Harnessing the Biological Responses Induced by Nanomaterials for Enhanced Cancer Therapy
Funding: National Natural Science Foundation of China (52302359, 82172736), Science and Technology Projects in Guangzhou (Yat-Sen Excellent Young Scientists Fund, 2025A03J4278), State Key Laboratory of Systems Medicine for Cancer (ZZ-94-2306, zz-RCPY-24-41), Guangzhou Science and Technology Projects, State Key Laboratory of Systems Medicine for Cancer, Science and Technology Planning Project of Guangdong Province (2023B1212060013).
ABSTRACT
Nanomaterials (NMs) have garnered decades of research interest owing to their unique physicochemical properties and unparalleled advantages in diverse applications. However, these distinctive characteristics simultaneously raise concerns regarding their biosafety. Recent advancements in understanding NMs–organism interactions have led to innovative strategies for mitigating their intrinsic toxicity. Notably, emerging studies reveal that through rational design and precise manipulation, the inherent toxicological effects of NMs can be strategically repurposed for cancer therapeutics. For instance, functionalized NMs may disrupt oxidative homeostasis, activate programmed cell death pathways, modulate immune responses, or regulate ion channel activities. Despite these promising discoveries, the systematic exploitation of NMs-induced biological responses in oncological interventions remains underexplored. Therefore, this review provides, for the first time, a comprehensive introduction to NM-mediated biological process modulation, focusing on their mechanisms and therapeutic potential in cancer treatment. We have summarized (1) key pathways through which NMs elicit cytotoxic effects, including redox homeostasis regulation, immunogenic cell death activation, and so on; (2) design principles for engineering NMs with controllable bio-interactions; and (3) innovative applications leveraging NM-triggered physical effects (e.g., photothermal conversion, reactive oxygen species generation) as targeted therapeutic modalities. Furthermore, we also highlight the translational significance of harnessing NM-specific bioactivities while discussing current challenges in clinical adaptation and possible solutions. By bridging the gap between nanotoxicology and therapeutic innovation, this manuscript offers novel perspectives for developing next-generation nanomedicine platforms with enhanced efficacy and safety profiles.
1 Introduction
The synthesis, applications, and explorations of nanomaterials (NMs) have attracted attention in various fields such as physics, chemistry, biology, medicine, and engineering [1-3]. Due to their unique chemical–physical properties, NMs are widely applied in medical fields, such as diagnosis, disease treatment, tissue engineering, and so on [4-7]. At present, NMs have already merged into electronic components, surface coatings, sports equipment, cosmetics, food additives, and many other commercial products, inevitably entering the environment, plants, animals, and humans, thereby increasing the proportion of interactions with the environment and organisms [8, 9]. Their toxicological effects on human health have aroused much consideration. The toxicological study on NMs remains buried in a vague and controversial situation. Some studies on the toxicity of carbon nanotubes claimed that it has no apparent toxicity or even therapeutic ability. However, some studies have shown the opposite experimental results [10, 11]. The same contradictions have been reported for graphene [12]. Such a dilemma appeared because the toxicity of NMs is governed by many complicated factors, including size, purity, surface properties, surface charge, hydrophilicity, surface modification, exposure dose, exposure time, and even reaction medium [13]. Thankfully, extensive research on the toxicity of NMs has led to the establishment of a vital subdiscipline, nanotoxicology. This discipline focuses on the interaction between NMs and biological systems, including tissues, organs, cells, and subcellular structures. It even specifies the interaction of biological macromolecules and the consequences of these contacts [14]. Understanding the mechanism of toxicity induced by NMs is of great importance for reducing related nanotoxicity and expanding the application of NMs in the biological field. Although there are many studies on the toxicity of NMs interacting with tissue, organ, cell, and even biomacromolecule levels, the mechanism of toxicity induced by NMs still needs to be completed.
So far, cancer is still one of the significant health problems seriously threatening human life. To date, most cancer treatment strategies have focused on conventional methods such as surgery, chemotherapy (CT), and radiation therapy (RT), where the efficacy of the treatments varies from tumor type. Tumors always fight back and force by increasing their malignancy via continuous mutation that helps them develop multiple resistances. Surgery is the most common and, until now, the most effective method for curing solid tumors. However, surgery can hardly deal with the recurrence and metastasis of cancer [15]. CT, solely or synergistically with other traditional or specialized approaches, is a crucial strategy to fight against cancer regardless of limitations in surgery [16]. At least 50 types of CT agents are available in the clinic to deal with 200 types of cancers [17]. However, the therapeutic outcome of CT is always compromised by tumor defenses, in which cancer cells exhibit versatile strategies to defend themselves against drugs, called multidrug resistance (MDR). MDR was first proposed in the late 1970s and occurs when chemotherapeutics are used for a while, contributing to almost 90% of therapeutic failure or tumor relapses in patients [18]. Moreover, traditional chemotherapeutics lack specificity and will damage both normal and tumor cells because of their nonspecificity, leading to systemic side effects. RT performed under a range of radiation doses has minimal impact on metastases and nonspecific damage to normal tissue, thus broadly limiting their use. Currently, the average curing percentage of tumors by RT is less than 40% [19]. The main reasons attributed to the poor or even disappointing therapeutic outcome generated by RT are the radiation resistance of tumor cells and the toxic side effects on normal cells, which are related to various factors, like the type of radiation, irradiation dose, and tumor cell type [20]. Compared with conventional cancer treatment strategies, targeted therapy exhibits advantages in efficacy and tolerability. Small molecular inhibitors are one of the primary targeted therapies. More than 80 small molecular inhibitors are used in clinical trials for cancer treatment and effectively improve prognosis efficacy [21]. However, the small molecular inhibitors still face multiple barriers, such as low response rate and duration, toxicity, and primary and recurrent resistance. Protein biologics are an emerging class of biopharmaceutical products that have seen rapid growth in approvals over the past few decades, with more than 180 new drugs approved by the United States Food and Drug Administration (US FDA) and European drugs Medicines Agency (EMA) between 2018 and 2022 [22]. Protein biologics have various modalities: monoclonal antibody, cytokine, recombinant protein, vaccine, antibody–drug conjugate, and so on. Due to three-dimensional structures, they can interact with specific targets in the body with significant selectivity and affinity, providing unique advantages. Nevertheless, protein's instability and aggregation issue is one of the major obstacles, as they are susceptible to various destabilizing factors. Besides, immunogenicity, high cost of production, and heterogeneity also hinder their efficacy and large-scale production. Immunotherapy is a burgeoning and promising method for cancer treatment. Since 1891, when William B. Coley first discovered that bacterial toxins could treat desperate patients with bone and soft-tissue sarcoma, immunotherapy has been dramatically and gradually flourishing for a century [3]. Although immunotherapy has shown promising therapeutic outcomes against various cancers, it is hard to generate a satisfactory tumor-killing effect, which is greatly hindered by inadequate infiltration of immune cells or a deeply immunosuppressive tumor microenvironment (TME) [23]. Therefore, an urgent need to improve immunotherapy available in anticancer therapy.
Currently, the US FDA and EMA have approved several nanodrugs for cancer therapy, including lipid-based (56%), protein-based (38%), and metallic-based nanoformulation (6%) [24, 25]. The lipid-based nanoformulation is the first approved and most used nanodrug. For example, Doxil (pegylated doxorubicin), the first US FDA-approved nano-drug in 1995, is used in ovarian cancer, Kaposi's sarcoma, and multiple myeloma treatment. Caelyx, DaunoXome, Myocet, and so on are lipid-based nanodrugs approved for various types of cancer. Protein-based nanoformulation drugs are synthesized using proteins, such as albumin, fibroin, lipoprotein, and gelatin, while albumin is the most widely used. For example, Abranane is an albumin-bound paclitaxel (PTX) and a practical option for most solid cancer treatment. Until now, NanoThermTM is the only metallic-based nanodrug approved for glioblastoma and prostate cancer and is always synergistically used with CT and RT. Research on nanodrugs is still in full swing, with more than 500 in clinical trials, most of which are in clinical phase I and II and are mainly targeting cancer.
Although some nanodrugs have shown clinical benefits, the contemporary use of NMs for cancer treatment has been limited to mere carriers for therapeutic agents [26]. Intriguingly, several studies have proved that specific nanoparticles (NPs) can selectively activate several cellular biological processes to independently regulate cell fate, including ferroptosis, pyroptosis, autophagy, or/and necroptosis, without an external stimulus under certain circumstances, through the interaction between the NMs and cancer cells or organism proteins or inducing immune correlated cell death (ICD). This may ultimately lead to cancer cell death with high selection and be free of inducing related resistance for the independence of specific therapeutic targets. This phenomenon is encouraging because NMs exhibit tremendous potential to influence cancer cells intrinsically without other extrinsic stimuli or triggering unwanted effects. Furthermore, the stimulation or activation capability of NMs can be tuned precisely by chemical manipulations or modifications. For all these reasons, it is necessary to systematically study or introduce the effects of NMs in stimulating biological processes from the perspective of nanotoxicology and therapeutics.
Therefore, for the first time, we provide a clear overview of NMs-mediated modulation of biological processes in this review, such as interfering with oxidative homeostasis, interacting with ion channels, regulating cellular metabolism, triggering programmed cell death (PCD), inducing immune activation, and their related investigations and potential applications in cancer therapy (Figure 1). We will also briefly discuss how the biological processes started by NMs can be manipulated and controlled. Finally, we will summarize how NM-induced physical effects can be exploited as a novel therapeutic tool and why they have significant and promising meanings.
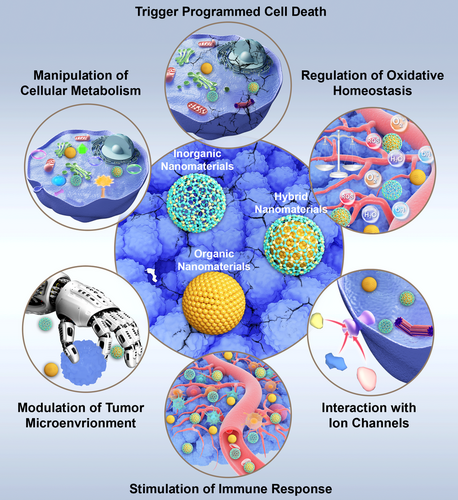
2 Results of Nano–Bio Interaction
With the burst of advancements in nanotechnology, more and more NMs have been subjected to industrial production. They are extensively used in many fields, increasing the possibility that a human being is exposed to NMs by significantly interacting with the environment, ecology, and workplace [27]. Due to the unique properties of NPs, especially the large specific surface area caused by their nano-scale volume, there are more active sites on the surface to participate in different biochemical reactions, so NPs possess a solid ability to penetrate tissues. Studies have confirmed that NPs can enter the human body by several routes, including ingestion, inhalation, dermal penetration, and blood circulation [28]. Therefore, NMs with different characteristics can affect organisms at multiple levels, including tissues/organs, cells, subcellular levels, proteins, and gene levels, ultimately leading to diverse responses.
2.1 Interference with Redox Homeostasis
Redox homeostasis is a crucial dynamic process balanced by intracellular reductive and oxidative reactions, which also play a pivotal role in managing biological events [29]. The regulation of reactive oxygen species (ROS) mainly achieves the maintenance of cellular redox homeostasis. ROS represents a category that indicates the chemical species produced by the incomplete reduction of oxygen, including the superoxide anion (O2•−), hydrogen peroxide (H2O2), singlet oxygen (1O2), and hydroxyl radical (•OH). These ROS participate in almost all biological processes and reactions, regulating various physiological functions in living organisms [30]. However, the abnormality of ROS may lead to severe oxidative stress associated with several diseases, such as cancer, neurodegenerative diseases, and inflammation [31]. The deregulation of ROS balance would result in disease initiation or even the promotion of cancer development [30]. For tumor cells, they always have reduced ROS clearance ability caused by their disturbed intracellular ROS levels, suggesting that they are more vulnerable to ROS than normal tissues; that is to say, regulating ROS may have a selective killing effect on cancer cells [32]. Modulating ROS content selectively by external excitation (such as light irradiation) or in situ catalytic reactions (chemical reactions initiated by the structure of nanoenzymes or chemistry of NMs that contain metal ions with variable valence) makes damaging and killing tumor cells possible by regulating oxidative stress. Thus, “oxidation therapy” or “nanocatalytic therapy” are also put forward [33]. The production of ROS mediated by NMs can induce oxidative stress and oxidative damage toward cancer cells, thus initiating the subsequent multiple cellular death processes like apoptosis or ferroptosis related to oxidative stress, which is favored by anticancer therapy [34-38].
2.2 Interaction with Ion Channel
The ion channel is a category of transmembrane biomacromolecule proteins with selective permeability to different ions, regulating or controlling various physiological activities of the body, such as signal transportation between neurons, neuromuscular excitation, cell proliferation, sensing of physiological conditions, blood pressure, learning, and memory [39]. Dysfunction in ion channels can generate a variety of serious diseases, such as epilepsy, arrhythmia, and diabetes. The relationship between ion channels and conditions is one of the hotspots of basic research in the biological field. Based on this, ion channels have been widely used as therapeutic drugs in clinical practice. As reported, various ion channel blockers can affect tumor cell proliferation, differentiation, viability, and metastasis at different stages [40]. Therefore, scientists began to consider the use of ion channel blockers as tumor therapeutics and have achieved encouraging progress. For example, calcium channel inhibitors, such as Verapamil, Nifedipine, Diltiazem, Bepridil, and so on, can reverse the MDR in many cancerous cell lines, which may be attributed to competitive inhibition of the function of P-gp that effluxes drugs out of cells [41]. Although many small molecules have shown high efficacy in ion channel inhibition for disease treatment, studies have found that NMs not only act as carriers for on-demand ion channel inhibitors, but some also exhibit intrinsic ion channel inhibition ability. They then presented the ideas by applying them to cancer therapies [42]. The following sections will discuss whether this effect can apply to cancer treatment.
2.3 Intervention in Cellular Metabolism
Cellular metabolism is formed by a set of chemical reactions that occur in living cells. Roughly, these reactions can be divided into catabolic and anabolic reactions. The catabolic reaction always refers to generating energy from nutrients, while the anabolic reaction represents the synthesis of various biomolecules. The reactants or products involved in these chemical reactions are named metabolites [43]. The connection between altered metabolism and cancer is an underdeveloped topic. A very different metabolism from normal cells or tissues is a typical characteristic of cancer, such as the most famous one: the Warburg effect. Besides, mutations in oncogenes and tumor suppressor genes lead to alterations in multiple intracellular signaling pathways that affect and redesign tumor cell metabolism to enhance survival and growth [44]. This difference in metabolism to generate energy gives us new opportunities to specifically target cancer cells while reducing the side effects to normal cells [45].
