Crown-Ether-Directed Assembly of One-Dimensional Silver(I) Coordination Polymers With Dramatically Enhanced Photoluminescence
Funding: K.-G. Liu is grateful for financial support from the National Natural Science Foundation of China (Grant No. 92161106) and the Natural Science Foundation of Ningxia (Grant No. 2023AA02004). J. Wei is grateful for financial support from the Natural Science Foundation of Ningxia (Grant No. 2024AAC03032). X. Yang is grateful for financial support from the Key Research and Development Program of Ningxia (Grant No. 2021BEB04066). The authors acknowledge the French Grand Equipment National de Calcul Intensif for HCP support (Project a0010807367).
Xiaojiao Yang and Xiao-Lin Ye contributed equally to this work.
ABSTRACT
Two novel 18-crown-6-ether (18-C-6) directed one-dimensional silver(I) coordination polymers (1D Ag(I) CPs), formulated as {[(18-C-6)Ag(bpy)]·X}∞ and {[(18-C-6)Ag(pyz)]·X}∞ {bpy = 4,4′-bipyridine; pyz = pyrazine; X = BF4− (Ia, IIa), CF3SO3− (Ib, IIb)}, are prepared and structurally determined. The protection of the 18-C-6 macrocycle not only efficiently prevents intermolecular interactions within each 1D Ag(I) CP but also significantly enhances the rigidity of the chain structures, allowing these polymers to exhibit remarkable phosphorescence with intense green-light emission at room temperature. Moreover, IIa and IIb show enhanced photoluminescence quantum yields and aggregation-induced emission properties, which can be attributed to their close-packed structure modes in the crystalline states. Interestingly, upon mixing with commercial resin, IIa can serve as an efficient and stable luminescent ink for 3D printing. The resulting printed structures demonstrate exceptional irradiation stability, retaining their luminescence properties without any quenching over a three-month period. This work not only provides a facile strategy to prepare luminescent 1D Ag(I) CPs but also shows the promise of their utilization in optical devices.
1 Introduction
The growing demand for new materials with molecular-scale properties in the past few decades has led to the rapid increase of one-dimensional (1D) nanostructures [1-5]. Among them, organometallic coordination polymers (OMCPs) have attracted great attention due to their remarkable structure derived from various controllable coordination modes and efficient electron transfer between metal centers and organic ligands [6-8]. This makes them promising candidate materials for various applications including nano-electronics [9], nano-devices [10], biomedicine [11], dye-sensitized solar cells [12], supercapacitors [13, 14], and nano-photonics [15]. Coinage metal(I) complexes with d10 electronic configurations are generally expected to show photoluminescence (PL) with high intensity due to their large HOMO–LUMO gaps which afford a substantial energy separation between their singlet ground state (S1) and lowest triplet state (T1) [16-19]. However, it is of note that a large part of coinage metal(I) complexes, especially Ag(I) ones, are in fact weakly emitting or even non-luminescent, including at low temperatures (77 K) [20-24]. Indeed, the emitting properties of this kind of Ag(I) complexes not only depend on the electronic characteristics of the Ag(I) centers but are also affected by their local environment, structural rigidity, intermolecular interactions, aggregation states, etc. [25-34]. Thus, constructing and controlling Ag(I) complexes with high PL remains very challenging.
In general, the Ag(I) cation tends to linearly coordinate to two 2-electron neutral donor ligands to form a 14-electron ML2 species such as, for instance, the diamine-silver(I) complex [Ag(NH3)2]+ [35]. This kind of compound hardly shows any luminescence since, upon excitation, the energy is dissipated via vibrational relaxation of the rotatable ligands. One way to possibly trigger the luminescence of linear Ag(I) complexes is to aggregate the metal centers, by using ditopic bridging ligands such as 4,4′-bipyridine (bpy) [36-38] or pyrazine (pyz) [39-43], to form a 1D infinite structure with enhanced regularity and rigidity. But still, this is not enough, and the linear chain-structured [Ag(bpy)]∞ or [Ag(pyz)]∞ coordination polymers (CPs) do not luminesce [44-47]. Indeed, in the solid state, the 1D chains are not isolated from each other but easily interconnect to form complicated networks via the coordination of counterions, π···π stacking, or argentophilic interactions [44, 45], resulting in luminescence quenching (Figure 1). Therefore, the PL properties of the “true” 1D Ag(I) CPs are rarely explored.
