Effect of oral itraconazole on the pharmacokinetics of tacrolimus in a hematopoietic stem cell transplant recipient with CYP3A5*3/*3
A 21-year-old woman was diagnosed with Hodgkin lymphoma and went into remission with ABVD combination chemotherapy, but relapsed with multiple nodules in the lung fields. She received an allogenic bone marrow transplant from an one-locus mismatched, unrelated donor in October 2009. She was conditioned using a reduced-intensity regimen of fludarabine 25 mg/m2 daily for 5 days and melphalan 90 mg/m2 daily for 2 days. Before starting the conditioning regimen, prospective analysis of the CYP3A5*3 allele was performed using PCR-RFLP [1]. Tacrolimus 0.03 mg/kg daily (from Day −1) and short-term methotrexate (Day 1, 15 mg; Day 3, 10 mg; Day 6, 10 mg) were also given to prevent graft vs. host disease (GVHD).
On Day 40, we switched from continuous infusion of tacrolimus (0.03 mg/kg/day) to tacrolimus capsules at a dose of 0.06 mg/kg/day, given in equally divided doses every 12 h (at 08:00 and 20:00). On Day 48, we started coadministration of itraconazole. After itraconazole instillation at 200 mg twice daily (10:00 and 22:00) for 2 days (Days 48 and 49), the patient was switched to itraconazole oral solution given at 6:00 each morning, beginning on Day 50. Each day for 2 weeks thereafter, venous blood samples were taken just prior to and 2 h (C2h) after tacrolimus administration (08:00) for determination of the tacrolimus, itraconazole, and hydroxyitraconazole concentrations.
The C0 of tacrolimus measured before coadministration of itraconazole were controlled in the range of 5.7 to 8.3 ng/mL. On Day 50, the C0 and C2h for tacrolimus had increased to 16 and 102.4 ng/mL, respectively. From Days 51 to 55, we gradually reduced the tacrolimus dosage from 1 mg/day (0.03 mg/kg/day) administered in two doses to 0.25 mg/day (0.004 mg/kg/day) administered in one dose. Clinically, acute skin GVHD (grade 1) appeared on Day 57, but it was relieved within several days by application of an external steroid. Consequently, the maintenance dosage of 4 mg/day (0.06 mg/kg/day) of tacrolimus alone administered in two doses was reduced to 0.10 mg/day (0.0015 mg/kg/day) with coadministration of itraconazole, which was 1/40 of the dose before combination with itraconazole (Figure 1).
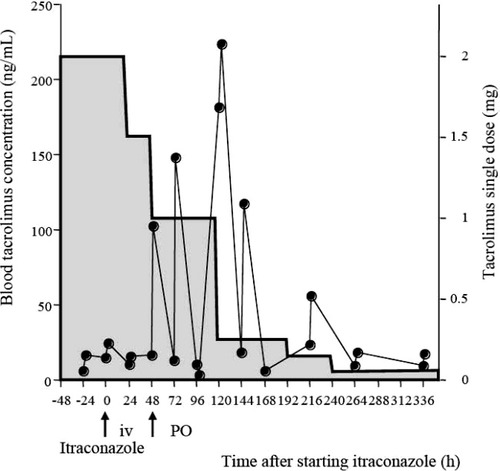
Blood concentration-time profiles (solid circles) after a single dose (gray box) of tacrolimus before and after coadministration of itraconazole to a patient carrying the CYP3A5*3/*3 genotype.
Itraconazole inhibits tacrolimus metabolism via CYP3A, thereby increasing its blood levels [2]. In this case, the C2h for tacrolimus was increased by itraconazole, but the elimination half-life of tacrolimus was always 6.0 ± 0.9 h (range 5.1–7.3 h), which was nearly the same as was obtained with tacrolimus alone. In addition, the drug interaction between tacrolimus and itraconazole was first noted on the day oral administration of itraconazole was begun, which suggests it occurs mainly via intestinal CYP3A.
We were able to predict an approximate next trough concentration for tacrolimus by monitoring the C0 and C2h, and to quickly adjust the daily tacrolimus dose to one appropriate for achieving the desired blood-trough target level (5 to 15 ng/mL). In addition, to reduce the peak concentration, we administered tacrolimus twice daily, even with itraconazole coadministration, and we avoided trying to control only the trough concentration to less than 20 ng/mL by administering the drug only once daily.
Patients with genetic variants of CYP3A5 can be very sensitive to tacrolimus, and the effect of itraconazole after combined administration can appear more quickly than in patients carrying the CYP3A5*1 allele [3]. Combining tacrolimus and itraconazole requires that very careful attention be paid; however, one may be able simply calculate the next tacrolimus dosage by the monitoring C2h, even in patients with the CYP3A5*3/*3 genotype. Prospective analysis of CYP3A5 polymorphism and monitoring of both C0 and C2h would seem to be extremely important for safe and reliable immunosuppressive therapy with tacrolimus.
References
Miho Nara*, Naoto Takahashi*, Masatomo Miura , Hirobumi Saitoh*, Hideaki Kagaya , Kenichi Sawada*, * Department of Hematology, Nephrology, and Rheumatology, Akita University Graduate School of Medicine, Akita, Japan, Department of Pharmacy, Akita University Hospital, Akita, Japan.




