular responses in PB vs. BM in patients with sequential monitoring. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Discussion
To our knowledge, our research is the first comparison of the differences and correlations of BCR-ABL mRNA levels between PB and BM assays associated with the molecular response. We are surprised to discover that although the amounts of BCR-ABL mRNA in PB and BM from the on-treatment samples strongly correlated as a whole, the differences and correlations shifted when data were grouped based on the molecular responses in BM.
Two important factors may have led to the disparate findings from the previous research [10-16]. First, it is worth noting that the samples in the previous studies were taken from patients with lower proportion and lower degree of cytogenetic and molecular responses to TKIs treatment, chemotherapy, or after transplantation, or from patients at earlier time points and with a shorter follow-up during imatinib therapy than those in our study. The dynamics of leukemia burden in sequential Q-PCR monitoring in our study revealed that the PB MRD was gradually converted from lower than to equal, and from equal to higher than the levels in simultaneous paired BM samples, and that the correlation of PB and BM results was converted from good to modest with the prolongation of imatinib therapy and the increase in effect of the molecular response. These results show why the level of BCR-ABL mRNA in PB was lower than that in BM and why PB values strongly correlate with BM values at lower levels of molecular response, but significantly higher than BM values and only modestly or poorly correlate at a high degree of molecular response. Second, the analyses in terms of differences and correlations between PB and BM Q-PCR values were performed as a whole in the previous reports, but our data were analyzed based on the depth of the molecular responses in BM.
Furthermore, we confirmed the lack of an agreement of BCR-ABL mRNA levels and a frequent and disparate molecular response (overall 71.0% of minimal discordance) in the simultaneously collected PB and BM samples. This was especially notable in the probability of MMR in the PB samples which was statistically significant lower than that in the BM samples. These findings may suggest that Q-PCR monitoring for CML MRD using PB is not as equally effective as monitoring using BM, especially when evaluating and monitoring the deeper molecular response of treatment with TKIs.
Our data suggested that BM may be more sensitive than PB in detecting MRD by Q-PCR for CML patients with a lower degree of molecular response and that greater accuracy can be achieved by analyzing PB samples in those patients with a higher degree of molecular response. The mechanisms are still not clear. Several factors may have contributed to the increased sensitivity of BM for CML MRD monitoring when there is a lower degree of molecular response, such as the higher percentage of myeloid precursor or the smaller amount of lymphocytes in BM compared to PB, a larger amount of BCR-ABL transcripts in BM precursors compared with more mature myeloid cells [21], the proliferation of CD34+ CML hematopoietic progenitor cells more easily inhibited by imatinib in PB than in BM [22]. We do not have a reasonable explanation for the greater sensitivity of PB for CML MRD monitoring when there is a stronger molecular response. Further studies focusing on the variation of BCR-ABL mRNA levels in different cellular compartments in PB and BM might be warranted. The retention of, but significant reduction in, BCR-ABL transcripts in hematopoietic stem cells in CML after imatinib therapy [23] may reflect significant disparities in the pathobiology of CML in PB and BM.
As a limitation to our current study, it has to be stated that the values of BCR-ABL/ABL were not converted to the international scale by the conversion factor. The PB and BM samples were measured and analyzed within the same experimental protocols of our lab and the standardized baselines were defined locally. A comparative trial to internationally standardize the Q-PCR values of PB and BM for use in monitoring the diagnostic and treatment responses in CML patients is needed.
In summary, our study is the first to discover that the differences and correlations of BCR-ABL transcripts between simultaneous paired PB and BM assays are associated with the molecular response in BM during imatinib therapy and molecular monitoring of MRD using PB is not completely equal to monitoring using BM. We caution against the interchanging of BM value with PB value for MRD testing. Although the clinical impact of our observations seems modest now, we cannot know the “truth”, it is unclear if PB overestimates the BCR-ABL burden, or BM underestimates that. A long-term follow-up study on the prognosis for the differences may be needed.
Acknowledgements
American Journal Experts (www.journalexperts.com) provided editorial assistance to the authors.