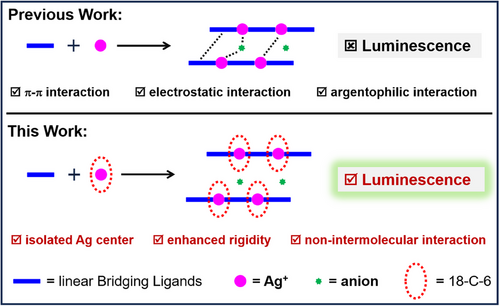
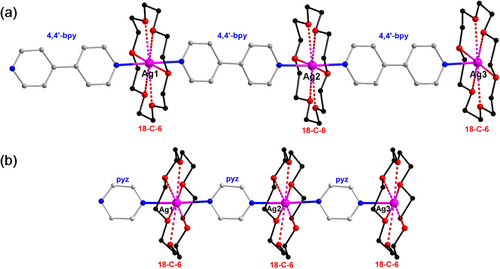
One potential strategy to suppress intermolecular interactions and trigger the PL of non-luminescent [Ag(bpy)]∞ and [Ag(pyz)]∞ CPs is to isolate each Ag(I) center using protective macrocycles [44-47]. Notably, 18-crown-6-ether (18-C-6) is a suitable candidate because its cavity size precisely matches the dimension of silver atoms, enabling multidentate coordination with Ag(I) centers. Herein, we report for the first time a crown-ether directed method to achieve the “true” 1D Ag(I) CPs ([Ag(bpy)]∞ and [Ag(pyz)]∞). In these CPs, each Ag(I) center is linearly coordinated to bpy or pyz ligands, and “nested” in an 18-crown-6-ether (18-C-6) macrocycle, leading to an infinite “Sugar-coated Haws”-like 1D structure (Figure 2). The obtained Ag(I) CPs show then dramatically enhanced room temperature phosphorescence and aggregation-induced emission (AIE). The protection of 18-C-6 prohibits any type of interaction between chains, making them well-separated and with enhanced regularity and rigidity (Figure 1). The structures, stability, and optical properties of these novel CPs were further explored by various characterization tools and density functional theory (DFT) calculations. The main results are reported and discussed here.
2 Results and Discussion
2.1 Synthesis and General Characterization
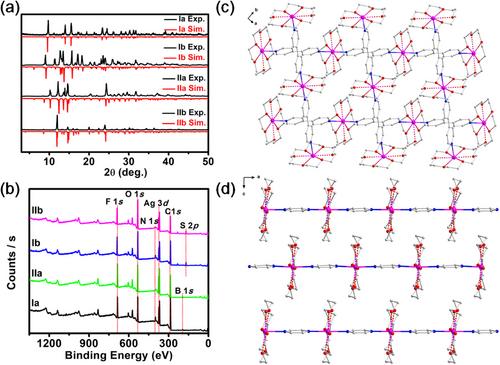
Four Ag(I) CPs were synthesized under similar solvothermal conditions. In brief, 18-C-6 was mixed with AgBF4 or AgCF3SO3 in a methanol solution, and after ultrasonic treatment for 5 min, a colorless solution was formed. Then, the bridging ligands (4,4′-bpy or pyz) were added, under ultrasonication for 10–15 min to obtain a white turbid solution. The mixture was then heated to 70°C and kept for 20 h. After cooling to room temperature, colorless elongated crystals of {[(18-C-6)Ag(bpy)]·X}∞ {X = BF4− (Ia), CF3SO3− (Ib)} [48] and {[(18-C-6)Ag(pyz)]·X}∞ {X = BF4− (IIa), CF3SO3− (IIb)} [48] were obtained directly with acceptable yields (see Supporting Information). Ia (IIa) and Ib (IIb) just differ from their counterions (Figure S1). A powder X-ray diffraction (PXRD) analysis was performed to determine the nature of these Ag(I) CPs. Their PXRD spectra were found to fit nicely with their homologs simulated from the single-crystal X-ray data described below (Figure 3a). X-ray photoelectron spectroscopy (XPS) confirmed the +1 oxidation state of Ag in all four CPs (Figure 3b). No other elements were detected, except C, N, O, F, B (or S), indicating that their elemental compositions were in line with their molecular formula. The prepared Ag(I) CPs were also characterized by IR spectroscopy (Figure S2). Peaks at 1596/1351 and 1601/1352 cm−1, were observed for Ia and Ib, respectively, assigned to C═N/C─N stretching vibrations of the 4,4′-bpy ligands [49, 50]. For IIa and IIb, peaks were observed at 1601/1352 cm−1, corresponding to C═N/C─N stretching vibrations of the pyz ligands [51]. The presence of 18-C-6 in all Ag(I) CPs was confirmed by observing their typical n(C-O) frequencies {1110 cm−1 (Ia), 1106 cm−1 (Ib), 1111 cm−1 (IIa), and 1106 cm−1 (IIb)} [52]. Infrared bands at 1051 and 1055 cm−1 in Ia and IIa, respectively, confirmed the presence of BF4− counterions. Finally, the presence of CF3SO3− in Ib and IIb was confirmed with bands at 1269 cm−1/636 cm−1 and 1268 cm−1/638 cm−1 for SO3, and 1028 and 1032 cm−1 for CF3, respectively.
