Mitoxantrone, etoposide, cytarabine, and melphalan (NEAM) followed by autologous stem cell transplantation for patients with chemosensitive aggressive non-Hodgkin lymphoma†
Conflict of interest: The authors have declared no conflicts of interest
Abstract
Patients with chemosensitive aggressive non-Hodgkin lymphoma (NHL) could benefit from high-dose chemotherapy (HDC) followed by autologous stem cell transplantation (auto-SCT). We report clinical outcomes of HDC using a novel regimen consisting of mitoxantrone, etoposide, cytarabine, and melphalan (NEAM) with auto-SCT. A total of 69 patients were consecutively enrolled. Median age was 42 years (range, 20–66 years). Median event-free survival (EFS) was 17.9 months. Median overall survival (OS) has not been reached yet and estimated 2-year OS was 64.2%. Among patients with measurable lesions, response rate was 79.5%. Median time to recovery of neutrophil (>500 mL) and platelet (gt;20,000 mL) was 12.5 and 13.5 days, respectively. Febrile neutropenia developed in 61 patients (88.4%). Grades 3 or 4 hepatic toxicity developed in 7 patients (10.1%), Grades 3 or 4 renal toxicity in 2 patients (2.9%), and Grade 3 or 4 cardiac toxicity in 2 patients (2.9%). Transplant-related mortality (TRM) developed in two patients (2.9%). Multiple prior treatments before transplantation, auxiliary bone marrow harvest for stem cell collection, and high serum lactate dehydrogenase level were related to unfavorable treatment outcomes. In conclusion, NEAM conditioning with auto-SCT demonstrated considerable efficacy with modest toxicity in patients with chemosensitive aggressive NHL. Am. J. Hematol. 87:479–483, 2012. © 2012 Wiley Periodicals, Inc.
Introduction
High-dose chemotherapy (HDC) with autologous stem cell transplantation is generally accepted as an important treatment modality of patients with non-Hodgkin lymphoma (NHL). This treatment was effective not only as a salvage treatment but also as a consolidative treatment during the prerituximab era although the efficacy has yet to be proven in the rituximab era [1-3]. A number of studies with different conditioning regimens prior to autologous transplantation have been reported. Among them, CBV (cyclophosphamide, carmustine (BCNU), and etoposide), BEAC (BCNU, etoposide, cytarabine, and cyclophosphamide), and BEAM (BCNU, etoposide, cytarabine, and melphalan) are popular regimens [1-3]. Although there have been no head to head studies comparing BEAM to BCV or BEAC, the BEAM regimen has been one of the most commonly used regimens for lymphoma. However, BCNU-related pulmonary toxicity has been reported in 7–27% of patients receiving BEAM [3-5].
Mitoxantrone, an anthracenedione compound, is similar to anthracyclines in clinical activity. This agent retains antineoplastic activity of anthracyclines with significantly less potential for cardiotoxicity [6-8]. Mitoxantrone also has demonstrated potent activity in patients with aggressive NHL [9, 10]. In a previous study, mitoxantrone in combination with cyclophosphamide, BCNU, and etoposide was used for HDC prior to autologous transplantation, and demonstrated promising efficacy and acceptable toxicity in patients with refractory lymphoma [11]. In another study, HDC using mitoxantrone in combination with thiotepa and carboplatin (TMJ) was effective and safe for treatment of patients with lymphoma [12]. In addition, pulmonary toxicity of these regimens was relatively rare compared to BCNU-containing high-dose regimens [11-13].
Based on these results, we made a novel HDC protocol consisting of mitoxantrone, etoposide, cytarabine, and melphalan (NEAM) with autologous transplantation for patients with chemosensitive aggressive NHL. We report clinical outcomes of the NEAM regimen with autologous transplantation in these patients along with analysis of treatment-related adverse events and risk factors affecting treatment results.