2.4 Induction of PCD
Many types of PCD and related pathways have been revealed, such as apoptosis, autophagy, ferroptosis, cuproptosis, and so on. Here, we will discuss some common PCD types that NMs can induce. Apoptosis, a regularly mentioned effect caused by NMs, refers to cell death programmed by genes with the typical process of maintaining the stability of the intracellular environment and adapting to the living environment based on animal demands [46]. Apoptotic cells feature specific characteristics, such as cell membrane blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation [47]. The mechanism of apoptosis is mainly concluded in two pathways: the extrinsic and the intrinsic. Intracellular stimuli activate the intrinsic apoptotic pathway, including DNA damage, growth factor deprivation, and oxidative stress. It relies on forming a complex termed the apoptosome, composed of procaspase-9, apoptotic protease-activating factor, and cytochrome c. The extrinsic apoptosis pathway is initiated by binding death receptors (DRs), such as Fas ligand, TNF-related apoptosis-inducing ligand, to DRs of the TNF receptor superfamily [48]. Some research on the safety of NMs has revealed their toxicological mechanism. As the manipulation of the physical properties of NMs is more developed and controllable, the selective induction of apoptosis in cancerous cells is becoming realistic, encouraging us to focus on their cancer-killing potential [49].
Autophagy is a fundamental and evolutionarily conserved process that supports cellular preservation by degrading cytoplasmic material in response to various stresses [50]. Since the overstimulation of this self-degradative process would lead to cell death, a complex signaling network tightly balances the level of autophagy [51]. There are commonly two levels of autophagy in the body: basal and induced autophagy [52]. Basal autophagy is essential for stabilizing cytosolic components. In contrast, induced autophagy is used to generate amino acids when cells face starvation, in which typical factors that induce autophagy in cells are nutrient deficiencies, such as hunger and stress conditions, including hypoxia, energy deficiency, high temperature, hormone stimulation, chemical drugs, and diseases [52]. Interestingly, some studies suggested that the autophagy process is generally labeled as a prosurvival pathway; however, it has been argued that excessive self-digestion can result in autophagic cell death [53]. In terms of cancer treatment, induced autophagy could be used to fight against cancer or combined with other applications to achieve higher efficiency. Different drug combinations have achieved notable clinical therapeutic outcomes, and the corresponding mechanism is related to autophagy induction [54]. Various NMs are reported to be capable of inducing autophagy at the cell or animal levels, featuring their potential applications in treating certain malignant tumors [54-56].
Ferroptosis is a kind of PCD dependent on iron and ROS [57]. Ferroptosis was first discovered when studying the mechanism of small-molecule erastin in killing tumor cells with RAS mutation [58]. RAS-mutated tumor cells, featuring increased intracellular iron content by upregulating transferrin receptor 1 and downregulating ferritin for iron outward transportation, treated by erastin would result in an “oxidative, nonapoptotic” cellular death pathway, which is preventable by iron ion chelators [59]. Such a phenomenon suggests that this kind of cellular death is iron ion dependent. Concerning morphological changes, cells with iron-dependent death are characterized by shrinking mitochondrial volume, increasing bilayer membrane density, and decreasing or disappearing mitochondrial ridges [58]. Regarding biochemical variations, intracellular glutathione (GSH) is depleted, glutathione peroxidase 4 (GPX4) activity significantly decreases, and GPX4 hardly catalyzes lipid oxides [60]. Until now, ferroptosis has attracted increasing attention, especially in NM-based cancer therapy [33, 61-65]. Based on the clarified regulation mechanisms and signaling pathways of ferroptosis, some NMs have been specifically designed to trigger the Fenton reaction, catalyzed by variable valent metal ions and hydrogen peroxide (H2O2), which will then be discussed in detail in the following part.
Cuproptosis is a newly arisen PCD identified by Tsvetkov et al. in 2022 [66]. It is a copper-dependent cell death highly correlated with the tricarboxylic acid (TCA) cycle. Copper is an indispensable inorganic trace element for humans. However, copper ion, similar to iron ion, is also cytotoxic when its intracellular concentration is far over the physiological threshold. Excessive copper ions can bind the acylation products in the TCA cycle and then promote the aggregation and malfunction of the acylated proteins, thereby leading to the blockade of the TCA cycle [66-68]. This process triggers proteotoxic stress, ultimately resulting in PCD. The authors also suggested that this discovery may help investigate copper discords. Researchers recently found that some NMs could induce cuproptosis of cancer cells, indicating the feasibility of triggering cuproptosis against cancer [69-74].
Pyroptosis is another caspase-dependent PCD, with DNA damage and nuclear condensation. Pyroptosis shares similarities with apoptosis; however, it is characterized by swelling and bubble-like protrusions on the cellular membrane before rupture, distinct from the blebbing seen in apoptosis [75]. Unlike apoptosis, pyroptosis triggers inflammation and cell membrane flattening due to leakage, leading to osmotic lysis and the release of inflammatory cytokines. Canonically, pyroptosis is mediated by inflammasome assembly and gasdermin D (GSDMD) cleavage and IL-1β and IL-18 release. Uncanonically, the upstream sensor of caspase 4/5 (human) and caspase 11 (mouse) is absent, and these caspases can be activated by directly binding to cytosolic lipopolysaccharide, following GSDMD cleavage and plasma membrane pore formation. Besides, the chemotherapeutic drugs that cause caspase-3/8-related apoptosis could induce gasdermin E (GSDME) cleavage, ultimately promoting pyroptosis of tumor cells. Notably, various NMs have been reported to induce pyroptosis in breast cancer and hepatocellular carcinoma models through the caspase pathway and oxidative DNA damage [76].
PANoptosis is a relatively new type of PCD, as proposed by Malireddi et al. in 2019 [77]. Indeed, the interactions among pyroptosis, apoptosis, and necroptosis are involved in PANoptosis, with each letter standing for one type of PCD: “P” stands for pyroptosis; “A” stands for apoptosis; “N” stands for necroptosis [78]. However, the mechanism of PANoptosis cannot be explained by any of these three. PCD/ PANoptosis is one type of inflammatory PCD mediated by PANoptosome complexes with key features of pyroptosis, apoptosis, and necroptosis. PANoptosis plays a significant role in various diseases, including cancer, infections, and inflammatory conditions, which also demonstrates that PANoptosis patterns in cancer can predict survival rates and responses to immunotherapy and CT [79, 80]. This highlights the potential of PANoptosis as a therapeutic target in cancer treatment. Additionally, PANoptosis also plays a vital role in limiting the spread of cancer cells by enabling their elimination through multiple cell death pathways. This approach may address various cancer treatment challenges, including drug resistance and immune evasion [81]. Moreover, some NMs can induce PANoptosis by activating RIPK1/RIPK, caspase 8/caspase 3, and MLKL pathways [74, 75, 82].
2.5 Stimulation of Immune Response
The immune system is an effective surveillance system that eliminates abnormal cells and invading organisms. The immune system consists of nonspecific immunity (innate immunity) and specific immunity (adaptive immunity), which are tightly correlated [83] and keep invaders, such as bacteria and viruses, under active surveillance. These loyal guardians also recognize and eradicate mutated cells, like cancer cells. These surveillance activities require the cooperation of different cells in the body, which are precisely controlled by the organics. Inevitably, the application of NMs will have a direct contact or impact on the immune system. The interactions between NMs and immune system components may cause different biological responses, like inflammatory allergic reactions, suggesting that the NMs may trigger immunotoxicity [84]. Immunotoxicity investigation is essential for preclinical safety examination. However, some NMs show reduced immunotoxicity by chemical modification. For example, nano-albumin-formulated PTX will not induce anaphylaxis, while pristine PTX is likely to be anaphylactic [85]. Interestingly, some NMs have been reported to be capable of stimulating various immune responses due to their specific physical and chemical properties, such as size, elemental compositions, surface modifications, and protein corona. Some NMs with specifically designed properties can promote antigen presentation or stimulate immune responses without the assistance of special immunological adjuvants [86, 87]. Therefore, understanding and exploiting the interactions and influences of NMs on the immune system is essential for the biomedical usage of NMs.
2.6 Modulation of TME
TME is a concept that the solid tumor is a stack of multiple cell types, not only containing cancer cells but also incorporating types of stromal cells, including fibroblasts, macrophages, lymphocytes, adipocytes, and so on, where each cell type plays its role and together nourishes tumor growth [88]. These cells are embedded in the extracellular matrix (ECM) composed of collagens and proteoglycans, which provide a hydrated matrix to support tumor growth, where the tumor blood vessels are inserted with irregular diameters and leaky structures. Some internal regions even lack endothelial cells or basement membranes. These factors comprise TME, which supports tumor growth and promotes metastasis [89]. Adversely, tumor cells also feed back into TME by secreting growth factors and proteases, featuring hypoxia, acidosis, high hydraulic pressure, nutrition deficiency, and so on, which has posed a considerable challenge for scientists to develop anticancer strategies [90], which results in modulating hypoxia by nanoenzymes, inducing macrophage polarization by NMs, regulating tumor-associated fibroblast proliferation by nanofibers, and broadly facilitating the anticancerous battle [91].
3 Applications of these Biological Effects Caused by NMs in Cancer Therapy
NMs have unique properties, including quantum size effects, surface effects, and macroscopic quantum tunneling. These properties give NMs different physical properties, such as light, heat, electricity, magnetism, mechanical, chemical properties, adsorption, dispersion, agglomeration, surface activity, catalysis, and photocatalytic properties [92]. Therefore, nanotoxicological concerns are always caused by the action and reactivity of NMs. Many investigations have been made in nanotoxicology in the past decade, including interactions of NMs with bio-systems in vitro and in vivo. The mechanism of toxic effects has been explored, and some basic conclusions have been obtained. A few studies have been conducted to evaluate the side effects caused by NMs, which regard the induction of cellular death as a harmful influence. However, as nanotoxicology is being strengthened, the detrimental outcome can be tailored for cancer therapeutic purposes. The representative investigations on the applications of biological effects caused by NMs against tumors are concluded in Table 1.
| Formulation | Induced biological responses | Cell/mouse models | References |
|---|---|---|---|
| Caffeic acid-functionalized Au–Fe3O4 and Pt–Fe3O4 nanoheterodimer | DNA fragmentation and ROS formation | MCF-7 breast cancer cell | [93] |
| Pt/MgO NPs | Oxidative stress-mediated apoptosis | HT29 colon cancer cell, A549 lung cancer cell | [94] |
| CeO2 NPs | Increase the intracellular ROS level | BxPC3-pancreatic cancer | [95] |
| PAA–CeOx@PANI–PEG NPs | Catalase-like activity overcomes hypoxia and generates ROS. | H22 hepatocellular cancer | [96] |
| PEG-modified NPs | Increase K+ efflux and induce mitochondrial apoptosis | BEL-7402 tumor | [97] |
| PTX–PP@Au NPs | Blockage of theTRPV6 cation channel enhances cell cycle arrest and generates ROS. | PC3 prostate tumor | [98] |
| HC-030031 loaded polymer NPs (NPs-H) | Inhibits TRPA1 ion channels and destroys Ca2+-dependent antiapoptosis pathways | HCC1569 breast cancer | [99] |
| TGZ@eM | Consume endogenous glucose and O2 to starve tumor cells | CT26 colon cancer | [100] |
| 2-DG conjugated black phosphorus nanosheet | 2-DG inhibits glycolysis, while black phosphorus nanosheet blocks consequent autophagic responses and compensatory energy supplies. | A357 melanoma | [101] |
| HZ@GD | Zn2+ mediated glycolysis inhibition and GLUT1 depletion for energy exhaustion. | B16F10 melanoma | [102] |
| Au@CaP–Flu@HA | Induce glycose deprivation, lactate efflux inhibition, and autophagic inhibition | 4T1 breast cancer | [103] |
| Peptide-golden NPs | Decrease antiapoptotic protein Mcl-1 increases proapoptotic protein Puma, resulting in marked mitochondrial transmembrane potential change | CNE1 nasopharyngeal cancer | [104] |
| CONPs | Downregulate the copper chaperone protein to disrupt copper transportation to the ER and mitochondria, inducing ER stress and mitochondrial-mediated apoptosis | SR786O renal cancer | [105] |
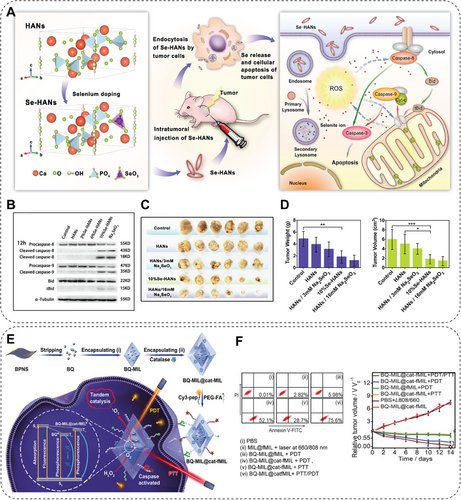
| Se–HANs | Induce caspase-dependent apoptosis and ROS production | Osteosarcoma | [106] |
| B-SeHANs | Induce apoptosis and autophagy via ROS-mediated JNK activation and Akt/mTOR inhibition | MNNG/HOS osteosarcoma | [107] |
| Catalase and black phosphorus quantum dot coloaded MOF | Catalyze H2O2 into 1O2, improving hypoxia and PDT efficacy, and inducing apoptosis | Hela breast cancer | [108] |
| P-Bec1 | Induce autophagic cell death | MCF-7 breast cancer | [109] |
| PLT@BPQDs–HED | Induce autophagic cell death by promoting the formation of autophagosomes | MCF-7 breast cancer | [110] |
| Organometallic gold(III) complexes | Induce mitochondrial dysfunction and increase ROS generation, resulting in autophagy and apoptosis | A549 lung cancer cell | [111] |