2.2 Crystal Structure Determination
The crystal structure of the four synthesized Ag(I) CPs was determined by single-crystal X-ray diffraction (Table S1). The structural arrangement shows each silver(I) coordinated to two N atoms of bpy (Ia, Ib) or pyz (IIa, IIb) in a head-to-tail mode, and nested in an 18-C-6 crown ether, leading to an infinite 1D linear structure. The Ag─N bond lengths range from 2.226(13) to 2.308(3) Å (Table S2), with an average bond length of 2.285(5) (Ia), 2.252(13) (Ib), 2.273(3) (IIa), and 2.296(3) (IIb). These bond lengths are similar to those previously reported in Ag-bpy or Ag-pyz coordination compounds [44, 45]. The N─Ag─N angles are close to 180° in all the CPs series, with 179.5(2)° for Ia, 175.9(6)∼178.4(6)° for Ib, 177.57(15) for IIa, and 174.42(12)∼177.96(12)° for IIb. Ag─O (oxygen atoms in 18-C-6) distances for all Ag(I) CPs are in the range of 2.654-2.859 Å (Table S2), comparable to distances usually reported in 18-C-6 silver assemblies, indicating relatively weak interactions between 18-C-6 and silver ions [53-55].
Although the 1D chains in I and II are comparable, they stack in a different manner. Layers of parallel 1D chains stack perpendicularly to each other along the c direction in I (Figure 3c and Figure S3), forming pores where the counterions are located (Figure S3). In II, layers of parallel 1D chains are densely packed on top of each other along the x direction with the 18-C-6 of one 1D polymer chain inserted in the space formed by two 18-C-6 of two neighboring 1D polymer chains (Figure 3d). These different stackings are indeed important for the PL properties of these CPs, which will be discussed in the following sections.
2.3 Stability Analysis
In general, silver(I) coordination compounds show poor thermal stability, and they usually are sensitive to light and air. Notably, they readily undergo photoreduction to metallic Ag nanoparticles upon light exposure or transform into Ag2O following photo-induced ligand dissociation under air conditions [56-58]. This is not the case here, with the protection of the 18-C-6 macrocycles, the CPs are indeed more inert, giving these polymers excellent stability. The thermal stabilities of the solid Ag(I) CPs were evaluated by thermogravimetric analysis (TGA) (Figure S4). The thermal decomposition process for all CPs includes two steps. In the case of Ia and Ib, the first decomposition starts at 165°C with mass losses of 45 % and 41 %, close to the theoretically calculated values of 43% and 40%, which are 18-C-6 losses. The second decomposition occurs at 265°C, releasing 4,4′-benzylpyridine and the counter ion. Similarly, for IIa and IIb, the first decomposition started at 170°C with mass losses of 14.5% and 13%, close to the theoretically calculated values of 14.8% and 13.5%, corresponding to the loss of pyrazine, while the second decomposition started at 223°C (IIa) and 211°C (IIb), associated with the release of 18-C-6 and the counter ion. After these two decomposition steps, the mass percentage of residue was 15.7% in Ia, 20.2% in Ib, and 19.4% in both IIa and IIb, indicating complete decomposition of the organic fraction. Variable temperature X-ray diffraction analysis (Figure S5) further confirmed that these Ag(I) CPs can be kept stable up to nearly 165°C. Their stability in solution was also evaluated by variable temperature ultraviolet (UV) spectroscopy (Figure S6). Results show that these Ag(I) CPs have good stability in CH3CN solutions at 80°C. These analyses indicate that they show very good thermal stability compared to other silver(I) coordination compounds [44, 45]. In addition, they can be exposed to natural light and air for over one week without showing any decomposition (Figures S7 and S8), indicating that the title Ag(I) CPs series exhibit excellent photo- and air-stabilities.