Patients and Methods
Patient enrollment
Three hospitals participated in this trial: the Seoul National University Hospital, Seoul, Korea; the Severance Hospital, Seoul, Korea; and the Inha University Hospital, Incheon, Korea. This study consecutively enrolled patients with chemosensitive aggressive NHL. Achievement of partial response (PR) or complete remission (CR) after the last chemotherapy before autologous transplantation was considered as “chemosensitive.” The other inclusion criteria were age between 18 and 70 years, no previous history of stem cell transplantation, adequate hepatic function (serum total bilirubin <1.2 mg/dL, serum aspartate aminotransferase <40 IU/L, and serum alanine aminotransferase <40 IU/L), adequate renal function (serum creatinine <1.4mg/dL), adequate bone marrow function which can produce 1 million CD34+ cells/kg during stem cell harvest, and Eastern Cooperative Oncology Group (ECOG) performance status 0, 1, or 2. Patients who had symptomatic congestive heart failure at baseline or those who had previously received a cumulative anthracycline dose more than 400 mg/m2 body surface area (BSA) of doxorubicin or equivalent were excluded from this study.
Stem cell collection
Several cycles of leukapheresis were performed after chemotherapy or intravenous administration of cyclophosphamide (single dose of 3.0 g/m2 BSA) to obtain at least 1 million CD34+ cells per kilogram of patient body weight for cryopreservation. If the number of peripheral blood stem cells collected did not reach 1 million per kilogram, auxiliary bone marrow harvest was performed to meet the required number of stem cells.
Treatment protocol
Combination regimen consisting of mitoxantrone, etoposide, cytarabine, and melphalan was used for HDC before autologous transplantation. Mitoxantrone 12 mg/m2 BSA was intravenously infused daily from day-6 to day-4. Etoposide 100 mg/m2 BSA and cytarabine 100 mg/m2 BSA was also administered intravenously every 12 hr from day-6 to day-3. In addition, melphalan 140 mg/m2 BSA was infused once at day-2.
Prevention of tumor lysis syndrome has been done in a usual manner. Electrocardiogram was monitored from day-6 to day-2. Antimicrobial prophylaxis was performed from day-6: antifungal prophylaxis with either oral fluconazole or intravenous micafungin and antibacterial prophylaxis with oral ciprofloxacin. Serotonin antagonist and benzodiazepine were used to prevent chemotherapy-induced emesis.
Recombinant granulocyte colony stimulating factor (G-CSF) was administered via an intravenous or subcutaneous route from day +1 until recovery of neutrophil count over 3,000 mm3.
Study end points
The primary objective was to evaluate event-free survival (EFS) of patients. The secondary objectives were to evaluate overall survival (OS) and response rate of patients with measurable lesions and treatment-related adverse events and to elucidate risk factors affecting treatment results. The candidate risk factors were as follows: age, sex, histology, Ann Arbor stage, number of prior treatments, prior anthracycline dose, response to prior therapy, stem cell source, serum lactate dehydrogenase (LDH; cutoff, 500 IU/L), serum albumin (cutoff, 4.0 g/dL), and hemoglobin (cutoff, 10.0 g/dL).
Evaluation of patient status and treatment results
Tumor histology was classified as the World Health Organization classification updated in 2008. Treatment outcomes were evaluated by the revised response criteria updated in 2007 [14]. EFS was calculated from the treatment initiation to any treatment failure including disease progression, toxicity, patient preference, initiation of new treatment without documented progression, or death. OS was defined as the time from the treatment initiation until death as a result of any cause. Positron emission tomography/computed tomography (PET/CT) scan using 18F-fluorodeoxyglucose (FDG) or computed tomography (CT) scan performed for response evaluation of prior chemotherapy were used as baseline scans. Measurable lesions were defined by either 18F-FDG-PET/CT scan in patients with positive 18F-FDG-PET/CT scans previously or CT scan in patients without positive 18F-FDG-PET/CT scans. To evaluate treatment response, either 18F-FDG-PET/CT scan or CT scan was performed as appropriate at 3 months after finishing treatment.
Echocardiography was routinely performed in all patients before treatment to measure baseline cardiac function. In patients who developed heart failure symptoms after treatment, a follow-up echocardiography was performed to evaluate changes in cardiac function.
Duration of hospitalization was defined as the time from the initiation of treatment to discharge. Adverse events were assessed in all patients with version 3.0 of the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE).