| HA–OXA | Transform mild autophagy to overactivated autophagy and induce ICD | CT26 colon cancer | [112] |
| NBP/TiO2 | Block autophagosome–lysosome fusion, inhibit cellular proteolytic activity, and sensitize cancer cells to proteasome inhibitors | U-87 MG glioblastoma | [113] |
| TNP-1 | Inhibit prosurvival autophagy | 4T1 breast cancer, MCF/MDR breast cancer, patient-derived breast cancer | [114] |
| PolyCAFe micelles | Promote ROS generation | SW620 colorectal cancer | [115] |
| Ce6@RMOF | Deplete GSH and inhibit GPX4, resulting in enhanced ferroptosis | 4T1 breast cancer | [116] |
| Ferumoxytol | Ferumoxytol induces oxidative stress, ROS generation, and ferroptotic cell death. | Blast crisis leukemia, patient-derived leukemia | [117] |
| VF/S/A@CaP | React with H2O2 to generate ROS and consume GPX4, inducing ferroptosis | H1975 lung cancer, patient-derived lung cancer | [61] |
| FA–pyrite nanoenzyme | Ultrahigh peroxidase-like and glutathione oxidase-like activities generate ROS while depleting GSH. | CT26 colon cancer | [118] |
| Pa–M/Ti–NCs | Cyclically induce synergistic immunomodulation and ferroptosis | B16F10 melanoma, 4T1 breast cancer | [119] |
| Cro–Fe@BSA NPs | Induce ferroptosis due to the release of Fe3+ and the reaction with GSH to afford Fe2+ | 4T1 breast cancer | [120] |
| ART@CuT/ETH HNP | Catalyzes H2O2 to generate ROS, enhances apoptosis and ferroptosis, initiates cuproptotic cell death | 4T1 breast cancer | [121] |
| NP@ESCu | Elesclomol and Cu ions induce cuproptosis and reprogramme immunosuppressive tumor microenvironment. | MB49 bladder cancer | [122] |
| PTC | Induce cuproptosis by promoting copper overload while inhibiting copper efflux | 4T1 breast cancer | [70] |
| Au@MSN–Cu/PEG/DSF | DSF chelated with Cu2+ causes apoptosis and cuproptosis by mitochondrial protein aggregation. | 4T1 breast cancer | [123] |
| CuET | Reverses cisplatin resistance by inducing cuproptosis | A549 lung cancer | [124] |
| As2O3 NPs | GSDME cleavage triggered by As2O3, resulting in pyroptosis | Huh7 hepatocellular carcinoma | [125] |
| LipoDDP | Activates the caspase-3 pathway to trigger pyroptosis in decitabine-pretreated cells with demethylation of the DFNA5 gene | 4T1 breast cancer | [126] |
| NP-GSDMA3, Phe–BF3 | Desilylation releases gasdermin from NP-GSDMA3 to induce pyroptosis | 4T1 breast cancer | [127] |
| PFH@Lipo–PpIX@EVs | Induce PANoptosis under ultrasound stimulation | 4T1 breast cancer | [128] |
| HSA@Tz–Ir | Bioorthogonal-activated membrane-targeting SDT modality to evoke tumor-specific immunogenic PANoptosis | 4T1 breast cancer | [129] |
| Ferumoxytol | Induces macrophage polarization into proinflammatory M1 phenotypes | MMTV-PyMTderived cancer | [130] |
| n-nHA | Depletes M2-like macrophages by promoting multinucleated giant cell formation and autophagy activation | 4T1 breast cancer | [131] |
| MRF | Induces oxidative-damaged mtDNA to trigger proinflammatory M1 polarization of macrophages | PANC-1 pancreatic cancer | [132] |
| MF-NPs | Deliver genetic materials to NK cells and generate CAR–NK in situ | MDA-MB-231 breast cancer | [133] |
| MNPs@PEI–FA/pDNA | NK activation and proliferation by mild magnetocaloric regulation-induced IL-2 overexpression | Hepa 1–6 liver cancer | [134] |
| Zn–CoFe2O4@Zn–MnFe2O4 | Activates UL16-binding protein and promotes NK cell activation | HepG2 hepatocellular carcinoma | [135] |
| mRNA loaded 113-O12B | CD8+ cytotoxic T cell response | B16F10 melanoma | [136] |
| mRNA-loaded polymeric NPs | Targeted release of gene modification agents in the nucleus to generate CAR-T cells | Eμ-ALL01 leukemia | [137] |
| Cytokine protein nanogel | Anchors on the T cell surface promote cytotoxic T cell activation and expansion. | B16F10 melanoma | [138] |
| Nano-sapper | Releases cytokine and enhances cytotoxic T cell infiltration | KPC1199 pancreatic cancer | [139] |
| Au–DOPC NPs | NIR(II) PTT triggers deeper ICD and elicits innate and adaptive immune responses. | 4T1 breast cancer | [140] |
| SGNP@PDA | PTT- and CDT-induced immune response | CT26 colon cancer, TC-1 head and neck squamous cell carcinoma | [141] |
| R837–OVA–PEG–MnFe2O4 NPs | PTT induces an immune response and reduces systemic immunosuppression by downregulating M2-associated cytokines. | 4T1 breast cancer | [142] |
| Gd@C82–Ala (Gd–Ala) | PDT promotes ROS generation and DC maturation, increases CD4+ and CD8+ T cell differentiation. | 4T1 breast cancer | [143] |
| PMPS NDs | SDT induces ER stress by generating ROS, promotes DC maturation, and induces ICD. | Panc02 pancreatic cancer | [144] |
| PARNs | PDT and SDT damage the vascular system, produce ROS, and induce antitumor immunity. | Hela breast cancer | [145] |
| CoFe2O4@MnFe2O4 | MHT-induced thermal ablation of the tumor and antitumor immunity | 4T1 breast cancer | [146] |
| Zn–CoFe2O4@Zn–MnFe2O4 | MHT induces tumor-associated antigens to promote the maturation and activation of DC. | HepG2 hepatocellular carcinoma | [135] |
| ZnCoFe2O4@ZnMnFe2O4–PBA | MHT promotes M1 polarization and DC maturation and awakens T cells. | 4T1 breast cancer | [147] |
| AlO(OH)–polymer NPs | Deliver vaccine to antigen-presenting cells and activate CD8+ T cells | B16F10 melanoma | [148] |
| pD–Al2O3 NPs | Promote the release of tumor-specific antigens and trigger a systemic immune response | B16F10 melanoma | [149] |
| CpG/ZANPs | Elicit antigen-specific humoral immune responses and activate cytotoxic T lymphocytes | EG7-OVA lymphoma | [150] |
| MnO2@PtCo | Intracellular oxidative damage induction | 4T1 breast cancer | [151] |
| GIM | Catalyzed cascade reactions offer the O2 and promote the ROS generation. | A549 lung cancer | [152] |
| Ce–MOFs | Oxidase-like activity induces oxidative damage and ATP deprivation. | H22 hepatocellular cancer | [153] |
| F56–PTX–NPs | Normalize tumor vasculature, enhance vascular perfusion | MDA-MB-231 breast cancer | [154] |
| LPD | Induces cancer cell apoptosis and reprograms CAFs | UMUC3 urinary bladder cancer | [155] |
| Collagenzome | Promote ECM degradation and drug penetration | KPC pancreatic cancer | [156] |
| NM–Ce6 | NM-Ce6 and PDT increase tumor perfusion and oxygenation level. | 4T1 breast cancer | [157] |
| LDH | LDH neutralizes the acidic microenvironment and blocks autophagy | CT26 colon cancer, B16F10 melanoma | [158] |
|
Zn–LDH |
Neutralizes acidic microenvironment, blocks autophagy, and induces ICD | 4T1 breast cancer, B16F10 melanoma | [159] |
| NaHCO3 NPs | Regulate lactic acid metabolism, activate pyroptosis, and ICD | 4T1 breast cancer | [160] |
3.1 NMs-Interfered Oxidative Homeostasis
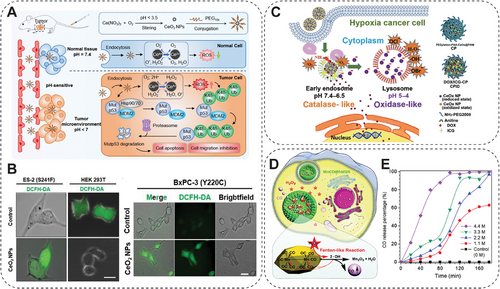
Moreover, the oxidative imbalance within the tumor site is an endogenous stimulation factor for prodrug, achieving precise targeted therapeutics release [165]. For instance, the H2O2 in TME is significantly higher than in normal cells/tissues, which can be used for H2O2-responsive therapeutic gas release to achieve the antitumor effect [165]. He et al. developed a hydrophobic manganese carbonyl prodrug loaded hollow mesoporous silica NP (MnCO@hMSN), with H2O2-triggered carbon monoxide (CO) gas release by a Fenton-like reaction to eliminate cancer cells (Figure 2D,E). The cytotoxic effect of MnCO@hMSN is highly dependent on the H2O2 level. Therefore, it exhibits potent cytotoxicity toward tumor cells with elevated H₂O₂ expression, while exerting minimal toxicity on normal cells with physiological H₂O₂ levels [164]. The dysregulation of redox homeostasis impacted by NMs can also profoundly affect cellular function and lead to various diseases. Chen and her team reveal for the first time that the dehydrogenation reaction in cellular metabolism is significantly influenced by mimicking dehydrogenase activity through NMs, which leads to the accumulation of reducing substances, such as NAD(P)H and GSH, and thus triggers the onset of reducing stress [166]. It was found that reduction stress triggered by NMs, especially for MoB2 not only alters the ratio of immune cell populations, but also leads to an increase in immunosuppressive cells (e.g., M2-type macrophages and Treg cells), as well as contributes to the secretion of immunosuppressive cytokines (IL-10, TGF-β, etc.). It was shown that MoB2-induced reducing stress further exacerbated the process of breast tumor metastasis and immune escape by altering the immunosuppressive microenvironment in lung tissues, raising essential safety considerations for future NMs applications in cancer therapy. Such a situation also reveals the mechanism by which the impact of oxidative stress on the adverse effects of NMs leads to the dysregulation of redox homeostasis and results in various diseases, like cancer metastasis [166].
3.2 NMs-Mediated Ion Channel Manipulation
Modulation of ion channels involves several traditional methods, including utilizing small-molecule agents to modulate the opening or closing of ion channels across the cellular membrane, implementing genetic modifications to regulate the expression of ion channels, or applying electrodes to manipulate the activity of ion channels. These methods successfully achieve the primary goal of ion channel modulation; however, challenges exist in meeting the growing demands for simplicity, high efficiency, precise spatiotemporal control, and nondestructive regulation with ideal safety and efficacy [167]. The interaction between ion channels and NMs has been discovered and investigated in recent decades. Researchers found that NP-mediated regulation of ion channels shows unique advantages. However, investigations into the underlying mechanisms of such regulation are a prerequisite. Gold NPs (AuNPs) have been extensively studied in biomedical applications by acting as drug carriers or theragnostic agents. Simon and coworkers [168] found that ultrasmall AuNPs with a diameter of 1.4 nm inhibited the hERG potassium ion channel activity and generated potential cytotoxicity. In contrast, thiol-stabilized ultrasmall AuNPs can hardly affect the hERG ion channel [168]. In addition, Duan's group [97] compared the influences of three different NPs, positively or negatively charged with or without PEG modification, on potassium current in cancer cells. Results indicated that PEG-modified NPs increased the K+ efflux, leading to more apoptotic cancer cells. However, negatively charged NPs displayed much less impact on the K+ efflux of normal cells and resulted in negligible side effects [97]. These studies provided a theoretical basis for researching fighting against cancer based on ion channel regulation by NMs. In further work by Duan's lab, they reported a multifunctional gold-caged NPs (PTX–PP@Au NPs) in treating androgen-resistant prostate cancer via photothermal therapy (PTT), photodynamic therapy (PDT), and CT effects, in a multimodal modality synergistically with ion channel inhibition mediated by ultrasmall AuNPs (Figure 3A). This synergistic nano platform achieved PTT/PDT caused by pluronic-polyethyleneimine micelles coated gold cage under NIR laser excitation, and the tumor cell cycle was arrested by CT conducted by PTX loaded in micelles. Also, the nanosystem exhibited remarkable performance in blocking transient receptor potential cation channel subfamily V6 (TRPV6) cation channel by releasing AuNPs, thus inhibiting the growth of androgen-resistant tumors. Overall, the PTX–PP@Au nanosystem successfully applies multimodal therapy combined with ion channel modulation to inhibit androgen-resistant prostate cancer [98]. Excessive influx of calcium ions (Ca2+) promotes resistance to ROS-related cancer CT; studies have proved that the transient receptor potential A1 (TRPA1) ion channel is essential in this process. TRPA1 ion channels are consistently overexpressed in breast cancer cells and promote Ca2+ influx to activate the oxidative stress defense by inducing the antiapoptotic pathway of cancer cells. Researchers designed an HC-030031 (a TRPA1 ion channel inhibitor) loaded polymer NPs (NPs-H) for PTT to overcome such ROS resistance. Triggered by 808 nm laser irradiation, HC, released from the NPs-H, blocks the Ca2+-dependent resistance by inhibiting TRPA1 and promoting apoptosis of breast cancer cells (Figure 3B). Compared with the traditional clinical CT drug carboplatin, the tumor volume decreased by 54.1% after being treated with NPs-H (Figure 3C,D) [99]. Researchers have also attempted to activate special environment-sensitive ion channels to induce Ca2+ overload for cancer inhibition (Figure 3E) [169-171]. These studies indicated that regulating Ca2+ concentration and related ion channels may be a possible way to conquer cancer. Another study also proved that ion channel is highly correlated with therapy resistance to cancer. Stauber et al. [172] identified that ion channel LRRC8A is crucial for cancer cisplatin resistance by next-generation sequencing, CRISPR/Cas9 knockout technology, and clinical data analysis. Based on this finding, they developed cisplatin-loaded poly-sarcosine-based core cross-linked polymeric NPs to overcome cisplatin resistance by bypassing the LRRC8A transport pathway [172]. Moreover, in another study, Kang et al. introduced a novel method for controlling macrophage polarization using an upconversion NP (UCNP)-based photoresponsive nanocarrier for NIR light-mediated control of intracellular calcium levels (Figure 3F) [173]. UCNP was coated with mesoporous silica (UCNP@mSiO2), loading calcium regulators that can either supply or deplete calcium ions, and was modified with photocleavable linker and Arg–Gly–Asp peptide-bearing molecular cap via cyclodextrin-adamantine. The upconverted UV light emission from the UCNP@mSiO2 under NIR light excitation triggered the cleavage of the cap and intracellular release of calcium regulators, thereby allowing temporal regulation of the intracellular calcium levels. Application of NIR light through skin tissue promoted M1 or M2 polarization of macrophages by elevating or depleting intracellular calcium levels, respectively (Figure 3G). This photoresponsive nanocarrier can be remotely manipulated in vivo immune functions via NIR light-controlled macrophage polarization, such as inflammation, tissue regeneration, and cancer treatment [173].
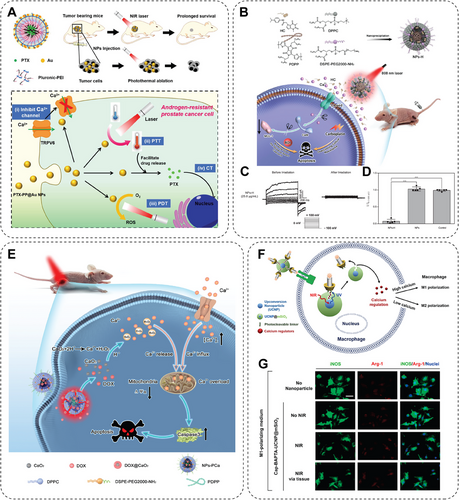
In summary, on-demand modulation of ion channels produces efficient inhibition of tumor progression and is helpful against cancer in both single and combination therapy.