2.4 Excitation Properties
Solid-state UV–vis absorption and UV diffuse-reflectance spectra of the four Ag(I) CPs are shown in Figure S9. They depict similar spectral patterns, with a broad absorption band between 220 and 450 nm. Their optical energy gaps, measured from the solid UV–vis diffuse reflectance spectra (Figure S9b), are calculated to be 3.11 eV (Ia), 3.06 eV (Ib), 2.92 eV (IIa), and 2.93 eV (IIb), consistent with colorless crystals. The UV−vis absorption spectra (Figure S10a) of I in a CH3CN solution exhibit a prominent peak at ∼ 238 nm (εmax ∼ 238 nm (3.04 × 104 L•mol−1•cm−1) for Ia, and εmax ∼ 238 nm (1.43 × 104 L•mol−1•cm−1) for Ib), which is consistent with the absorption peak of 4,4′-bpy ligands. Those of II (Figure S10b) in the same solution show two prominent peaks at ∼ 260 nm (εmax ∼ 260 nm (5.60 × 103 L•mol−1•cm−1) for IIa, and εmax ∼ 260 nm (1.43 × 104 L•mol−1•cm−1) for IIb), and at ∼ 313 nm (εmax ∼ 313 nm (712.0 L•mol−1•cm−1) for IIa, and εmax ∼ 313 nm (1020.0 L•mol−1•cm−1) for IIb), consistent with absorption peaks of pyz ligands.
The geometrical, electronic, and optical properties of the Ag(I) CPs were further investigated by means of DFT calculations at the PBE0/Def2-TZVP/D3(BJ) level of theory. Calculations were first performed on the oligomeric models [{(18-C-6)Ag}3(bpy)4]3+ (Ag-bpy-tri) and [{(18-C-6)Ag}3(pyz)4]3+ (Ag-pyz-tri) used to mimic 1D I and II cationic chains. Their optimized geometries of lowest energy (Figures S11 and S12) were found to be of C1 symmetry (namely Ag-bpy-tri (C1) and Ag-pyz-tri (C1)) and to exhibit non-coplanarity of their ligand rings, in contrast with the crystal structures of I and II in which the ligands are all nearly coplanar (Figure 3c,d). Consequently, calculations were also performed under the C2 symmetry constraint, which provided the optimized structures Ag-bpy-tri (C2) and Ag-pyz-tri (C2) of nearly C2h symmetry (Figures S11 and S12) in which the ligands can be considered as co-planar. These two structures are less stable than their C1 counterparts by 0.39 and 0.14 eV, respectively, but their electronic structures are quite similar, as exemplified by the nature and axial properties of their HOMOs and LUMOs plotted for both structures in Figures S11 and S12. As expected, the HOMO in both models and for both C1 and C2 geometries is mainly of σ(Ag-bpy/pyz) character, whereas the LUMO is π*(bpy/pyz) in nature (Figures S13 and S14).
For each model, time-dependent DFT (TD-DFT) calculations were performed to simulate the UV–vis spectra of their C1 and C2 optimized geometries. The simulated UV-vis spectrum of Ag-bpy-tri (C1) is in very good agreement with the experimental one recorded for Ia (Figure 4a), with two slightly red-shifted (less than 20 nm) absorption bands. The electronic transitions associated with the simulated optical bands of Ag-bpy-tri (C1) are given in Table S3 and Figure S15. The nature of the electronic transitions, investigated by a natural transition orbital (NTO) analysis, indicates that the band α is mostly due to a strong metal (4d(Ag))–to–ligand (π*(bpy)) charge transfer (MLCT) (Figure 4a,b). The band β involves mixed metal and ligands (4d(Ag), π(18-C-6) and π(bpy)) to π*(bpy) MLCT and ligand–to–ligand charge transfer (LLCT) transitions (Figure 4a,b). In contrast, the two above-discussed bands are more red-shifted in the simulated spectrum of Ag-bpy-tri (C2) (265 and 284 nm). Furthermore, the latter exhibits an experimentally non-observed weak band at 322 nm (Figure 4a), which is mainly attributed to HOMO−7→LUMO and HOMO−8→LUMO+1 transitions (Table S3 and Figure S16). This weak band is also computed around 330 nm in the C1 structure but with a very small oscillator strength. This result suggests that in solution compounds I likely lose their ligand coplanarity, the latter being imposed by the crystal forces in the solid. In order to understand the variations of the excitation properties between I and the individual ligand, the absorption spectrum of bpy was also simulated. The TD-DFT simulated UV-vis spectrum reproduces well the characteristics of the experimental one. The absorption bands α and β involve HOMO → LUMO and HOMO−2 → LUMO transitions, respectively (Figure S17). Both transitions are of π(bpy) → π*(bpy) nature (Figure S17).