Statistical analysis
The median EFS and OS were calculated using the Kaplan-Meier method. Univariate analysis and multivariate analysis of risk factors for survival data were made using the Cox proportional hazard model. Statistical analysis of categorical variables was performed using either the Pearson's chi-square test or the Fisher's exact test as appropriate. The Mann-Whitney U test was used for comparison of two independent nonparametric samples. Variables with P value of univariate analysis below 0.05 were selected for multivariate analysis. Two-sided P values below 0.05 were considered statistically significant. SPSS version 17.0 (SPSS, Chicago, IL) software was used for all statistical analysis.
Ethical considerations
A signed informed consent form was obtained from all patients before treatment. This study was reviewed and approved by the institutional review board of each participating hospital and conducted in accordance with the precepts established by the Helsinki Declaration.
Results
Baseline characteristics
A total of 69 patients were consecutively enrolled from 3 participating hospitals between January 2005 and September 2009. Baseline characteristics are shown in Table I. Median age was 42 years (range, 20–66 years). Forty-nine patients (71.0%) had B-cell lymphoma and 20 patients (29.0%) had T/NK-cell lymphoma. Before autologous transplantation, patients had received median two prior treatments (range, 1–6 treatments). Sixty-five (94.2%) patients had been previously treated with anthracycline before NEAM therapy. Among these patients, median cumulative dose of doxorubicin was 300 mg/m2 BSA (range, 30–400 mg/m2 BSA). All patients had at least one previous positive 18F-FDG-PET/CT scan. Among them, 25 patients (36.2%) achieved CR after prior therapy and had no measurable lesion defined by 18F-FDG-PET/CT scan at baseline. The other 44 patients (63.8%) attained PR after previous treatment and had measurable lesions defined by 18F-FDG-PET/CT scan at baseline. In seven patients (10.1%), CD34+ stem cells collected from peripheral blood was <1 million cells per kilogram. To collect sufficient stem cells, auxiliary bone marrow harvest was performed in these patients. Median number of CD34+ cells collected was 4.60 × 106 kg (range, 1.18 × 106 − 38.00 × 106 kg). Baseline serum LDH level was median 243 IU/L (range, 144–1224 IU/L). Eight patients (11.6%) had serum LDH level ≥ 500 IU/L. Baseline serum albumin level was median 3.2 g/dL (range, 3.5–4.9 g/dL). Eleven patients (15.9%) had serum albumin level ≥ 4.0 g/dL. Baseline hemoglobin level was median 10.2 g/dL (range, 7.7–14.4 g/dL). Thirty-nine patients (56.5%) had hemoglobin level ≥ 10.0 g/dL. Baseline left ventricular (LV) ejection fraction was median 58.5% (range 47–78%).
| Characteristics | No of patients (%) | |
|---|---|---|
| Age | <40 years | 29 (42.0) |
| ≥40 years | 40 (58.0) | |
| Sex | Male | 43 (62.3) |
| Female | 26 (37.7) | |
| Histology | Diffuse large B-cell lymphoma | 41 (59.5) |
| Peripheral T-cell lymphoma | 8 (11.7) | |
| Mantle cell lymphoma | 6 (8.8) | |
| Anaplastic large cell lymphoma | 5 (7.2) | |
| Extranodal NK/T-cell lymphoma, nasal type | 3 (4.3) | |
| Blastic NK-cell lymphoma | 3 (4.3) | |
| Intravascular large B-cell lymphoma | 1 (1.4) | |
| Burkitt lymphoma | 1 (1.4) | |
| Subcutaneous panniculitis-like T-cell lymphoma | 1 (1.4) | |
| Ann Arbor stage at diagnosis | I | 5 (7.2) |
| II | 10 (14.5) | |
| III | 14 (20.3) | |
| IV | 40 (58.0) | |
| A | 49 (71.0) | |
| B | 20 (29.0) | |
| IPI at diagnosis | Low risk | 20 (29.0) |
| Low-intermediate risk | 27 (39.2) | |
| High-intermediate risk | 13 (18.8) | |
| High risk | 9 (13.0) | |
| Prior treatment regimens | 1 | 26 (37.7) |
| 2 | 33 (47.8) | |
| 3 or more | 10 (14.5) | |
| History of prior anthracycline therapy | Absent | 4 (5.8) |
| Present | 65 (94.2) | |
| < 350 mg/m2 | 52 (75.4) | |
| ≥ 350 mg/m2 | 13 (18.8) | |
| Disease status at treatment | CR1 | 22 (31.9%) |
| CR2 | 3 (4.3%) | |
| PR1 | 41 (59.5%) | |
| PR2 | 3 (4.3%) | |
| ECOG performance status at treatment | 0 or 1 | 67 (97.1) |
| 2 | 2 (2.9) | |
| Bone marrow involvement at treatment | Absent | 66 (95.7) |
| Present | 3 (4.3) | |
| Stem cell source | Peripheral blood only | 62 (89.9) |
| Peripheral blood and bone marrow | 7 (10.1) | |
- CR1, first complete remission; CR2, second complete remission; ECOG, Eastern Cooperative Oncology Group; IPI, International Prognostic Index; PR1, first partial response; PR2, second partial response.