3.3 NMs-Mediated Cellular Metabolism Manipulation
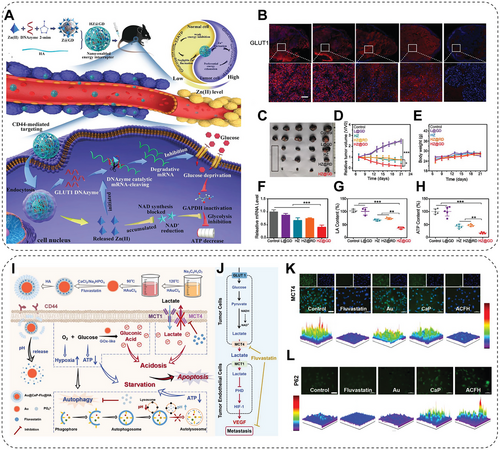
The reprogramming of cellular metabolism plays a crucial role in tumorigenesis, in which variation in glycolytic metabolism is a significant target for antitumor attempts [174]. Compared with normal cells, cancer cells metabolize glucose by glycolysis preferentially rather than oxidative phosphorylation, which promotes cancer progression and metastasis. Therefore, metabolic regulators have been developed to interfere with the glycolysis pathway to improve the therapeutic outcomes based on the glycolytic dependence of the tumor. However, the complexity of TME hindered the clinical effects of these metabolic regulators. To solve this, the assistant delivery of these regulators by NMs is becoming an efficient therapeutic strategy to regulate glycolysis in cancer cells by targeting glucose and lactate, two critical molecules in the glycolysis pathway [45, 175-177]. One of these approaches, starvation therapy, interferes with the metabolic processes of cancer cells by depriving glucose through glucose oxidase (GOx) or its analogs. Nevertheless, the application of free GOx or its analogs is greatly hindered by the poor delivery efficiency and the self-limiting effect. To address these, researchers designed a biomimetic nanomedicine, a MOF cloaked by an erythrocyte membrane and loaded with GOx and prodrug tirapazamine (TPZ) (TGZ@eM). TGZ@eM exhausts the glucose and oxygen at tumor sites, leading to much-enhanced cancer starvation and enhanced hypoxia to activate TPZ release and activity in killing colon cancer [100]. Shi's group [101] developed a synergetic strategy to augment tumor starvation therapy. Herein, they conjugated the 2-deoxy-d-glucose (2-DG), which is a glucose analog that serves as an antiglycolytic agent undergoing clinical trials (Phase I/II), into the black phosphorus (BP) nanosheets. In this system, 2-DG inhibits lactate production in A375 and HeLa cells, and the maximum inhibition rate reaches 77.6%. Since cancer cells always initiate autophagy when facing stress or are deficient in nutrition and metabolism, BP nanosheets can block the autophagic flux and nutrition supplement. The cooperation of 2-DG and BP sheets places cancer cells in severe energy deprivation, resulting in significant cancer inhibition [101]. Further research in the same lab addressed the problem of starvation therapy being often associated with nonspecific and incomplete energy blockades. Scientists designed a synergistic energy deprivation strategy of Zn2+ interference-mediated glycolysis inhibition and Zn2+-activating glucose transporter 1 (GLUT1) specific energy depletion (Figure 4A). A hyaluronic acid shell and DNAzyme-loaded zeolitic imidazole frameworks (ZIF)-8 core nanostructures were established herein. The zinc, controlled by the hyaluronidase (HAase)-response and pH-sensitive gates, can decrease the NAD+ and inactivate GAPDH, leading to glycolysis inhibition (Figure 4A). At the same time, the specially designed DNAzyme can downregulate GLUT1 expression to cut off glucose supply (Figure 4B). This dual-gate controlled starvation system achieves 80.8% tumor growth inhibition in vivo without systemic toxicity (Figure 4C–H) [102]. Another study has simultaneously performed glycose deprivation and lactate efflux inhibition to induce toxic acidosis and enhance starvation. They synthesized the CaP-coated Au-nanocomposites nanoreactor encapsulating the monocarboxylate transporter 4 (MCT4) inhibitor Fluvastatin, Au@CaP–Flu@HA (Figure 4I). Herein, MCT4 inhibition by Fluvastatin promotes lactate accumulation in cells, leading to energy insufficiency and reduced acidosis (Figure 4K). The GOx-mimic AuNPs consume glucose to shield the energy source of cancer cells. Later, the CaP shell inhibits the autophagic activity, further exacerbating the nutritional deficiency (Figure 4L). This combinational strategy significantly inhibits cancer progression by severely disrupting the “Warburg Effect” (Figure 4M) [103]. In addition to glucose metabolic modulation, NMs have already been proven to regulate other types of metabolic activity, such as lipid and iron metabolism, autophagy, or glutaminolysis [174, 178, 179].
3.4 NMs-Induced PCD
3.4.1 Apoptosis
The generated H2S, transported to the anterior cingulate cortex (ACC) in the brain by the bloodstream, increases the expression of glutamate transporter 1 in ACC, thus alleviating anxiety-like behavior by reducing extracellular glutamate levels and attenuating the hyperactivity of glutamatergic neurons [186]. Besides, He and his teams [184] found that NO-mediated degradation of ECM may help promote nanomedicines in penetrating solid tumors. They designed cross-linking nanomicelles self-assembled with dendrimers containing phenylboronic acid and lactobionic acid, through borate esterification. In response to the intratumoral over-expressed GSH, the nanomicelles produce NO via a loaded NO donor, which mediates the expression of matrix metalloproteinases for the degradation of ECM in the tumor, facilitating the penetration of nanomicelles. Meanwhile, the generated NO triggers mitochondrial dysfunction, further enhancing the therapeutic outcomes [184]. Other gas molecules, such as CO and SO2, also exhibit significant induction of apoptosis, which has been widely studied and investigated [165].
3.4.2 Autophagy
Autophagy is an essential cellular process with positive and negative effects on organisms. Therefore, the NMs with autophagy modulation ability are becoming an appealing opportunity for overcoming some barriers in cancer treatment, such as MDR, radioresistance, or even the tumor immunosuppressive microenvironment [188-191]. Several studies have proved that certain types of NMs can selectively stimulate or inhibit autophagy activity in cancerous cells with selectivity, resulting in significant cell death and tumor inhibition. Moreover, research on synergistic therapy with autophagy modulation nanomedicines and other treatments has grown recently. For such an appealing reason, it is essential to study the effect of NMs on the modulation of autophagy from both nanotoxicological and therapeutic perspectives.
As reported, dioleoyltrimethylammonium propane (DOTAP), a cationic lipid, is commonly used as a transfection agent and can effectively induce autophagy in HeLa cells. The results indicated that DOTAP enhances mTOR-independent autophagosome formation. Autophagy activation may be caused by some cells not degrading the manufactured DOTAP. As a result, those cells increase their total degradative capacity as feedback, that is, by the elevation of autophagy activity. At the same time, the phenomenon may imply that inhibition of autophagy helps improve transfection efficiency. Interestingly, treatment with charged-free lipids (i.e., dioleoylphosphatidyl-ethanolamine) failed to induce an autophagy regulatory effect [192]. Polymeric NPs have exhibited significant clinical potential in revolutionizing cancer treatment for decades by effectively delivering diverse therapeutics, including autophagy regulators, while minimizing nonspecific side effects. For example, research has proved that the upregulation of Beclin-1 could induce autophagy, resulting in a notable reduction of cell viability in vitro and significant progression of breast cancer in vivo. Accordingly, Bec1, an autophagy-inducing peptide combined with PEG, is self-assembled into a micelle-like NP (P-Bec1) (Figure 6A). The P-Bec1 is pH-responsive, which triggers bec1 release in mildly acidic TME and induces significant cancer cell autophagy, as shown by GFP–LC3 positive cells and related quantification (Figure 6B). Moreover, compared with other groups, the cancer cells treated with P-Bec1 displayed the most significant elevation of autophagosomes, stained by AO (Figure 6C), suggesting the induction of autophagy, which was further confirmed by the western blotting results of autophagy-related protein LC3 and P62 (Figure 6D) [109]. Gradient alloyed QDs have emerged as exceptionally promising NMs for biomedical imaging due to their superior fluorescent properties compared with conventional QDs. However, the biological performances of QDs are highly associated with surface modification. A study verified the influence of two commonly used modifications of QDs, 3-mercapto propionic (MPA) acid modification and PEG modification, on the autophagy pathway. Interestingly, MPA-modified QDs improved the lysosomal function and inhibited ROS generation. In contrast, the PEGylated QDs promoted lysosomal impairment and ROS production and further induced autophagic cell death of breast cancer cells [193]. Moreover, further study attempts to increase the bioavailability of antitumor agents delivered by QDs. Hence, a platelet membrane camouflaged hederagenin-loaded BP QDs was successfully prepared (PLT@BPQDs–HED). Due to the physicochemical characteristics of the platelet membrane, PLT@BPQDs–HED enhanced the tumor target and increased the accumulation of hederagenin in breast cancer cells. Moreover, this nanoplatform promoted the formation of autophagosomes by upregulating Beclin-1 and LC3-II to overcome breast cancer [110]. Between autophagy and apoptosis, intricate and complex interactive regulations are involved; they can be coactivated by various stress stimuli, share multiple regulatory molecules, and even coordinate transformation. For instance, ER stress is a vital inducer of apoptosis and can stimulate autophagy. Liang et al. [111] reported that Au nanocomplexes (NCs) triggered the overproduction of ROS by inducing mitochondrial dysfunction, ultimately resulting in the simultaneous activation of autophagy and apoptosis in A549 cancer cells. A combination of CT and immunotherapy is currently a thriving and developing method against tumors; however, according to some researchers, the notable antitumor outcome highly depends on autophagy induction. Modulation of autophagy accurately and timely could vastly improve cancer cell mortality and promote antigen presentation or secretion of immune cytokines, as reported in the following. Hence, on-demand autophagy cascade amplification NPs (ASNs) were designed. ASNs were designed and prepared in two steps as an on-demand nanosystem: self-assembly of autophagy-sensitive micelles (C-TFG micelles) and then electrostatic binding to oxaliplatin grafted hyaluronic acid prodrug (HA–OXA) (Figure 6E). When ASNs were internalized by cancer cells, the HA–OXA degraded and released oxaliplatin, inducing immunogenic cell death (ICD) and mild autophagy in CT26 cells (Figure 6F,G). Then, C-TFG micelles release autophagy inducer STF-62247 in response to autophagic activity, transforming autophagy from “mild” to “overactivated,” thereby enhancing the antitumoral immune response (Figure 6H,I). This research demonstrated the application of controlled autophagy in improving antigen presentation and secretion of antigens, indicating a vital combination mechanism for cancer chemo-immunotherapy [112].
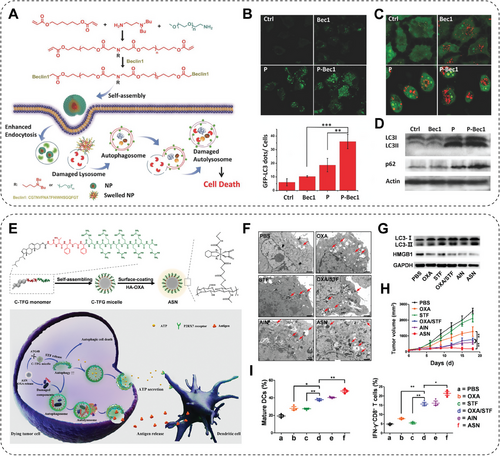
However, autophagy is always two-faced toward cancer cells. During the early stage of tumorigenesis, autophagy acts as a suppressor by degrading potentially harmful agents or damaged organelles, thus avoiding spreading damage, including DNA mutations [194]. Accumulating evidence has proved that autophagy also plays a prosurvival role in cancer cells and strongly correlates with chemoresistance [195]. Hence, the rational inhibition of autophagy could also suppress tumor progression. Targeted protein degradation is a feasible strategy in cancer treatment. The ubiquitin–proteasome system and the autophagy–lysosome pathway are two pivotal routes responsible for intracellular protein degradation, critical in cancer pathogenesis and therapy. A recent clinical trial suggested that the anticancer efficiency of bortezomib, a first-generation proteasome inhibitor, is greatly enhanced when combined with autophagy inhibitors [196]. Based on this, Zhu et al. [113] reported a TiO2-coated Auno-bipyramids (NBPs) nanostructures (NBP/TiO2), which act as a novel autophagy inhibitor in human glioblastoma U-87 MG cells. NBP/TiO2 effectively blocks autophagosome–lysosome fusion with high efficacy, thereby impeding cellular proteolytic activity by suppressing cathepsin B maturation. Besides, NBP/TiO2 also exhibits inhibitory effects on trypsin-like proteolytic activity. More importantly, the disruption of autophagy flux by NBP/TiO2 can even enhance the susceptibility of cancer cells to bortezomib [113]. Combining autophagy inhibitors and other cancer treatment strategies can eradicate drug-resistant cancer. The integration of CT and PTT, known as chemo-PTT, presents a promising prospect for achieving notable tumor elimination [197]. Unfortunately, when employing conventional chemotherapeutic agents, the therapeutic outcomes of chemo-PTT may also be compromised by drug resistance. Thankfully, combining autophagy inhibitors, 3-methyladenine or chloroquine, instead of traditional chemotherapeutic agents, in synergy with copper–palladium alloy tetrapod NPs (TNP-1) exhibits much higher efficiency in eliminating drug-resistant breast cancer [114].
3.4.3 Ferroptosis
Ferroptosis, an iron-dependent cellular death pathway, has attracted significant attention owing to its effectiveness in killing cancer cells for years [33]. Investigations mainly focused on developing both iron-based and iron-free NMs to induce ferroptosis in cancer cells by the upregulation of ROS generated by the broad-applicable Fenton reaction. For example, benzoyloxycinnamaldehyde (BCA), derived from cinnamaldehyde, can exert antiproliferative activities and thus cause apoptotic cell death via H2O2 generation. Lee and colleagues [115] designed and fabricated a polymer (PolyCAFe) micelle as a new class of Nano-Fenton reactors. It acts as an anticancer therapeutic agent, incorporating BCA and iron-containing compounds in its backbone. When injected intravenously, PolyCAFe micelles can accumulate in tumors, preferentially to remarkably suppress tumor growth by effectively inducing ferroptosis with negligible toxicity to normal tissues [115]. PDT is an appealing antitumor modality for its ability to kill tumor cells by mediating ROS production. Zhao and colleagues [116] confirmed that the ROS in PDT can deplete GSH and thus activate the ferroptosis process. In this study, researchers designed a disulfide-bearing imidazole ligand coordinated with zinc to form an all-active MOF nanocarrier with encapsulated photosensitizer (chlorin e6, Ce6). The Ce6-loaded nanocarrier depleted intracellular GSH via the disulfide-thiol exchange reaction, resulting in the inactivation of GPX4 and inducing ferroptosis accordingly in 4T1 cells. Therefore, this nanocarrier significantly suppresses tumor growth and a much-improved animal survival rate in vivo, further validating the potential of a ferroptosis-inducing agent as a practical therapeutic approach. In addition, the excellent antitumor effect is alleviated by ferroptosis inhibitors, which further verifies the mechanism of inhibiting tumor growth by upsurge and the recovered GPX4 activity. This study reveals that ferroptosis is a novel mechanism of antitumor PDT, which redox-responsive nanocarriers can further employ [116]. Ferumoxytol, or Feraheme, is an US FDA-approved iron oxide NP for iron deficiency anemia treatment and is a safe pharmaceutics [198]. Guzman and colleagues [117] recently demonstrated that ferumoxytol exhibits an efficient antileukemia effect. Under the investigation of a mechanism using leukemia cell lines and primary acute myeloid leukemia patient samples, researchers figured out that the ferroptosis induction in these cell lines is due to low expression of the iron exporter ferroportin, thus resulting in a susceptibility toward an increase in intracellular iron from ferumoxytol. The generation of ROS due to the presence of free ferrous iron leads to elevated oxidative stress and cell death. Ferumoxytol treatment significantly reduces leukemia in a murine model and a patient-derived xenograft leukemia-bearing model with low ferroportin expression. This discovery highlights the potential of clinically available inorganic NPs in dealing with leukemia characterized by low ferroportin levels [117]. A finding unveils a strong association between ferroptosis and therapy resistance in cancer cells. Briefly, therapy-resistant cancer cells with high expression of ZEB1 undergo epithelial-mesenchymal transition, which exhibits vulnerability to ferroptotic cell death induced by lipid peroxidase pathway inhibition [199]. According to this novel finding, Duan et al. [61] designed a nanocatalytic sensitizer VF/S/A@CaP, coloading Vc–Fe(II) NCs and gene inhibition drugs (Figure 7A). Vc–Fe(II) NCs react with H2O2 to generate ROS and consume GPX4, thus inducing ferroptosis and killing drug-resistant cancer cells with high efficiency (Figure 7B). Using AZD9291-resistant non-small cell lung cancer (NSCLC) as a subcutaneous tumor model and a patient-derived xenograft model was also established to confirm the antitumor effect of such nanocatalytic sensitizer (Figure 7C,D). This study reveals that the AZD9291-resistant NSCLC is vulnerable to ferroptosis induced by a specifically designed nanocatalytic modality [61].