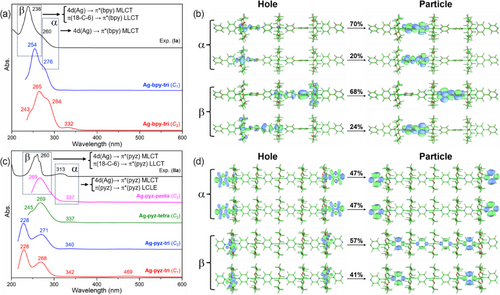
The simulated UV-vis spectrum of Ag-pyz-tri (C1) shows four absorption bands at 469, 342, 268, and 228 nm (Figure 4c). The weak band at 469 nm, describing a HOMO−1 → LUMO transition (Table S4 and Figure S18), which is not observed in the experimental spectrum of IIa, disappears in the simulated spectrum of Ag-pyz-tri (C2) (Table S4 and Figure S19), suggesting that, contrarily to compounds I, their II relatives tend to preserve ligand coplanarity in solution. In order to get a better agreement between the simulated and experimental spectra, calculations on the larger oligomers [{(18-C-6)Ag}4(pyz)5]4+ (Ag-pyz-tetra) and [{(18-C-6)Ag}5(pyz)6]5+ (Ag-pyz-penta) were carried out under C2 symmetry constraint. One can note that when going from Ag-pyz-tri (C2) to Ag-pyz-penta (C2) the two absorption bands of higher energy are gradually merging, with a good agreement between the simulated spectrum of Ag-pyz-penta (C2) and the experimental spectrum of IIa (Figure 4c, Table S4 and Figure S20). An NTO analysis of Ag-pyz-penta (C2) indicates that the absorption band α involves both 4d(Ag) → π*(pyz) MLCT and σ(Ag-pyz) → π*(pyz) ligand-centered local excitation (LCLE) (Figure 4c,d). The absorption band β corresponds to mixed 4d(Ag) and π(18-C-6) to π*(pyz) ligand transitions (Figure 4c,d). In addition, the absorption properties of the individual pyz ligand were also investigated. TD-DFT simulated UV-vis spectrum exhibits a good match with the experimental one, with minor blue-shifted excitation energies (less than 50 nm). The absorption bands α and β of pyz ligand are associated with HOMO → LUMO and HOMO−1 → LUMO transitions, respectively (Figure S21). Both transitions are π(pyz) → π*(pyz) in nature (Figure S21).
In order to check a potential bias caused by the ligand lone pairs situated at the two ends of the oligomers, we have also simulated the UV-vis spectra of the hypothetical species [{(18-C-6)Ag}3(bpy)2(bpyH)2]5+ and [{(18-C-6)Ag}5(pyz)4(pyzH)2]7+ (Figures S22–S25), in which the lone pairs are protonated. They exhibit the same shape as those of their Ag-bpy-tri (C1) and Ag-pyz-penta (C2) homologs and the transitions are of the same nature. Unsurprisingly, they are red-shifted by ∼100 nm due to the large cationic charge of these species. These results indicate that the largest considered oligomers are reasonably good models for the real polymers I and II.