Treatment outcome
During a median follow-up of 24.7 months (range 1.8–51.3 months), 33 patients (47.8%) experienced treatment failure and 21 patients (30.4%) died. Median EFS was 17.9 months [95% confidence interval (CI), 0.0–38.6 months]. Median OS has not yet been reached and estimated 2-year OS was 64.2% (Fig. 1). Among 44 patients who had measurable lesions at baseline, response rate was 79.5%: 25 patients (56.8%) had CR and 10 patients (22.7%) had PR. Among them, 16 out of 21 patients with diffuse large B-cell lymphoma (76.2%) and 9 out of 12 patients with T-NK-cell lymphoma (75.0%) responded to treatment.
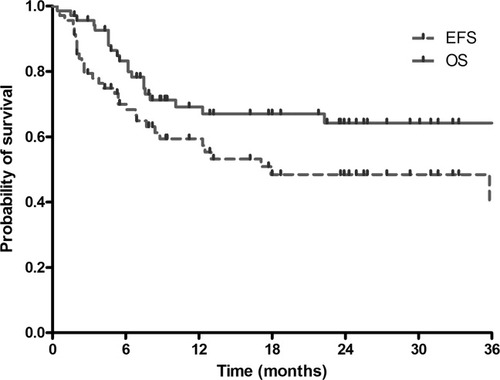
Kaplan-Meier analysis of EFS and OS. The median EFS was 17.9 months (95% confidence interval, 0.0–38.6 months). The median OS has not been reached yet and estimated 2-year OS was 64.2%.
In patients who achieve CR after therapy, median EFS was 39.5 months and estimated 2-year OS was 78.2%. In patients with PR, median EFS were 5.4 months (95% CI, 2.5–8.3 months) and estimated 2-year OS was 64.8%. In patients with stable disease or progressive disease, median EFS was 2.2 months (95% CI, 1.6–2.8 months) and estimated 2-year OS was 20.5%.
Transplant-related adverse events
Transplant-related adverse events are listed in Table II. Among patients with successful engraftment, median time to recovery of neutrophil (>500/mm3) and platelet (>20,000/mm3) was 12.5 days (range 3–50 days) and 13.5 days (range 0–175 days), respectively.