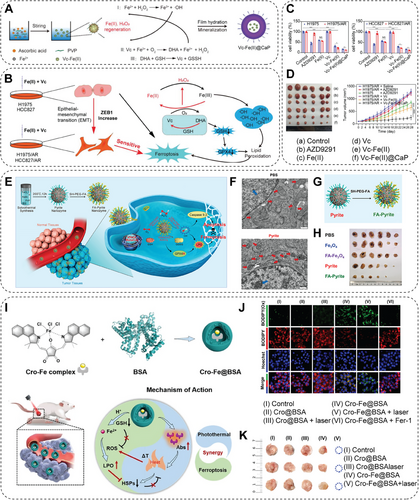
A combination of ferroptosis with other antitumoral modalities may lead to avenues in efficient cancer treatment. Fan and his team utilize pyrite peroxidase nanozyme by triggering apoptosis in synergy with ferroptosis for tumor therapy (Figure 7E) [118]. This addresses the dilemma of low levels of H2O2 in TME and low affinity between nanozyme and H2O2. This pyrite nanozyme shows a 4144–3086 fold increase of catalytic activity compared with the traditional Fe3O4 nanozyme (Figure 7F). Moreover, the folic acid modification of pyrite nanozyme improves tumor accumulation (Figure 7G), resulting in an enhanced antitumor effect (Figure 7H) [118]. Besides, a biomimetic magnetosome composed of Fe3O4 core and pre-engineered leukocyte membrane shell is constructed to promote ferroptosis and immunomodulation in various kinds of cancer [119]. Combination therapy, targeting different cell death mechanisms, has presented potential in tumor therapy. However, the design of such integrated therapies often fails to achieve the optimal synergy between the various approaches, thereby limiting the therapeutic outcomes. To solve this, the iron (III)-coordinated croconaine NP encapsulated by bovine serum albumin (BSA), Cro–Fe@BSA NP, is conducted, which is designed for mutually beneficial combination therapy by attentively coordinating the mechanism of photothermal effect and ferroptosis cancer theranostics (Figure 7I). Cro–Fe@BSA NPs release Fe3+ ions in tumor sites, which GSH reduces to Fe2+ ions. The photothermal effect can further enhance the Fenton reaction efficiency catalyzed by Fe2+, resulting in a significant elevation of lipid peroxidation and subsequent ferroptosis (Figure 7J). Due to the formation during the ferroptosis process, the ROS can disrupt the heat-induced formation of heat shock proteins (HSPs), impeding the self-protective mechanism of cancer cells against heat stress. Additionally, the synergy of photoacoustic and magnetic resonance imaging (MRI) in tumors offers a promising approach for effective and secure cancer theranostics (Figure 7K). Overall, this study provides a groundbreaking perspective on the development of combination therapy, introducing the possibility of maximizing the synergistic therapeutic effects in cancer therapy [120].
3.4.4 Cuproptosis
With the discovery of cuproptosis, cuproptosis-based cancer therapy has been put forward, especially in synergistic cancer treatment with PDT, PTT, ferroptosis-based therapy, and immunotherapy. For example, some nanomedicines were designed to simultaneously activate cuproptosis, apoptosis, and ferroptosis processes against cancer [121, 200]. In detail, copper-dithiocarbamate chelate-doped and artemisinin-loaded hollow nanoplatforms (ART@CuT/ETH HNP) are synthesized via a chelation competition-induced hollowing strategy (Figure 8A). Artemisinin, a world-renowned antimalaria drug, has been found toxic to cancer cells due to its endoperoxide bridge-containing sesquiterpene structure, which can be cleaved by intracellular oxidative stress amplified by the Fenton reaction (Figure 8B). When the ART@CuT/ETH HNP enters cancer cells, Cu2+ and artemisinin are released due to the responsiveness to an acidic and GSH-rich intracellular environment (Figure 8C). Cu2+ then catalyzes H2O2 to generate ROS and further enhances apoptosis and ferroptosis induced by oxidative stress. At the same time, Cu2+ could effectively initiate cuproptotic cell death (Figure 8D,E). This discovery proposes an effective cuproptosis/ferroptosis/apoptosis synergetic strategy for cancer treatment [121]. Xing and colleagues [122] also prepared a ROS-responsive polymer loaded with elesclomol and Cu ion named NP@ESCu. The NP@ESCu initiates cuproptosis of cancer cells and reprograms TME to enhance immune responses (Figure 8F). Moreover, the NP@ESCu and PD-L1 antibody combination can synergistically inhibit bladder cancer growth in mouse models (Figure 8G) [122]. The copper efflux mechanism is a crucial obstacle that prevents the application of cuproptosis-based cancer therapy. Therefore, some photosensitizers have been utilized to enhance cuproptosis by promoting copper overload while inhibiting copper efflux in cancer cells. As reported, TBP-2, an AIE photosensitizer, can generate ROS to consume GSH and inhibit copper efflux after extrinsic light irradiation. To prolong blood half-life and promote copper ions accumulation in cancer cells, CONPs (Cu2O) and TBP-2 are coated by platelet membrane to induce cuproptosis (Figure 8H). This nanosystem can markedly influence the cuproptosis of tumor cells and inhibit breast cancer metastasis and relapse (Figure 8I,J) [70]. Photothermal-triggered drug release is a highly controllable strategy to minimize the toxicity to nontargeted cells. Therefore, mesoporous silica-coated Au nanorods (Au@MSN), triggered by NIR light, are designed. To enhance the load efficiency and achieve controllable cuproptosis therapy, the DSF-loaded copper-doped Au@MSN nanoplatform (Au@MSN-Cu/PEG/DSF) is fabricated, which can accumulate in tumor cells and release copper ions to induce apoptosis and cuproptosis after NIR laser triggering, thereby resulting in marked inhibition of breast cancer [123]. Cuproptosis-based strategy is also capable of overcoming drug resistance. The Pt-based drug is widely used clinically to treat ovarian, breast, or lung cancers. However, the resistance of cancer cells to Pt-based drugs developed by cancer cells is inevitable. The copper-organic complex is proposed to overcome Pt-based drug resistance. For example, the GSH resistance of copper(II) bis(diethyldithiocarbamate) (CuET) can reverse the cisplatin resistance of NSCLC via cuproptosis [124].
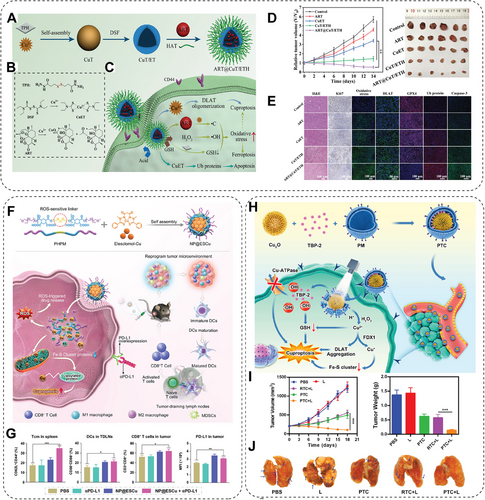
3.4.5 Pyroptosis
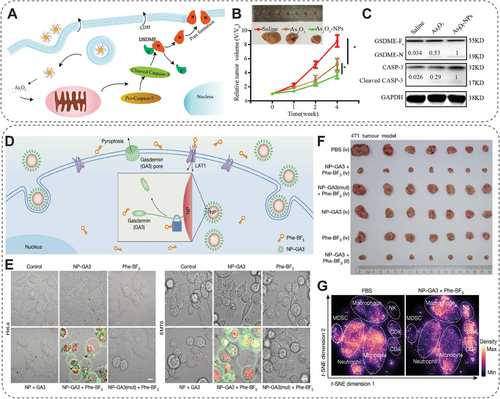
Various NMs can induce pyroptosis, including liposome-based, polymer-based, protein-based, oligonucleotide-based, and MOF nanoformulations. For example, arsenic trioxide (As2O3) can reduce tumor malignancy and metastasis, but it has severe toxicity. Duan et al. developed As2O3-loaded NPs (As2O3 NPs) using mPEG–PLGA–PLL to maintain therapeutic levels in tumors and avoid systemic toxicity [125]. As2O3 NPs activated caspase-3 in tumor cells, leading to GSDME-mediated pyroptosis of Huh7 and HepG2 cells (Figure 9A). In hepatocellular carcinoma models, intratumorally administrated As2O3 NPs reduced DNA methyltransferase expression, increased GSDME-N levels, and enhanced antitumor activity without systemic toxicity, showing promise for hepatocellular carcinoma therapy via the pyroptosis pathway (Figure 9B,C) [125]. Pyroptosis-induced NMs often synergize with immunotherapy in cancer treatment since pyroptosis is an inflammatory form of PCD and plays a significant role in the body's immune response. For example, Zhang et al. reported a strategy combining decitabine (DAC) with CT nanodrugs to induce pyroptosis in tumor cells through the epigenetic method, further enhancing the immunological effect of CT [126]. In detail, they pretreated the tumor cells with DAC for demethylation of the DFNA5 gene, which is hypermethylated, to reduce GSDME protein expression in most tumor cells. Subsequently, cisplatin-loaded nanoliposome (LipoDDP) treatment activates the caspase-3 pathway and triggers pyroptosis. This pyroptosis-based CT strategy not only enhances the antitumor activities, inhibits metastasis, and prevents recurrence but also improves the immunological effects of CT by inducing ICD and recruiting CD8+ T cells [126]. Liu and colleagues also established a bioorthogonal chemical system, Phe-BF3, enabling drug-controlled release [127]. This system furtherly synergizes with a gasdermin A3 (GSDMA3) NP conjugate (NP-GSDMA3) (Figure 9D). In vitro assay indicated that about 40%, 35%, and 20% of HeLa, EMT6, and 4T1 cells treated with NP-GSDMA3 and Phe-BF3 underwent pyroptosis (Figure 9E). Intravenous and Intratumoral injection with NP-GSDMA3 and Phe-BF3 caused repression of 4T1 tumors by pyroptosis and promotion of cytotoxic T cells and CD4+ T helper cells (Figure 9F,G). This application of the system suggests that pyroptosis-induced inflammation triggers robust antitumor immunity and can synergize with checkpoint blockade therapy [127].
3.4.6 PANoptosis
Since PANoptosis is a relatively new form of PCD, research on NMs-induced PANoptosis is currently limited. A recent study designed a nanovesicle-sensitized ultrasound-controlled immunoengineering therapy of tumor (NUITT) tactics. In NUITT, engineered extracellular vehicles (EVs) are an essential part of NUITT, and they are generated through genetically engineered cancer cells (Figure 10A). In detail, the pyroptosis protein GSDMD-overexpressed 4T1 cell is produced by drug pretreatment and transient transfection with designed siPD-L1 and PD-1 plasmids. After obtaining the GSDMD and PD-1 overexpressed EVs, EVs-sonosensitive liposome complexes, PFH@Lipo–PpIX@EVs (PLPE), were synthesized through the membrane extrusion (Figure 10B). It is hypothesized that the PD-1 on the surface of PLPE disrupts the immune resistance machinery of tumor cells against killer T cells. Under ultrasound stimulation, the PLPE induces immunogenic PANoptosis of tumors through triple catalysis, namely biological catalysis, chemical catalysis, and physical catalysis, thereby promoting an immune response. After verification, the NUITT significantly reduced PD-L1, increased GSDMD on 4T1 cells, and activated related pathways associated with pyroptosis, apoptosis, and necroptosis (Figure 10C,D). In vivo, the NUITT induced highly immunogenic PANoptosis in triple-negative breast cancer and iteratively initiated the energization of the innate immunity cycle by repeatedly releasing damage-associated molecular patterns. This process primes sufficient antigen-specific T cells and shapes a protective immune response by activating cGAS–stimulating the interferon genes (STING) signaling pathways (Figure 10E,F) [128]. Another study developed an acid-responsive, membrane-anchoring clickable iridium(III) nanosonosensitizer with superior sonodynamic activity and ICD induction ability, which can induce PANoptosis and boost antitumor immunity in TME [129]. Overall, PANoptosis is a promising new target for cancer therapy, though it is still in its early stages of development. Further research is needed to develop more effective and safer NMs-based treatments for cancer.
3.5 NMs-Based Immunotherapy
As a newly thriving tumor treatment, immunotherapy is based on activating the immune system to detect and remove tumor cells, ultimately leading to long-term immune memory, which is considered an ultimate approach to curing cancer. Cancer immunotherapy was listed by Science magazine as one of the top ten scientific breakthroughs in 2013 [201]. Currently, investigations on immunotherapy include adoptive lymphocyte therapy (chimeric antigen receptor T-cell therapy and tumor-infiltrating lymphocyte therapy), immune checkpoint (PD-1/PD-L1, CTLA-4) blocking therapy, monoclonal antibodies, cancer vaccines, and so on. Although different kinds of immunotherapy have been successfully applied in clinical settings, developing novel immunotherapeutic approaches is still necessary to overcome critical challenges, such as off-target side effects, low immunogenicity, low response rate to immunotherapy of solid tumors, and so on. One potential solution to address these issues is the combination of nanotechnology and other technologies. This cutting-edge technology enables targeted delivery of diverse types of immunotherapeutic agents, leading to remarkable advancements in cancer immunotherapy [202, 203]. This section will briefly discuss subsets of NMs-based immunotherapies of interest to us.