2.5 PL Properties
The PL properties of the Ag(I) CP series were explored at room temperature. Their emissions are very weak in solution, but dramatically enhanced in the solid state. With excitation at 365 nm, Ia exhibits intense green emission with a maximum wavelength at 505 nm (Figure S26a). With excitation at 362 nm, Ib exhibits intense cyan emission with a maximum of 482 nm (Figure S26b). Excitation of IIa at 368 nm results in a cyan emission with a maximum of 525 nm (Figure 5a), whereas that of IIb at 366 nm shows a green emission at a maximum of 485 nm (Figure S26c). More importantly, the absolute photoluminescence quantum yield (PLQY) and lifetimes of I and II are very different from each other in the solid state. The PLQYs and lifetimes measured for Ia and Ib are comparable (15.3% and 2.28 ms, and 14.5% and 13.94 ms, respectively) (Figures S27 and S28). Values measured for II are much higher (31.2% and 58.28 ms for IIa, and 35.8% and 123.54 ms for IIb). Among them, the proportion of long-lived components in IIa and IIb has significantly increased (Table S5). The Knr value (Table S6) of samples IIa and IIb is significantly lower than that of Ia and Ib, indicating that their non-radiative decay paths are effectively suppressed, which explains their significantly enhanced PLQYs and lifetimes for the later series originates, in turn, from the higher rigidity of their packing structures (Figure 3c,d). The high QYs, microsecond-scale lifetimes, and large Stokes shifts suggest that the photoemissions of these Ag(I) CPs series are phosphorescent in nature. In addition, these four Ag(I) CPs also exhibit temperature-dependent luminescence behavior. Their PL spectra and lifetime were investigated in the solid state from 97 to 298 K (room temperature), and the Ia and IIa quantum yields were studied at low temperatures of 77 K. Their color and emission wavelength did not change with temperature variation, but their emission intensities, lifetime, and quantum yield (68.1% and 82.3%) increase gradually with the decrease of temperature (Figure 5b and Figures S26d–f, S29, and S30). A good correlation between the maximum emission intensity (Imax) and the temperature (T) was found for the whole Ag(I) CP series (Figure S31), with correlation coefficients of 0.99 (Ia), 0.99 (Ib), 0.99 (IIa), and 0.99 (IIb).
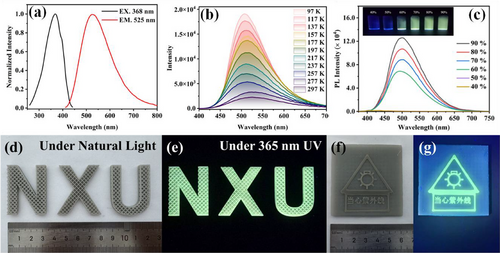
Interestingly, the large difference in the PL properties of compound II between solution and solid state strongly encouraged us to further explore their AIE activity, which was investigated by controlling the polarity of mixed-solvent systems. IIa was analyzed in a DCM/ether solvent solution, in which the aggregation degree was varied by the volume fraction of ether in the mixed solvent (). The dilute solution of IIa in DCM was completely non-emissive. A weak green emission was observed when fe increased to 60%, followed by a further enhancement of the PL intensity with a further increase of the ether fraction. Finally, strong green emission was observed after fe increased to 90%, indicating the formation of well-dispersed colloids of denser aggregates (Figure 5c). IIb displayed similar AIE responses to that of IIa in an acetone/ether solvent system, where its emission was triggered when the ether fraction increased to 80% (Figure S32).
Owing to the remarkable phosphorescence properties recorded for both I and II CPs due to a T1 → S0 decay process, the geometric and electronic structures of the T1 state for the trimer models of I (Ag-bpy-tri (C1)) and II (Ag-pyz-tri (C2)) were then investigated using DFT calculations. The optimized T1 geometry of the first one shows an average Ag–N distance (2.20 Å) slightly shorter than that computed for the S0 geometry (2.25 Å) (Figure S33). Moreover, the chain structure with T1 geometry is slightly bent with an angle of 174° between the centered and the two side Ag atoms. The MO diagram of the T1 state of Ag-bpy-tri (C1) is shown on the left side of Figure 6. The resulting electronic structure of the T1 state involves a slight stabilization of the π*(bpy)-type LUMO and a destabilization of the σ(Ag-bpy)-type HOMO, leading to a calculated T1 → S0 emission at 495 nm (2.51 eV), in an excellent agreement with the values measured from the PL experiments of Ia and Ib (505 and 482 nm, respectively). The spin density of the T1 state of I (Ag-bpy-tri (C1)) is mainly distributed on the Ag(I) center and its coordinated bpy ligand, indicating that the triplet excitons are coming from the σ(Ag-bpy)-type HOMO (S0) to the neighboring π*(bpy) type orbitals (Figure S34).