| Adverse events | No of patients (%) |
|---|---|
| Oral mucositis–total | 69 (100.0) |
| Grade 1 | 17 (24.6) |
| Grade 2 | 26 (37.7) |
| Grade 3 | 26 (37.7) |
| Grade 4 | 0 (0.0) |
| Pulmonary toxicity–total | 0 (0.0) |
| Hepatic toxicity–total | 46 (66.7) |
| Grade 1 | 31 (45.0) |
| Grade 2 | 8 (11.6) |
| Grade 3 | 6 (8.7) |
| Grade 4 | 1 (1.4) |
| Renal toxicity–total | 14 (20.3) |
| Grade 1 | 11 (16.0) |
| Grade 2 | 1 (1.4) |
| Grade 3 | 2 (2.9) |
| Grade 4 | 0 (0.0) |
| LV dysfunction–total | 9 (13.0) |
| Grade 1 | 6 (8.8) |
| Grade 2 | 1 (1.4) |
| Grade 3 | 1 (1.4) |
| Grade 4 | 1 (1.4) |
| Fever | 61 (88.4) |
| Documented sepsis–total | 16 (23.2) |
| Gram (+) | 9 (13.1) |
| Gram (−) | 5 (7.2) |
| Combined Gram (+) and Gram (−) | 2 (2.9) |
| TRM | 2 (2.9) |
All patients suffered from oral mucositis during the therapy. Grade 3 oral mucositis developed in 26 patients (37.7%). Grade 4 oral mucositis was not observed. Pulmonary toxicity did not develop. Grades 3 or 4 hepatic toxicity occurred in seven patients (10.1%). Venoocclusive disease developed in two patients (2.9%). Grade 3 or 4 renal toxicity developed in two patients (2.9%). Grade 3 or 4 cardiac toxicity developed in two patients (2.9%): one patient with Grade 4 had a baseline LV ejection fraction of 47% and another patient with Grade 3 had a cumulative doxorubicin dose of 400 mg/m2 BSA at baseline.
Febrile neutropenia developed in 61 patients (88.4%). Among them, 16 patients (23.2%) had microbiologically documented sepsis. No candidemia was reported. Transplant-related mortality (TRM) developed in two patients (2.9%): all resulted from uncontrolled infection.
Risk factors for survival
Univariate and multivariate analysis of risk factors for EFS and OS are shown in Tables III and IV, respectively. In the univariate analysis, EFS was related to the number of prior treatment regimens, stem cell source, and high serum LDH (≥500 IU/L; Table III). OS was associated with the number of prior treatment regimens, stem cell source, high serum albumin (≥4.0 g/dL), high serum LDH (≥500 IU/L), and high hemoglobin (≥10.0 g/dL; Table IV). These variables were selected for multivariate analysis. The multivariate analysis revealed that the number of prior treatment regimens, stem cell source, and serum LDH ≥500 IU/L were significant factors for both EFS (Table III) and OS (Table IV).
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI)a | P | HR (95% CI) | P | ||
| Age | <40 years | 1 | − | − | |
| ≥40 years | 0.67 (0.34–1.33) | 0.256 | |||
| Sex | Male | 1 | − | − | |
| Female | 0.91 (0.64–1.32) | 0.630 | |||
| Histology | B-cell | 1 | − | − | |
| T/NK-cell | 1.24 (0.60–2.57) | 0.566 | |||
| Ann Arbor stage at diagnosis | I–III | 1 | − | − | |
| IV | 1.59 (0.77–3.28) | 0.215 | |||
| No. of prior treatment regimens | 1 | 1 | 1 | ||
| 2 | 4.59 (1.71–12.27) | 0.002 | 6.20 (1.78–21.56) | 0.004 | |
| 3 or more | 5.21 (1.70–16.02) | 0.004 | 6.35 (1.58–25.43) | 0.009 | |
| Prior anthracycline doseb | <350 mg/m2 | 1 | – | – | |
| ≥350 mg/m2 | 0.92 (0.40–2.12) | 0.840 | |||
| Disease status at treatment | CR | 1 | – | – | |
| PR | 1.37 (0.65–2.89) | 0.406 | |||
| Stem cell source | PB | 1 | 1 | ||
| PB + BM | 5.80 (2.43–13.85) | <0.001 | 4.87 (1.86–12.78) | 0.001 | |
| Serum albumin | <4.0 g/dL | 1 | – | – | |
| ≥4.0 g/dL | 0.46 (0.20–1.04) | 0.061 | |||
| Serum LDH | <500 IU/L | 1 | 1 | ||
| ≥500 IU/L | 2.92 (1.24–6.88) | 0.014 | 3.94 (1.59–9.81) | 0.003 | |
| Hemoglobin | <10.0 g/dL | 1 | – | – | |
| ≥10.0 g/dL | 0.56 (0.28–1.12) | 0.101 | |||
- BM, bone marrow; CI, confidence interval; CR, complete remission; HR, hazard ratio; LDH, lactate dehydrogenase; PB, peripheral blood; PR, partial response.
- a A higher hazard ratio represents a shorter survival.