3.5.1 NMs-Regulated Innate Immune Cell-Based Immunotherapy
Over the past few years, researchers have explored modulating the innate immune system by NMs for therapeutic benefit. For example, the macrophage is a pivotal effector cell of the innate immune system, responsible for phagocytosing bacteria and secreting both proinflammation and antimicrobial mediators, which also play a crucial role in the fate of NPs inside the body [204]. A complete understanding of the interaction of macrophages and NPs and the rational design of nanomedicines with unique macrophage interactions will be valuable. Tumor-associated macrophages (TAMs) play a crucial role in the immunosuppressive solid TME, yet in situ engineering of TAMs to enhance tumor immunotherapy remains a major challenge in translational immuno-oncology. Gustafson et al. [205] verified that silica NP systems-initiated macrophages polarized to the M1 phenotype, while M1 macrophages exhibit tumor suppression ability. Daldrup-Link et al. [130] presented a result that ferumoxytol (a US FDA-approved iron supplement) has an intrinsic therapeutic effect on early mammary cancers and lung cancer metastasis. The mechanism study showed that ferumoxytol significantly inhibited subcutaneous tumor growth and metastasis development by increasing the presence of M1 macrophages in the tumor tissues, suggesting an attempt to utilize the intrinsic macrophage modulation ability of NMs [130]. Zhang and his team studied representative inorganic NPs: needle-shaped hydroxyapatite (n-nHA), granule-shaped hydroxyapatite, and silicon dioxide, and found that all of them can impair tumor progression effectively (Figure 11A) [131]. The n-nHA promotes the formation of multinucleated giant cells (MNGCs) (Figure 11B). In addition, the STXBP6 protein is proven to be upregulated in n-nHA-treated macrophages, triggering autophagy activation, thereby significantly promoting macrophage fusion into MNGCs. In this way, n-nHA depletes the M2-like macrophages (promoting immunosuppressive TME) in the TME and suppresses tumor growth and metastasis (Figure 11C,D) [131]. Besides, researchers successfully induce significant M1 polarization of TAMs by tumor-specific mitochondrial DNA (mtDNA) released from cancer cells’ mitochondria damaged by Fenton reaction mediated by Fe2+–Ru2+-loaded mesoporous silica (MRF) nanocatalytic medicine [206, 207]. Some metal ions, such as Fe2+, Zn2+, Mn2+, and Ca2+, elicit strong immune activation and are intensively focused on [208]. Thus, such effects push forward the emergence of metalloimmunology, suggesting that the chemistry of NMs, such as metallic-based NMs, can be a potent immune modulator [202, 209]. Thus, Shi and his team developed a platform featuring citrate-loaded iron-doped mesoporous silica NPs modified by dextran (DFHC), which efficiently modulates iron metabolism in TAM, the vital iron supply of cancer stem cells (CSCs), to induce iron death [210]. Such a situation interferes with the proliferation and stemness of CSCs and thus ultimately inhibits the onset and progression of early-stage cancers through nebulized delivery. After nebulized delivery, the prolonged lung retention of DFHC can lead to efficient accumulation in lung cancer microfoci. TAM then internalizes the DFHC via the interaction between dextran and CD206 receptors and is localized in the lysosome. Due to the strong chelating strength of citrate to Fe(III), degradation of the –Si–O–Fe-hybrid framework in response to the lysosomal acidic environment will result in the corelease of Fe(III/II) and citrate and the subsequent formation of Fe(III/ II)-citrate complex. Levels of iron metabolism are significantly elevated in protumorigenic microscopic lesions coexisting with CSCs and TAM. Iron death occurred in CSCs due to CD44-mediated/enhanced iron phagocytosis and glucose-6-phosphate-induced redox imbalance [210]. As one of the antigen-presenting cells (APCs), efficient activation of macrophages may promote the responses of T cells. Therefore, Dai and his team report a simple aluminum-like alkaline nano-adjuvant (MgAl-based hydrotalcite, bLDH) for enhanced cross-presentation of tumor antigens in macrophages triggered by radiotherapy, thereby significantly enhancing adaptive antitumor immune responses [211]. They found that cytidylic acid monophosphate guanosine oligodeoxyribonucleic acid can promote phagocytosis of irradiated cancer cells by macrophages. Introduction of the alkaline nanoadjuvant bLDH increased the acidity required for lysosomal proteases. It inhibited vacuolar-type ATPases that control low pH, thereby successfully retaining tumor antigens for presentation on MHC-I molecules. After radiotherapy, alkaline nano-adjuvants in peripheral tumors facilitated cytosine–phosphate–guanine oligodeoxynucleotides (CpG) penetration and increased tumor antigen cross-presentation by macrophages tenfold. In a highly metastatic 4T1 breast tumor model, the involvement of bLDH significantly inhibited primary tumor progression and radiotherapy-related lung metastases, indicating a novel strategy that markedly improved adaptive immune response after radiotherapy. Manganese ion (Mn2+) is an immune activator that enhances the activation of cGAS and STING proteins. STING signaling activation and subsequent immune responses are mainly associated with the ER. Therefore, targeting Mn2+ to the ER in subcellular compartments would promote the activation of the STING signaling pathway. Given this, Shen and his team [212] reported the design of ER-targeted manganese-based NCs (NCs) by complexing Mn2+ with the amphiphilic ionic polymer poly[2-(N-oxide-N, N-dimethylamino)ethyl methacrylate] (OPDMA). The Mn/OPDMA NCs (Mn/OPDMA NCs) could contribute to tumor accumulation, maintain prolonged blood circulation, and trigger adsorption-mediated cell extravasation and deep tumor penetration. Mn/OPDMA NCs could preferentially translocate to their ER in TAMs, significantly enhance cGAS–STING pathway activation, and promote TAM polarization and IFN-β secretion, resulting in potent antitumor potency in colon and hepatocellular cancer models. This study introduces a novel and effective metal ion delivery strategy for cancer immunotherapy [212].
Dendritic cells (DCs) are the most potent and specialized APCs in low abundance in the circulation and tissues, bridging innate and adaptive immunity [213]. In tumors, DCs play a critical role in initiating, recruiting, and activating tumor-specific T cells in secondary lymphoid organs. However, the effective activation of DCs is always inhibited by the immunosuppressive TME and inadequate antigen exposure [214]. To solve this, efforts have been made through multidisciplinary research. Nie and his colleagues demonstrate that zinc gluconate in oral supplements assembles with plasma proteins to form ZnO NPs that selectively accumulate into papillary Caki-2 renal tumors and promote the recruitment of DCs and cytotoxic CD8+ T cells to tumor tissues in both tumor animal models and human renal tumor samples [215]. The specific renal tumor targeting is mediated by the preferential binding of zinc ions to metallothionein-1X proteins, which are constitutively overexpressed in Caki-2 renal tumor cells. This binding event further upregulates intracellular metallothionein-1X expression to induce additional NP binding and retention. Such a phenomenon, ZnO NPs derived from supplements, can serve as a paradigm for developing a multifunctional drug delivery and cancer immunotherapy platform [215]. In another study, Nie and his colleagues propose a novel method to modulate the electrophysiological behavior of DCs, harnessing metal ion chelated l-phenylalanine (l-Phe) nanostructures to enhance ICB immunotherapy [216]. Magnesium ions (Mg2+), ferrous ions (Fe2+), and zinc ions (Zn2+) were assembled with l-Phe to form nanospheres (Ph–Mg), nanopins (Ph–Fe), and nanosheets (Ph–Zn), respectively. These NMs were destabilized and disintegrated in the acidic environment of lysosomes. Simulations showed that the metal ion-chelated l-Phe dimer was the most stable conformation and was able to open the potassium channel Kv1.3, accompanied by K+ efflux, depolarization-induced Ca2+ influx into the DCs, which activated the calmodulin-regulated nuclear factor-κB (NF-κB) pathway, promoting DC maturation and triggering the secretion of proinflammatory cytokines. In addition, the uptake of nanostructures in DCs may induce the release of histone B, which, together with K+ efflux, activates the inflammasome pathway and promotes DC maturation. Notably, DC maturation was further enhanced under nutrient-limited conditions. The present study suggests that induction of DC maturation by metal ion chelating nanostructures may contribute to immunotherapy based on programmed death receptor-1 (PD1) or programmed death ligand-1 (PD-L1) [216]. A NanoAlum proposed by Zhang and his team, composed of aluminum immune adjuvant (Alum) and Mg(OH)2. When injected into the peritumor of melanoma, NanoAlum supplies Mg2+ and neutralizes acidic TME by Mg(OH)2, which could effectively activate TAMs and T cells to attack tumors, thus remodeling the immunosuppressive TME [217]. Moreover, NanoAlum was internalized by tumor cells, blocked the autophagy pathway, interfered with tumor catabolic processes, and reduced tumor immune tolerance. This study provides a new paradigm for the rational adaptation of tumor immunomodulators from clinical adjuvants and may inspire the development of more candidate immunomodulators to enhance tumor metal immunotherapy [217]. Chen and her colleagues designed a dual-functional antitumor peptide (N-Pep) as a metal ion immunomodulator carrier [218]. The rationally designed antitumor peptide self-assembles into a hydrogel through coordination with Mn2+ ions (N-Pep–Mn gel). The multiporous hydrogel network allows efficient antiprogrammed death-1 antibody (αPD-1) loading. The hydrogel served as a depot for the sustained release of Mn2+ ions. It encapsulated αPD-1, effectively activating DCs, polarizing TAMs, and enhancing effector T cell infiltration, thereby effectively inhibiting tumor growth through intratumoral and systemic immune responses. This study underscores the potential of Mn2+ ion-coordinated antitumor peptide hydrogel as an advanced platform for enhancing antitumor immunotherapy [218].
Natural killer (NK) cell is another innate immune cell that can exert potent antiviral function and antitumor effect by different means. Multiple NK cell-based and NM-based strategies have been developed and are currently being tested. An example is using nanoscale graphene oxide (NGO) as a template to produce biomimetic nanocluster antibodies to activate NK cells by the CD16 receptor. The activation efficiency of the NGO-template nanocluster to NK cells is higher than that of antibodies [219]. Currently, the genetic manipulation of NK cells is a highly dynamic area of research. It focuses on enhancing their in vivo persistence, targeted migration to specific locations, overcoming resistance within the TME, and augmenting their cytotoxicity against tumor cells upon adoptive transfer. Thus, the chimeric antigen receptor–NK (CAR–NK) is emerging as an attractive method. CAR–NK cells offer several advantages over other cell therapies. They demonstrate superior safety profiles, with minimal or no cytokine release syndrome and neurotoxicity occurrences. Another noteworthy benefit is the feasibility of “off-the-shelf” manufacturing, allowing for readily available, standardized products that can be readily administered. To simplify the production process, scientists developed core–shell NPs (MF-NPs), which consist of a magnetic core and a polydopamine shell. MF-NPs could deliver genetic materials into the NK cells and induce EGFR-targeting CAR on the surface. Overall, MF-NPs possess the ability of CAR–NKs in situ establishment and in vivo cell tracking monitoring [133]. NK cells are severely insufficient and inactivated in solid liver tumors due to the highly immunosuppressive intra-TME, resulting in poor clinical therapeutic efficacy. To deal with it, researchers designed and constructed a magnetogenetic nanoplatform (MNPs@PEI–FA/pDNA (MPFD)) for modulating the IL-2 expression, critical for NK proliferation, in cancer cells by transfecting the specifically designed heat-inducible plasmid (HSP70–IL-2–EGFP) (Figure 11E). The magnetothermally responsive MPFD induces the gene transcription of IL-2 cytokine in orthotopic liver tumors (Figure 11F), leading to in situ expansion and activation of tumor-infiltrating NK cells through the IL-2/IL-2 receptor pathways (Figure 11G), resulting in prominent tumor inhibition (Figure 11H) [134]. Pan et al. [135] found that mild MHT can activate NK cells by inducing the expression of UL16-binding proteins in hepatocellular carcinoma cells. In contrast, such a modality significantly inhibits orthotopic liver cancer in Balb/c nude mice [135].
3.5.2 NMs-Mediated Cancer Vaccine Delivery
Cancer vaccines usually entail the external administration of specific tumor antigens and adjuvants designed to activate antigen-presenting and DCs. The critical point in the therapeutic cancer vaccines includes delivery of adequate antigens to DCs, optimal DC maturation, and induction of robust cytotoxic T (CD8+) cell responses [220]. Anderson et al. screened a combinatorial library of ionizable lipid-like materials to achieve intracellular delivery of therapeutic messenger RNA (mRNA) vaccine and robust immune activation more effectively (Figure 12A) [221]. They evaluated the mRNA delivery efficacy via loading mRNA on lipid NPs (LNPs) composed of the lipid library (Figure 12B). They identified that the head group is the vital component: lipids containing a cyclic amino head group. The lipid-containing cyclic amino head group acts as an mRNA delivery vehicle and induces DCs to mature by STING pathway. The LNPs fabricated by these lipid materials and mRNA demonstrated marked improvement in the survival of several tumor models after being treated with the LNPs (Figure 12C) [221]. Xu's group has developed various LNP systems that could target the liver, spleen, and lung without selectively targeting ligands by adjusting their components [136]. The latest study by his lab fabricated a lymph node-targeting LNP, 113-O12B, which can enhance the CD8+ cell-dependent responses and promote therapeutic effects (Figure 12D). 113-O12B reduces the liver's mRNA expression and increases lymph node expression compared with ALC_0315, a demethylated structure of US FDA-approved Seminary. Moreover, the 113-O12B cannot only deliver short-peptide-based or antigen-encoding mRNA but also deliver full-length proteins. The 113-O12B system combined with PD-1/PD-L1 blocking significantly generates suppression or complete eradication of the B16F10 tumor (Figure 12E,F) [136].
3.5.3 NMs-Assisted T Cell Therapy
T cell-based immunotherapy has excellent potential in curing cancers, with many pipelines of T cell-based treatment undergoing clinical trials [222]. Despite significant advances, such a promising strategy faces strict challenges that prevent it from clinical application, for example, insufficient effector T cells infiltrated in a solid tumor, effector T cell exhaustion, and inactivation due to immunosuppressive TME [223]. Fortunately, recent studies have successfully applied NMs for assistance in T cell-based immunotherapy to enhance efficacy, suggesting that NMs have great potential in improving the clinical effectiveness of T cell-based therapy [224-226]. For example, effector T-cell engineering and expansion can be conducted with the assistance of NMs in delivering genes, cytokines, or small molecules, which include polymeric, lipid-based, and inorganic NPs [137, 227-230]. Polymeric NPs are simultaneously coupled with the T-cell targeting fragment and the nuclear localization signal peptide on the surface (Figure 13A). Therefore, such NPs can be taken by T cells and then release gene modification agents in the cell nucleus (Figure 13B). The T cells reprogrammed by NPs can express the CAR effectively for weeks and differentiate into memory T cells, generating sustained antitumoral immunity [137]. Cationic polymer, poly(hydroxyethyl methacrylate)-grafted poly(2-(dimethylamino)ethyl methacrylate), exhibits remarkable transfection efficacy in Jurkat human T cells (up to 50%) with only minimal toxicity [231]. Ionizable LNPs can deliver mRNA into human T cells to trigger transient CAR expression with much reduced adverse effects. A library consisting of 24 synthesized ionizable lipids is constructed. The transfection efficiencies of NPs fabricated by lipids in the library are tested by delivering luciferase mRNA into Jurkat cells, and C14-4 is ultimately selected as the optimal vehicle for CAR mRNA delivery. The NPs containing C14-4 display comparable transfection efficiency compared with the conventional electroporation method. The resultant CAR-T cells elicit significant cancer-killing effects as verified in vivo [229]. Besides, T cells attached by NMs, called “backpacking,” can generate effective expansion in vivo. In this approach, NMs are bound to T cells and thus deliver their contents to T cells primarily in a pseudo-autocrine pattern [232]. One recent study described a T-cell backpacking strategy using protein nanogels that load various supporting protein drugs. This nanogel attaches to the T cell surface stably by noncovalent attachment via CD45 antibody and electrostatic association, which ensures that the nanogel remains at the T cell surface rather than being internalized by T cells (Figure 13C). When T cells recognize and bind to cancer cells, the drugs loaded in the nanogel are released due to the increased reduction potential at the T cell's surface (Figure 13D). By backpacking the interleukin (IL)-15 super-agonist complex with nanogel, this system induces T cell expansion over 16-fold in tumors compared with that caused by free cytokines (Figure 13E) [138]. In addition to promoting T cell expansion, NMs are also helpful in improving T cell penetration into solid tumors. In recent research, they designed a core–shell calcium phosphate liposome, encapsulating α-mangostin and plasmid encoding the stimulatory cytokine LIGHT loaded, named Nano-sapper (Figure 13F). In tumor sites, released α-mangostin reduces the physical barrier, and secreted LIGHT enhances cytotoxic T cell infiltration, resulting in a practical antitumor effect in an immune-excluded tumor [139]. Nonetheless, Gu and his team [233] reported that mild hyperthermia can improve the efficacy of CAR-T cells, which immunosuppressive TME continuously deactivates. Applying mild hyperthermia to the tumor reduces its compact structure and interstitial fluid pressure. It thus increases blood perfusion and antigen release, ultimately promoting the recruitment and activity of intratumoral CAR T cells. Therefore, it was found that the much higher CAR T cells infiltrate the human melanoma WM115 in mouse models and achieved superior antitumor activity after photothermal ablation of the tumor [233].