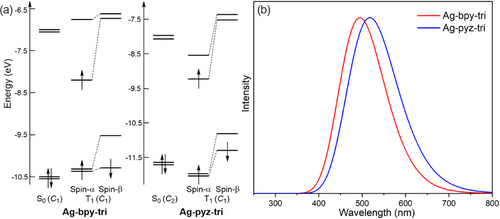
The T1 geometry of Ag-pyz-tri (C2) was optimized in C1 symmetry (not far indeed from C2 symmetry). The optimized average Ag–N distance (2.18 Å) is notably shorter than that in the S0 geometry (2.30 Å) (Figure S35). In addition, the chain structure of the T1 geometry is also slightly bent with an angle of 178.8° between the centered and the two side Ag atoms. The electronic structure of this T1 state of Ag-pyz-tri (C2) indicates a slight stabilization of the π*(pyz)-type LUMO and a destabilization of the σ(Ag-pyz)-type HOMO (Figure 6a), leading to a calculated T1, l0 emission at 518 nm (2.39 eV) (Figure 6b), which is consistent with the PL emissions measured experimentally for IIa and IIb (525 and 485 nm, respectively). The spin density of the T1 state of II (Ag-pyz-tri (C2)) is mainly distributed on the pyz ligands and to a lesser extent on the Ag(I) centers, indicating that the triplet excitons are transferring from the σ(Ag-pyz)-type HOMO (S0) to the neighboring π*(pyz) type orbitals (Figure S34).
2.6 3D Printing of Ag(I) CPs-Based Luminescent Materials
With respect to its remarkable emission properties and good solubility, IIa was used as a luminescent ink for the production of 3D architectures including the logo letters of Ningxia University (NXU, Figure 5d,e), and a UV detector label (Figure 5f,g). IIa exhibits indeed excellent compatibility with the commercial 3D printing resin that was used. As shown in Figure 5d, the printed letters feature W × H × D about 41.2 mm × 32.8 mm × 4.8 mm with a 1.6 mm × 1.6 mm × 1.6 mm cubic-topological hollow structure. Excitation of these letters with 365 nm UV light results in a bright cyan light at = 501 nm (Figure 5e), which is slightly blue-shifted in comparison to that of the IIa powder ( = 525 nm) (Figure S36a). This slight difference is attributed to the interaction of IIa with the printing resin [59]. The printed logo letters display outstanding irradiation stability which can remain luminescent for three months without any quenching. Even under acidic (1 M HNO3 solution) or alkaline (1 M NaOH solution) conditions (Figure S36b,c), the emission intensity of the logo letters did not change within three days. According to the excellent stability of IIa, we further produced a UV detector label by dual-color 3D printing technique (Figure 5f,g), which could be used as a signboard for UV irradiation. The above results strongly encourage further exploration of Ag(I)-based coordination polymers as stable luminescent inks in the application field of 3D printing.
3 Conclusion
In summary, we have reported here for the first time a crown-ether-directed method to construct luminescent 1D Ag(I) CPs. In their structure, each Ag(I) center is linearly coordinated with bridging bpy or pyz ligands, then nested by an 18-crown-6 (18-C-6) moiety, leading to an infinite “Sugar-coated Haws”-like 1D structure. DFT calculations indicate that the excitation properties of these Ag(I) CPs series originate from a significant metal-to-ligand charge transfer (MLCT) involving the 4d orbitals of the Ag(I) centers and the p* orbitals of the bpy (or pyz) ligands. The remarkable phosphorescence observed in these Ag(I) CPs, which arises from a T1 → S0 decay process, is facilitated by the significantly enhanced structural rigidity and suppressed intermolecular interactions between Ag(I) chains. Indeed, this macrocycle-protection-concept is expected to be extended to other Ag(I) CPs systems containing different types of ditopic bridging ligands and macrocycles in the future. The AIE behavior observed for CP IIa and IIb holds the promise to employ Ag(I) coordination polymers in various biological applications such as bioimaging. Moreover, we show that CP IIa can successfully be used as a stable luminescent ink component for 3D printing when mixed with a commercial resin. The resulting material maintains excellent irradiation stability for up to three months without any observable luminescence quenching. The present study provides indeed a new avenue for the construction of 1D Ag(I) CPs with exceptional PL and AIE behavior, opening new horizons for investigating Ag(I) CPs for future functional optical applications.