- b Expressed in doxorubicin equivalent doses.
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI)a | P | HR (95% CI) | P | ||
| Age | <40 years | 1 | – | – | |
| ≥40 years | 0.71 (0.30–1.66) | 0.426 | |||
| Sex | Male | 1 | – | – | |
| Female | 0.83 (0.52–1.33) | 0.443 | |||
| Histology | B-cell | 1 | – | – | |
| T/NK-cell | 1.49 (0.62–3.60) | 0.375 | |||
| Ann Arbor stage at diagnosis | I–III | 1 | – | – | |
| IV | 1.95 (0.78–4.87) | 0.151 | |||
| No. of prior treatment regimens | 1 | 1 | 1 | ||
| 2 | 14.67 (1.94–111.17) | 0.009 | 9.51 (1.22–73.89) | 0.031 | |
| 3 or more | 15.92 (1.86–136.42) | 0.012 | 5.70 (0.53–61.10) | 0.151 | |
| Prior anthracycline doseb | <350 mg/m2 | 1 | – | – | |
| ≥350 mg/m2 | 0.63 (0.18–2.13) | 0.453 | |||
| Disease status at treatment | CR | 1 | – | – | |
| PR | 0.76 (0.32–1.81) | 0.539 | |||
| Stem cell source | PB | 1 | 1 | ||
| PB+BM | 5.09 (1.95–13.30) | 0.001 | 3.75 (1.17–12.01) | 0.026 | |
| Serum albumin | <4.0 g/dL | 1 | 1 | ||
| ≥4.0 g/dL | 0.37 (0.14–0.96) | 0.041 | 0.68 (0.19–2.39) | 0.546 | |
| Serum LDH | <500 IU/L | 1 | 1 | ||
| ≥500 IU/L | 2.90 (1.06–7.94) | 0.039 | 3.64 (1.11–11.89) | 0.033 | |
| Hemoglobin | <10.0 g/dL | 1 | 1 | ||
| ≥10.0 g/dL | 0.39 (0.16–0.94) | 0.035 | 0.45 (0.16–1.26) | 0.128 | |
- BM, bone marrow; CI, confidence interval; CR, complete remission; HR, hazard ratio; LDH, lactate dehydrogenase; PB, peripheral blood; PR, partial response.
- a A higher hazard ratio represents a shorter survival.
- b Expressed in doxorubicin equivalent doses.
Risk factors for adverse events
Patients with auxiliary bone marrow harvest required a longer duration of neutrophil recovery over 500/mm3 (P < 0.001) and platelet recovery over 20,000/mm3 (P = 0.004). However, the relationship between stem cell source and febrile neutropenia, documented sepsis, or TRM was not significant. In addition, there was no TRM among patients with poor peripheral stem cell mobilization.
Patients with high baseline serum LDH (≥500 IU/L) had significantly higher incidence of TRM than those with low serum LDH (P = 0.013). The other adverse events were not correlated to serum LDH level.
Discussion
In a previous study using the BEAM regimen, the 3-year OS of patients with aggressive NHL was 55–56% [15]. In another study, the 5-year EFS and OS were 59 and 63%, respectively [16]. In this study, the median EFS was 17.9 months and the estimated 2-year and 3-year OS was both 64.2% (Fig. 1). Although the follow-up duration of median 24.7 months in our study is relatively short, our results seem comparable to the results of previous studies.
In another study of HDC using mitoxantrone in patients with refractory lymphoma, escalating doses of mitoxantrone (15–90 mg/m2) followed by the CBV regimen was used and CR was observed in 60% of patients [11]. In this study, mitoxantrone was administered at 12 mg/m2 daily for 3 days and 56.8% of patients with measurable disease achieved CR, which seems comparable to the result of the former study of mitoxantrone plus CBV.
BCNU-related interstitial pneumonitis has been reported in 7–27% of patients receiving BEAM with autologous transplantation [3-5]. In contrast, among patients receiving mitoxantrone-based HDC, the incidence of pulmonary toxicity seems relatively low [11-13]. Besides, melphalan is also reported to induce pulmonary toxicity although there is relatively a paucity of reports on this side effect and the causal mechanism is still not defined [17-19]. In this study, no pulmonary toxicity was reported. This finding suggests that the NEAM regimen could be a useful option for patients with pre-existing pulmonary disease.
