Necrosis following skull base irradiation and stem cell transplant for multiple myeloma†
Conflict of interest: Nothing to report.
A 58-year-old Caucasian man with no significant past medical history presented with rapid onset diplopia and blurred vision. Neurological examination revealed bilateral sixth as well as right fourth and fifth cranial nerve palsies, with no other focal neurological deficits. MRI of the head revealed a 6.0 cm × 4.7 cm × 3.2 cm soft tissue mass in the sella turcica, replacing most of the clivus and enhancing strongly after gadolinium administration (see Image 1). The radiological differential diagnosis of the tumor included a clival chordoma, an atypical invasive pituitary adenoma, a solitary plasmacytoma or a bone metastasis. Pituitary function testing demonstrated a markedly elevated serum prolactin (1994 ng/dL, normal 3–13 ng/dL), and bromocriptine therapy was initiated. The patient returned 1 month later with anorexia, nausea, fatigue, and new headache. Blood tests revealed renal dysfunction (creatinine 2.34 mg/dL, normal 0.7–1.2 mg/dL) and hypercalcemia (11.96 mg/dL, normal 8.5–10.3 mg/dL), with a decrease in prolactin (411 ng/dL, normal 2.1–17.7 ng/mL). A renal biopsy revealed nonspecific changes, while urine protein electrophoresis showed large free kappa monoclonal peaks. Bone marrow aspirates showed areas with 40–100% plasmocytes. Hemoglobin was 148 g/L (normal 140–180 g/L), β2-microglubulin 6.2 mg/L (normal 0–2.5 mg/L) and albumin 34 g/L (normal 38–50 g/L). A skeletal survey demonstrated multiple lytic lesions. These new findings were diagnostic of kappa light chain myeloma, ISS Stage III. A transsphenoidal debulking of the sellar lesion was performed, revealing a collision tumor consisting of a prolactinoma and a plasmacytoma. Treatment for MM was initiated with thalidomide and dexamethasone, with supportive pamidronate.
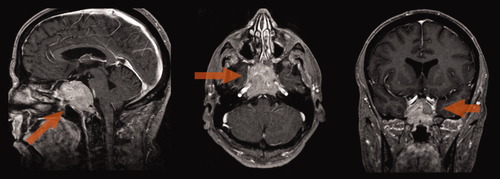
T1 Gadolinium multiplanar MRI of the head showing the large clival mass (arrows).
After surgery, the patient was referred to radiation oncology for consideration of radiation therapy for palliation of worsening cranial nerve deficits. A total of 50.4 Gy in 28 daily fractions was delivered using a multifield intensity-modulated photon plan (1.8 Gy/fraction). The treatment was well tolerated with subtle improvement of the right fifth cranial nerve deficit. On a contrast-enhanced MRI performed 3 months later, the lesion appeared largely necrotic and was decreased in size. Six months after completion of radiation therapy, the patient underwent an autologous stem cell transplant following stem cell mobilization with cyclophosphamide and G-CSF, and melphalan 200 mg/m2.
One year post radiotherapy, the patient presented with a 10-day history of lethargy, nausea and vomiting, new complete left seventh nerve palsy, decreased hearing on the left side, and a left third nerve deficit. A repeat CT scan revealed an unchanged clival lesion, and a new hypodensity at the left cerebellopontine angle measuring 4.8 cm × 2.6 cm, compressing the left cerebellar peduncle and 4th ventricle. An MRI showed new high signal intensity on T2 images involving the left side of the medulla, left inferior and middle cerebellar peduncles, left pons, and left cerebral peduncle. The abnormal signal involved predominantly the white matter without significant mass effect. These findings were compatible with radiation necrosis, infectious changes, or a neoplastic process. The patient had no infectious symptoms, and a lumbar puncture was negative by cytology. Myeloma restaging revealed no serum or urine monoclonal peak. In view of these findings and the overlap of the radiological changes with area irradiated, a clinical diagnosis of radiation necrosis was made (see Image 2). The patient was prescribed dexamethasone without any improvement in his new neurological deficits. Three months later, the patient reported worsening fatigue, lethargy, and depressed mood. A repeat MRI showed progressions of the necrosis, with new lesions in the right and left medial temporal lobes. These areas were hypointense on T1, hyperintense on T2, enhanced and had minimal mass effect relative to their size and location—all in keeping with the diagnosis of radiation necrosis. The patient was referred for hyperbaric oxygen therapy. After 32 sessions over 10 weeks, there was a reduction in the size of the brainstem lesion without effect on the temporal lobe lesions. Now, 36 months since presenting with symptoms of radiation necrosis, the patient is alive and in complete remission of his MM with complete resolution of the sellar lesion on MRI, but with persistent left seventh and eighth cranial nerve deficits.