4 Experimental Section
Synthesis of {[(18-C-6)Ag(bpy)]·BF4}∞ (Ia): AgBF4 (0.0584 g, 0.3 mmol) was dissolved in CH3OH (6 mL) solution containing 18-C-6 (0.0793 g, 0.3 mmol) to obtain a colorless solution. 4,4′-bpy (0.3 mmol, 0.0468 g) was then added under ultrasonication for 10–15 min to obtain a white turbid solution. The reaction mixture was sealed and kept at 70°C for 20 h. After cooling to room temperature, colorless elongated crystals of Ia were obtained (yield: 12.6% (0.023 g, based on AgBF4)). IR (KBr, cm−1): 2900 (m, nC─H); 1596 (m, nC═N); 1351 (m, nC─N); 1100 (s, nC─O─C); 1051 (s, nB─F).
Synthesis of {[(18-C-6)Ag(bpy)]·CF3SO3}∞ (Ib): AgCF3SO3 (0.0770 g, 0.3 mmol) was dissolved in CH3OH (6 mL) solution containing 18-C-6 (0.01584 g, 0.6 mmol) to obtain a colorless solution. 4,4′-bpy (0.3 mmol, 0.0468 g) was then added under ultrasonication for 10–15 min to obtain a white turbid solution. The reaction mixture was sealed and kept at 70°C for 20 h. After cooling to room temperature, colorless elongated crystals of Ib were obtained (yield: 32.4% (0.064 g, based on AgOTf)). IR (KBr, cm−1): 2899 (m, nC─H); 1601 (m, nC═N); 1352 (m, nC─N); 1269 (s, nS═O); 1104 (s, nC─O─C); 1028 (s, nC─F), 636 (s, nS─O).
Synthesis of {[(18-C-6)Ag(pyz)]·BF4}∞ (IIa): AgBF4 (0.0584 g, 0.3 mmol) was dissolved in CH3OH (6 mL) solution containing 18-C-6 (0.0793 g, 0.3 mmol) to obtain a colorless solution. Pyrazine (0.6 mmol, 0.048 g) was then added under ultrasonication for 10–15 min to obtain a white turbid solution. The reaction mixture was sealed and kept at 70°C for 20 h. After cooling to room temperature, colorless elongated crystals of IIa were obtained (yield: 71.5% (0.1157 g, based on AgBF4)). IR (KBr, cm−1): 2888 (m, nC─H); 1601 (m, nC═N); 1352 (m, nC─N); 1111 (s, nC─O─C); 1054 (s, nB─F).
Synthesis of {[(18-C-6)Ag(pyz)]·CF3SO3}∞ (IIb): AgCF3SO3 (0.0770 g, 0.3 mmol) was dissolved in CH3OH (6 mL) solution containing 18-C-6 (0.0793 g, 0.3 mmol) to obtain a colorless solution. Pyrazine (0.6 mmol, 0.048 g) was then added under ultrasonication for 10–15 min to obtain a white turbid solution. The reaction mixture was sealed and kept at 70°C for 20 h. After cooling to room temperature, colorless elongated crystals of IIb were obtained (yield: 57.7% (0.103 g, based on AgOTf)). IR (KBr, cm−1): 2887 (m, nC─H); 1601 (m, nC═N); 1352 (m, nC─N); 1268 (s, nS═O); 1107 (s, nC─O─C); 1032 (s, nC─F), 638 (s, nS─O).
Preparation of IIa-based printing ink: Under ultrasonication, crystals of IIa (5 g) were fully dissolved in 25 mL of CH3CN at room temperature. After 10–15 min, the solution was added into 175 g of the light-cured resin (type: FabPro Proto GRY), then alternatively stirred and sonicated for 3 min at room temperature to achieve a uniform ink.
CCDC 2419774 (for Ia), CCDC 2419775 (for Ib), CCDC 2419776 (for IIa), and CCDC 2419777 (for IIb) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Acknowledgments
K.-G. Liu is grateful for financial support from the National Natural Science Foundation of China (Grant Number. 92161106) and the Natural Science Foundation of Ningxia (Grant Number. 2023AAC02004). J. Wei is grateful for financial support from the Natural Science Foundation of Ningxia (Grant Number. 2024AAC03032). X. Yang is grateful for financial support from the Key Research and Development Program of Ningxia (Grant Number. 2021BEB04066). The authors acknowledge the French Grand Equipment National de Calcul Intensif for HCP support (Project a0010807367). X. Yang and X.-L. Ye contributed equally to this work.
Conflicts of Interest
The authors declare no conflicts of interest.