Incidence of Grades 3 or 4 hepatic and renal toxicity of the BEAM regimen has been reported as 2–8% and 0–2%, respectively [15, 16, 20, 21]. The incidence of Grades 3 or 4 hepatic and renal toxicity of the TMJ regimen was 19 and 2%, respectively [12]. In our study, Grades 3 or 4 hepatic toxicity (10.1%) tended to be more prevalent than that of the BEAM regimen and less prevalent than that of the TMJ regimen. It is known that mitoxantrone-induced hepatic toxicity is because of the reduction in antioxidant defenses of liver [22]. Hence, it is speculated that mitoxantrone may be responsible for the increase in hepatic toxicity of the NEAM regimen compared with the BEAM regimen. However, in our study, it was transient and no one died of hepatic failure. The incidence of Grades 3 or 4 renal toxicity (2.9%) was comparable to that of the other regimens. TRM of the BEAM regimen and the TMJ regimen has been reported as 3–8% [3, 4, 15, 16, 20, 21] and 4% [12], respectively. TRM of our data (2.9%) did not seem poor.
Mitoxantrone is known to lack the ability to produce free radicals, which is thought to be responsible for the anthracycline-associated cardiac toxicity, and markedly less cardiotoxicity has been reported compared with doxorubicin [23]. However, in this study, symptomatic dilated cardiomyopathy developed in two patients (2.9%) of our study population: one patient had a baseline LV ejection fraction of 47% and another patient had a cumulative doxorubicin dose of 400 mg/m2 BSA at baseline. In a previous study using 40 mg/m2 BSA of mitoxantrone for HDC, 7 out of 100 patients developed Grades 3 or 4 cardiac toxicity [12]. In contrast, the cardiac toxicity was not reported or relatively rare in previous studies using the BEAM regimen [3, 4, 15, 16, 20, 21]. Even though mitoxantrone may have contributed to cardiac toxicity during NEAM therapy, low baseline LV ejection fraction, or high cumulative doxorubicin dose prior to the therapy should also be responsible for cardiac toxicity since mitoxantrone is significantly less cardiotoxic than anthracyclines. Hence, attention should be paid to patients with low baseline LV ejection fraction or high cumulative anthracycline dose when the NEAM regimen is used.
However, it is generally accepted that poor peripheral stem cell mobilization is associated with inferior outcomes in terms of EFS or OS after autologous transplantation in patients with lymphoma [24, 25] although controversies exist [26]. Our results showed that these patients had shorter EFS and OS as well as engraftment. However, these patients did not have higher incidence of febrile neutropenia, documented sepsis, or TRM than the others. All of these patients with poor mobilization previously received more than three treatments, whereas only 3 of 62 patients (4.8%) with good mobilization did. However, the effects of stem cell source on treatment outcomes remained significant even after adjustment for the number of prior treatment regimens in the multivariate analysis (Tables III and IV). The reason for this finding has yet to be clarified.
Serum LDH level was also a well-known risk factor for unfavorable prognosis in patients with lymphoma [27, 28]. However, the relationship between serum LDH level and TRM is still uncertain. In this study, serum LDH level is a significant factor for EFS and OS, and is significantly associated with TRM. These novel findings need to be validated in further studies.
Hemoglobin level and serum albumin level were also known prognostic factors for lymphoma [29, 30]. This relationship was also observed on the univariate analysis of this study. However, these variables were not significant on the multivariate analysis.
In conclusion, HDC using the NEAM regimen with autologous transplantation demonstrated considerable efficacy with modest toxicity as a treatment of patients with chemosensitive aggressive NHL. Multiple prior treatments before transplantation, auxiliary bone marrow harvest for stem cell collection, and high serum LDH level were related to unfavorable treatment outcomes.
Acknowledgements
The authors would like to thank Ms. Eun Mi Kim and Ms. Su Jin Kim for coordinating the procedure of transplantation and Ms. Nam Hee Koo for helping the procedure of stem cell collection.