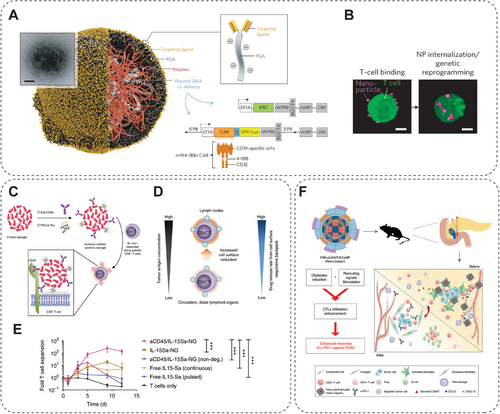
3.5.4 NMs-Based Synergistic Strategy of Immunotherapy
Combining immunotherapy with other therapeutic modalities, like RT, CT, PTT, PDT, MHT, chemodynamic therapy (CDT), and sonodynamic therapy (SDT), always provides superior antitumor activity than a single treatment [234-240]. PTT is a promising cancer treatment modality that transfers light absorbance to heat emission for the localization ablation of solid tumors. However, it hardly deals with deep, disseminated, or metastatic tumors. Accordingly, the immune activation synergy with PTT is a solution for overcoming such clinical limitations. In this work by Wang et al., the Au–DOPC NPs induce a photothermal effect under irradiation by NIR(II) light and activate the ICD process, thereby eliciting robust innate and adaptive immune responses [140]. The Au–DOPC NPs exhibit significant suppression of distant or metastatic tumors when combined with the checkpoint block inhibitor. Moreover, the systemic administration of a two-dimensional polypyrrole nanosheet, an NIR(II) transducer, achieves a more remarkable effect on aggressive whole-body metastasis. This study suggested using an effective NIR(II) transducer meets the striking therapeutic effects against both primary and metastatic tumors via a synergistic photothermal-immunological response [140]. In another work by Moon and his colleagues, PTT combined with CT-induced antitumor immunity against invasive cancer (Figure 14A) [141]. They engineered doxorubicin-loaded polydopamine-coated spiked AuNPs to elicit potent antitumor effects in animals bearing CT26 colon cancer by only a single round of PTT and a much lower dosage of doxorubicin. The robust antitumoral response eliminates almost all local tumors and more than 85% of distant tumors. Their strategy also works in TC-1 submucosa-lung metastasis, a highly aggressive model for advanced head and neck squamous cell carcinoma [141]. The photothermal-stable and biocompatible immunologic nanoplatform, ovalbumin (OVA)-coated PEGylated MnFe2O4 NPs encapsulated R837 immunoadjuvant (R837–OVA–PEG–MnFe2O4 NPs), could elicit significant antitumor response under PTT as well as work as an efficient MRI imaging agent [142].
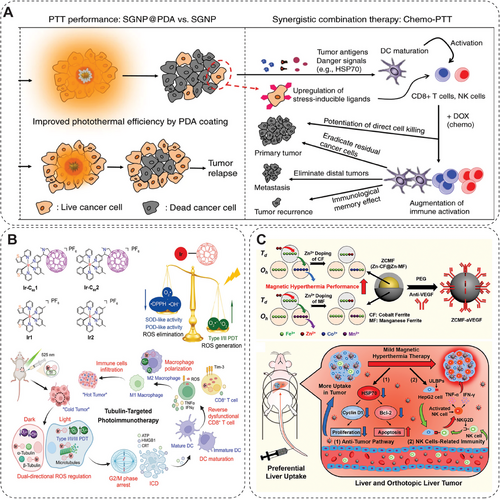
Both PDT and SDT can generate ROS to induce the death of tumor cells when subjected to light or ultrasound. At present, PDT and SDT are found to have three main effects on tumors: (1) direct killing of tumor cells, (2) damage to tumor blood vessels, and (3) induction of systemic antitumor immunity [145, 242, 243]. Since ROS is a double-edged sword in tumor, Tan's team synthesized Ir(III)–fullerene(C60) conjugates to manipulate ROS for cancer treatment. They synthesized two conjugates, Ir–C601 and Ir-C602, as type I/II photosensitizers targeting tubulin (Figure 14B) [241]. In the dark, the conjugates act as scavengers to reduce O2•−, •OH, and 2,2-diphenyl-1-picrylhydrazyl to reduce dark cytotoxicity, as well as reverse the dysfunctional CD8+ T cells. Upon 525 nm irradiation, Ir-C601 and Ir-C602 O2•− and •OONO− and 1O2 to overcome tumor hypoxia. The Ir-C601 exhibits antitumor effects by photo-oxidizing the cell cytoskeleton and inducing ICD [241]. Shu et al. reported a versatile photo-triggered nano-gadofullerene (Gd-Ala) that induces malignant tumor vascular disruption by efficiently producing ROS by shortening the light interval between Gd-Ala administration and illumination [143]. Then, the irradiated Gd-Ala promotes DC maturation and increases the secretion of TNF-α and IL-12, resulting in upregulated differentiation of CD4+ and CD8+ T lymphocytes. Resultantly, tumor metastasis is much inhibited by both antitumor immunity and downregulation of matrix metalloprotein 2 and matrix metalloproteinase 9 expression [143]. SDT has attracted broad attention due to its ability to induce ICD and deep tissue penetration. Assisted by cavitation, ER-targeted sonodynamic nanodroplets (PMPS NDs) plus PD-L1 antibodies overcome orthotopic and distant pancreatic cancer. When subjected to ultrasound, PMPS NDs liquid-to-gas bubbles undergo oscillation and cavitation, promoting the penetration of NDs into deeper tissue. After reaching deep tumor tissues, the cancer cells internalize the NDs and induce ER stress by generating ROS, promoting ICD and DC maturation. Such a combination therapy of SDT and immune checkpoint blockade could effectively suppress pancreatic cancer [144]. A multifunctional, peptide-engineered amphiphile-Rose Bengal (RB) loaded nanocapsules (PARNs) were developed for realizing PDT, SDT, and antitumor immunity effects against superficial malignant tumors. Herein, the RB achieves immune-response activation by acting as both PDT and SDT sensitizer. At the same time, the contribution balance of PDT and SDT efficacy is modulable by changing the RARN formulation. The PARNs promote cancer cell elimination efficacy by directly damaging the vascular system, ROS generation, and antitumor immunity [145].
MHT is a therapeutic modality that is already available in the clinic. Magnetic NPs are prepared as transducers, enabling the highly efficient conversion of electromagnetic energy into heat when subjected to an alternating magnetic field (AMF), which can elevate the local temperature of the tumor to around 42–50°C, resulting in notable cell death [244-248]. MHT has already exhibited promising clinical results in the treatment of brain cancer, prostate cancer, and esophageal cancer. For example, an MHT device was approved by EMA as an adjunct therapy for patients with recurrent glioblastoma who are also receiving RT in 2012 [249]. A clinical trial testing a similar approach for tumor ablation in men with prostate cancer received ethics approval (NCT05010759) in 2021 [250]. Based on the considerable optimal potential in clinical cancer therapy, attempts have been made in this field with great effort. Pan and his colleagues [146] propose a combined MHT and checkpoint blockade immunotherapy for primary tumor ablation and metastatic tumor inhibition. High-performance superparamagnetic CoFe2O4@MnFe2O4 NPs are synthesized and used for effective MHT-induced thermal ablation of primary tumors [146]. Simultaneously, tumor-associated antigens are abundantly released to promote the maturation and activation of DCs and cytotoxic T cells for effective immunotherapy of distant metastatic tumors in a tumor-bearing mouse model by synergizing with the immune checkpoint blockage (α-PD-L1). Meanwhile, different from traditional MHT, mild MHT (around 43°C) has proved effective in tumor killing without leading to irreversible damage to the surrounding organs and tissues, which is always caused by traditional MHT tumor ablation (>50°C). Chester et al. [251] found that the tumor antigen delivery efficiency of DCs and infiltration of CD8+ T cells could be significantly enhanced after MHT. Pan et al. [135] found that the mild magnetothermia mediated by ZCMF effectively activates the expression of UL16-binding proteins in hepatocellular carcinoma cells, significantly promoting NK cell activation (Figure 14C). The same team also successfully induced M1 polarization of TAMs by mtDNA released due to the damage caused by MHT [132]. These studies also led to a much-enhanced tumor inhibition from immune activation caused by mild MHT. Based on this research, Fu et al. [147] introduced a novel strategy of concurrently stimulating innate and adaptive immunity while enhancing immune cell infiltration in solid tumors using a specifically designed magnetic nanocatalysis medicine (ZnCoFe2O4@ZnMnFe2O4–PBA, ZCMFP) for MHT-mediated immune response activations. ZCMFP generates ROS catalyzed by released Fe2+/3+ ions in response to acidic TME, provoking the M1 polarization of TAMs. Meanwhile, the coreleased Mn2+ promotes the maturation of DCs via the STING pathway for antigen presentation, which awakens T cells and the adaptive immunity. The mild MHT also regulates vascular endothelial growth factor (VEGF) intratumorally, resulting in the elevated expressions of adhesion molecules (ICAM-1/VCAM-1) and thus facilitating the recruitment and infiltration of effector immune cells, which leads to the marked inhibition of both orthotopic and metastatic tumor growth. This strategy provides a new insights for solid tumor immunotherapy in clinics [147].
3.5.5 NMs-Based Immunoadjuvant
Therapeutic cancer vaccination is considered a highly promising strategy for cancer immunotherapy because of the induction of tumor-specific T-cell activation and the formation of a long-lasting immune memory effect [203]. Immunoadjuvants are constantly added to cancer vaccines to augment the specific immune response. For example, aluminum hydroxide, AlO(OH), has been an efficient immune adjuvant for over 80 years [252]. However, this traditional adjuvant fails to activate CD8+ T cell response efficiently, a crucial element to cancer vaccines. To solve this, aluminum hydroxide is transformed from gel to nano-sized carrier (the AlO(OH)-based NPs) (Figure 15A), which are developed to deliver vaccine to APCs and activate CD8+ T cells subsequently and thereby resulting in remarkable tumor suppression (Figure 15B–E) [148]. Besides, another aluminum-based material, Al2O3, can also act as an immunoadjuvant when combined with toll-like receptor 9 agonist CpG and can trigger robust immune responses (Figure 15F–I) [149]. Researchers attempted to develop different vehicles for delivering cancer vaccines and immunoadjuvants in the following work. They managed to fabricate aluminum-containing ZIF-8 that encapsulate the antigen OVA with CPG absorbed (donated as CpG/ZANPs). The CpG/ZANPs could target lymph nodes and are taken up by lymph node-resident APCs, which therefore enhances antigen and adjuvant presentation after decomposition in the lysosomes, leading to much-promoted inhibition of EG7-OVA tumors by activating strong antitumor responses [150].

3.6 NMs-Based TME Modulation
The TME features several hallmarks, including hypoxia, acidosis, and aberrant vascular system, which supports tumor growth, aggressiveness, metastasis, resistance to therapy, and hinders antitumor therapy efficacy [253, 254]. Many researchers have attempted to elucidate the therapeutic challenges associated with the TME and how nanotechnology can be applied to reshape the TME and increase the sensitivity of cancers to therapeutic methods.
3.6.1 Relief from Hypoxic Conditions
Unlike natural enzymes, typically featuring high catalytic activity and substrate selectivity, nanozymes usually have lower activity, nonspecificity, and limited enzyme mimetic types, severely preventing their practical application. Efforts have been made to improve the catalytic efficiency of nanozymes, including regulating substrate selectivity, improving catalytic activity, and developing multienzyme mimetic activity. Most importantly, nanozymes are modulable according to the requirements; for example, by catalytically generating abundant ROS selectively in TME, nanozymes with peroxidase or oxidase-like activity can be used for antitumor therapeutics [265]. Using the ROS scavenging capability, nanozymes with catalase or superoxide dismutase-like activity can help detoxify ROS and thus prevent inflammatory and aging-related diseases. MnO2 NPs have attracted increasing attention for cancer therapy applications due to their excellent catalytic activity and TME-responsive characteristics [266]. Wu's lab conducted a systematic in vivo characterization of MnO2 NPs for the first time. They demonstrated that polyelectrolyte–albumin-modified MnO2 NPs simultaneously regulate hypoxia and acidity of TME, resulting in elevation of oxygenation by 45% and pH from 6.7 to 7.2 [267]. In the following research, two different NPs were fabricated and embedded with polyelectrolyte–MnO2 in the hydrophilic terpolymer/protein–MnO2 (TMD) or hydrophobic polymer/lipid–MnO2 (LMD) matrices (Figure 16A). TMD and LMD relieve hypoxia and acidity by showing different oxygen generation rates (Figure 16B,C). Fast-acting TMD is used in local injections, reducing about 70% of hypoxia, while slow-acting LMD displays remarkable tumor accumulation via systematic administration, and the hypoxia reduction rate is about 45% (Figure 16D) [268]. To generate lethal ROS in hypoxia conditions, MnO2@PtCo nanoflowers are engineered, in which the MnO2 component with the catalase-like activity can induce the decomposition of H2O2 into O2, thereby relieving hypoxia (Figure 16E). The PtCo nanoenzyme exhibits oxidative reaction, cascades catalytic activity, and causes intracellular oxidative damage, leading to potent therapeutic outcomes. By modulating ROS production, such nanozyme platforms can overcome the dependence on oxygen status in generating ROS and circumvent hypoxic tumor limitations. As a result, the MnO2@PtCo nanoflowers have greatly relieved the hypoxia condition in tumor sites while promoting apoptotic cancer cell death (Figure 16E,F) [151]. The GOx-like activity of AuNPs has been discovered. However, after entering the body, surface passivation caused by strong protein absorption shields the catalytic function of AuNPs. The AuNPs are doped into iron-based MOFs (GIM) to maintain their function. In the tumor sites, the glucose is consumed by AuNPs, and H2O2 is generated, while the iron ion from GIM reacts with H2O2 to produce ROS by the Fenton reaction. Then, the H2O2 is catalyzed into O2 rapidly, which modulates the hypoxia of TME and provides O2 for glucose oxidation catalysis by AuNPs. Besides, the NIR irradiation effectively promotes the catalytic cascade reaction-mediated GIM, further increasing ROS generation and induction of apoptosis [152]. CeO2 is also a representative oxidase-mimic nanozyme. Still, some areas for improvement, such as irreversible aggregation and lower catalytic activity under acid conditions, prevent it from further application. Ce–MOFs have been designed and fabricated to surmount this problem. The Ce–MOFs display a high oxidase-like activity to induce desired oxidative damage toward cancer cells and ATP deprivation capacity [153].