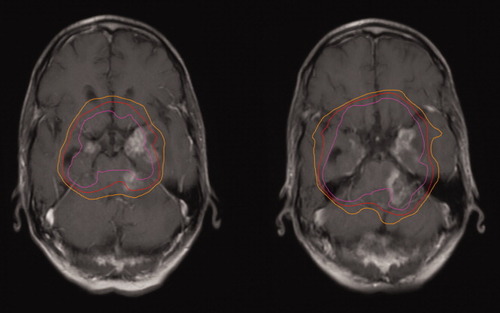
Axial T1 Gadolinium MRI of the brain showing the necrotic lesions within the previously irradiated volume. The pink, red, and orange lines represent overlays of the regions previously treated to 50, 45 and 40 Gy, respectively.
Radiation necrosis is a well documented complication following therapeutic irradiation for cerebral tumors. Known risk factors include the total radiation dose, fraction size, treatment duration, irradiated volume, and concurrent chemotherapy [1, 2]. Although high doses of focal radiation, such as in stereotactic radiosurgery or brachytherapy, are most commonly associated with radiation necrosis, it can also be a complication of external beam radiation therapy—especially when the target lesion is malignant and concurrent chemotherapy is used. In this case, the dose administered (50.4 Gy in standard fractions) is associated with a less than 1% risk of necrosis [3].
The diagnosis of radiation necrosis is difficult as biopsy is often not feasible and, in the case of malignant brain tumors, necrosis can be intermixed with tumor recurrence. On MRI, necrotic regions are enhancing, often with central unenhancing cores. These lesions can have a “soap bubble-like” or “swiss cheese-like” interior [4]. Radiation necrosis can mimic recurrent tumor and evolution over time must often be used to clarify the diagnosis. Magnetic resonance spectroscopy, perfusion-sensitive MRI, fluorodeoxyglucose positron emission tomography and thallium single photon emission computed tomography have all been used as noninvasive means of establishing a diagnosis, with limited success [5]. We considered the clinical and radiological findings specific enough not to pursue these other imaging modalities. The lesions were predominantly in the white matter, had central unenhancing areas and were surrounded by limited edema. Most convincingly, superimposing the radiation planning images over the MRI confirmed that the pathology developed within the dose lines outlining the areas of maximal radiation. In addition, there was no serological evidence of activity of either of the patient's two known malignancies.
We postulate that this very rare complication of skull base irradiation may have been the result of interactions between the radiotherapy and systemic therapy directed at the MM. Prior reports have suggested that particular chemotherapeutic agents have interacted with radiation to increase the risk of radiation necrosis [6]. In recent reports, patients with primary malignant astrocytomas treated with temozolomide and radiotherapy have an up to fourfold increase in radiation necrosis compared to patients receiving radiation therapy alone [1, 7-9]. Our patient was treated with high dose melphalan which, as an alkylating agent, has a similar mechanism of action and is known to be a radiosensitizer [10, 11]. Patients with plasma cell neoplasms are rarely treated with cranial radiation, thus are rarely at risk of radiation necrosis of the brain. In our case, standard dosing of radiotherapy resulted in severe morbidity and illustrates the need to be prudent if irradiating patients with planned exposure to high-dose chemotherapy.