3.6.2 Targeting Vascular Abnormality
Vascular abnormality in TME enables the accumulation of NPs based on passive targeting, while the active targeting of NPs depends on specific targeting ligands. For developing passive tumor-targeting NPs, the enhanced permeability retention (ERP) effect was the foundation, first proposed by Matsumura and Maeda in 1986 [269]. The EPR effect introduces a pathophysiological phenomenon and mechanism for the leaky tumor vasculature and impaired lymphatic drainage. The NPs with diameters between 50 and 200 nm are considered the optimal size to accumulate in the tumor tissues via the EPR effect [270]. Unfortunately, the results of clinical trials conducted by several therapeutic NPs failed to realize significant accumulation in tumor sites. The active-targeting NPs are fabricated by targeting the molecules highly expressed on the endothelial cells of abnormal tumor blood vessels, such as the cell adhesion molecules and integrins [253]. Furthermore, research has demonstrated that improving the abnormal tumor vessels is helpful for nanomedicine accumulation in tumors [254]. Antiangiogenic agents can modulate the perturbed signaling transduction and lead to transient normalization of the vasculature, thus improving interstitial fluid pressure, increasing tumor oxygenation, and enhancing drug penetration [254]. For instance, VEGF is an essential component in the angiogenic signaling pathway and an effective target for antiangiogenic therapy. Combining CT and anti-VEGF antibodies results in a remarkably prolonged patient survival rate [271]. Another research introduced a tumor inhibition strategy by tumor vascular endothelial cell-targeting NPs (F56–PTX–NPs), modified with endothelial cell-targeting F56 peptide and loaded with PTX (Figure 17A). The “normalization window” induced by F56–PTX–NPs is maintained for at least 9 days, and the TME is temporarily modulated, during which time PTX released from F56–PTX–NPs exerts a significant tumor-killing effect (Figure 17B–E) [154]. More interestingly, NMs could even modulate the vessel by the “NP-induced endothelial leakiness” effect (NanoEL), featuring that certain NPs with unique properties can induce micrometer-sized gaps between endothelial cells (Figure 17F) [272]. Researchers demonstrated that the AuNPs with diameters between 10 and 30 nm may display significant NanoEL phenomenon within 30 min after administration (Figure 17G,H). Investigation on mechanisms revealed that the NanoEL induced by AuNPs is due to the disruption of the VE–cadherin connection rather than endocytosis. This study provides the foundation for designing tumor-targeting nanomedicines without relying on the EPR effect, which is only effective in mature tumors. Besides, zinc oxide NPs, Ag NPs, TiO2, and silica NPs have also exhibited the NanoEL phenomenon [273]. However, further research in the same group found that NanoEL-inducing NPs may promote the possibility of cancer cells' intravasation into the surrounding vessels and forming distant metastasis by extravasation [274]. They compared the NanoEL among three different NPs, TiO2, silica, and AuNPs, administered intravenously. Results showed that all these NanoEL-inducing NMs have accelerated the migration and invasion of breast cancer both in vitro and in vivo. However, inducing tumor endothelium leakiness in the control is an alternative method for eliminating the systemic adverse effects caused by off-target situations. Therefore, some researchers have developed novel therapies by inducing gaps between endothelial junctions through stimuli that only focused on tumor sites, such as acoustic, hyperthermia, or radiation, to avoid the off-target effect [273].
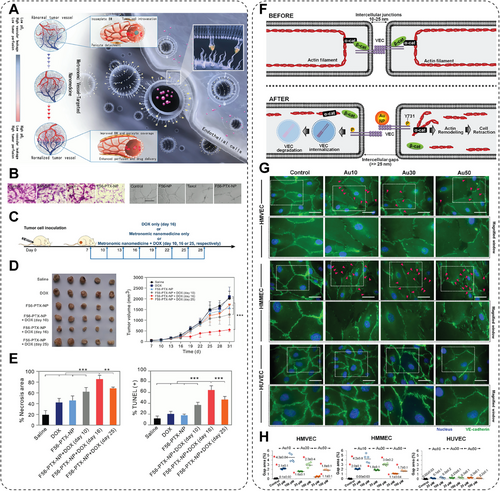
3.6.3 ECM Regulation
The ECM is a challenging part that significantly regulates the exchange of NMs across biological barriers. However, the infiltration efficiency across the tumor ECM of NMs could be better. Therefore, methods have been developed to enhance the diffusion of NMs across the tumor ECM, including NP characteristics (size, shape, surface charge, rigidity) and TME modification [275]. Cancer-associated fibroblasts (CAFs) produce large amounts of collagen and proteoglycans, significant components in the tumor ECM. Moreover, significantly increased collagen deposition and promoted ECM stiffness facilitate cancer cell survival and proliferation [276]. CAFs will also grow to surround the blood vessels at tumor sites and construct dense obstacles for NMs penetration and delivery [277, 278]. Since they are the primary cause for building these obstacles, CAFs are excellent targets for in situ modification and motivate many researchers. A lipid-coated, protamine DNA complex (LPD) was developed to confirm this proof of concept, within which a secreting form of TNF-related apoptosis-inducing ligand (sTRAIL) plasmid was loaded. CAFs efficiently internalize the sTRAIL-loaded LPDs; over 70% of CAFs secrete sTRAIL in situ. Then, the secreting sTRAIL induces significant apoptosis in adjacent tumor cells, reprograms residual CAFs, and facilitates the delivery of laterally administrated therapeutic NPs [155]. Using ECM-digesting enzymes to pretreat tumors is also an effective method to improve NMs penetration [254]. NPs, modified by enzymes, have achieved much deeper penetration into tumor tissues by active digestion of ECM. Collagenzome, a collagenase-loaded liposome, protects collagenase from being degraded in the blood circulation while maintaining sustained release at the tumor sites (Figure 18A–D). In the orthotopic pancreatic ductal adenocarcinoma model, the tumor-bearing mice are pretreated with Collagenzome and then followed by PTX micelles administration, which showed over 87% suppression of tumor size compared with that without Collagenzome pretreatment (Figure 18E,F). Additionally, they detected that circulating cancer cells showed no significant difference between different treatment groups, indicating the ECM incompetence of ECM digestion and no metastasis promotion (Figure 18G–I) [156]. Hyaluronan is another abundant component of ECM that exists in tumors and can be rapidly degraded by HAase. The combination of NPs (NM-Ce6) of the PDT agent and HAase improves the antitumor efficacy by enhanced tumor uptake of NM-Ce6 due to the increased perfusion after HAase treatment (Figure 18J). NM-Ce6 also augments the therapeutic efficacy of PDT, inhibiting lymph node metastasis because of the targeting ability of Haase (Figure 18K–M) [157].
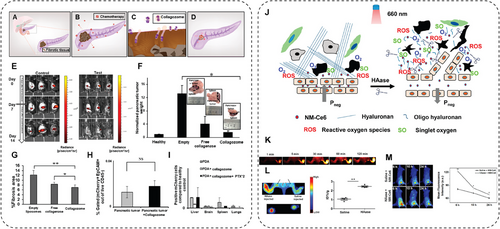
3.6.4 Neutralization of Acidic TME
Because of the rapid proliferation, cancer cells stack into a tight tumor tissue with a limited oxygen supply, resulting in a hypoxic microenvironment. Therefore, cancer cells tend to conduct anabolic glycolysis, named the Warburg effect, to support their growth, leading to abnormal accumulation of lactic acid and acidic TME [279]. Such an acidic microenvironment provides a hostile milieu that limits the activity of normal cells, especially the immune cells. Cancer cells survive in an environment different from normal cells by elevating their glycolytic activity or the expression of proton transporters that normalize intracellular pH for survival. More unfortunately, the adaptation of acidosis even increases the aggressive phenotype of tumor cells, exaggerating their invasion, proliferation, and drug resistance [280]. Besides, the adaptation of acidic microenvironment also facilitates immune escape, proliferation, metastasis, or even further mutation of tumor cells, which strongly maintains tumor growth and development. Furthermore, the immune cells are profoundly influenced by the acidic microenvironment. For example, acidic TME is capable of inducing macrophage polarization toward M2 phenotype for supporting the immune suppressive TME and promotes tumor growth; the acidic TME facilitates the production of VEGF and thereby results in the exhaustion of cytotoxic T cells; such a hostile low pH condition also inhibits the immune response to tumor antigens, worsening the immunosuppressive TME [281]. Thus, researchers have made great efforts to inhibit tumor growth by modulating the acidic TME to enhance the therapeutic outcomes of tumor therapies [282]. Layer double hydroxides (LDHs) have gained increasing focus, featuring excellent biocompatibility, pH-responsive biodegradability, easy surface modification, and especially acidic neutralization. Typically, LDHs consist of positively charged host layers with guest anions and water molecules intercalated in interlayer galleries. The LDHs are a type of hydroxide with a mild alkaline pH, resulting in their sensitivity to acidic environments and ability to neutralize the acidic microenvironment. To remodel the immunosuppressive acidic TME, Liu et al. [158] introduced drug-free LDHs with weak alkaline to neutralize the excess acid while blocking autophagy of tumor cells for much enhanced neoadjuvant cancer immunotherapy (Figure 19A). LDH NPs, peritumoral injected, efficiently neutralized the excessive acid in the TME, resulting in increased levels of antitumor TAMs and T cells. The buffers of H+ by LDHs are also capable of inhibiting the acidification of intracellular autophagolysosomes, thus leading to the accumulation of autophagolysosomes and blockage of the lysosome-mediated autophagy pathway in tumor cells and ultimately causing tumor cell death and antigen release. Furthermore, these LDHs captured and released tumor antigens. They effectively activated the in situ vaccination, thus triggering antitumoral immune responses and inhibiting the growth of both melanoma and colon tumors in vivo. This research validates that LDHs promote autophagy-related tumor cell deaths and subsequent immune system activation by modulating the acidic microenvironment of the intrinsic mild alkaline property. In another work conducted by Ling and his team [159], they prepared a Zn2+-doped layered double hydroxide (Zn–LDH)-based immunomodulating adjuvant for relieving immunosuppressive TME of solid tumor while eliciting robust antitumor immunity (Figure 19B). The Zn–LDH was peritumorally injected, thus neutralizing the acidic TME and releasing abundant Zn2+, which promotes the antitumoral activity of M1 TAMs, cytotoxic T cells, and NK cells. Moreover, the Zn–LDH effectively disrupts endo-/lysosomes in tumor cells due to the ability of acid neutralization, thereby blocking the autophagy activity and thus inducing mitochondrial damage (Figure 19C). Moreover, the released Zn2+ activates the cGas–STING signaling pathway to induce ICD (Figure 19D,E), further promoting the release of tumor-associated antigens to induce antigen-specific cytotoxic T lymphocytes (Figure 19F,G). Such a method for inducing robust immune responses by mere injection of Zn–LDH adjuvant, without using any cytotoxic inflammatory cytokines or immune agonists, provides a rational bottom-up design of a potent adjuvant for cancer metalloimmunotherapy against solid tumors by efficiently modulating the acidic TME. In a recent study, Lin and his team [160] synthesized a simple and drug-free inorganic NM, alkalescent sodium bicarbonate NPs (NaHCO3 NPs), for cancer immunotherapy. The alkalescent NaHCO3 NPs neutralized acid–base and thus regulated lactic acid metabolism, reversing the mildly acidic immunosuppressive tumor environment. Besides, NaHCO3 NPs induce a surge in intracellular osmolarity by releasing high amounts of Na+ ions inside tumor cells and thus activate the pyroptosis pathway and ICD. Such effect then triggers significant activation of antitumoral immune responses, resulting in the much-enhanced inhibition of primary/distal tumor and tumor metastasis. This work provides insights into regulating acidic TME caused by abnormal lactic metabolism and inducing pyroptosis via modulating the intracellular osmolarity, which has potential applications in clinical tumor immunotherapy. In conclusion, the regulation of acidic TME by acid neutralization of NMs can significantly modulate the immunosuppressive microenvironment in solid tumors without drugs or other agents, thus activating the antitumoral immune responses and subsequently enhancing the therapeutic outcomes of current tumor therapies, which is promising in the future combats against tumors.
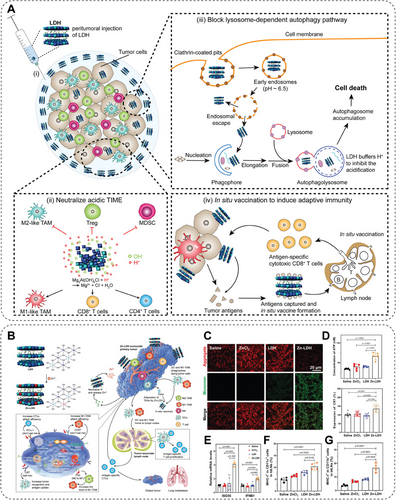
4 Conclusion and Outlook
Cancer is a long-standing cause of mortality, with more than 10 million new cases yearly. Traditional therapies in clinics once brought light to this continued battle. However, they have also encountered huge and challenging barriers. The development of nanotechnology has presented NMs as a newly flourishing technique in fighting against tumors, which features several advantages for in vivo targeted drug delivery with much reduced off-target and side effects generated by their specific size distribution. Here, we summarized that certain NMs could selectively activate several cellular biological processes to independently regulate cell fate, including ferroptosis, pyroptosis, autophagy, necroptosis, inducing ICD, and so on, mainly relying on modulating the interaction between the NMs and cancer cells or organism proteins. This may ultimately lead to cancer cell death with highly selective and free of inducing related resistance for the independence of specific therapeutic targets. This phenomenon is encouraging because NMs exhibit tremendous potential to influence cancer cells intrinsically without other extrinsic stimuli or triggering unwanted effects. Furthermore, the stimulation or activation capability of NMs can be tuned precisely by chemical manipulations or modifications. Such an NMs-induced regulation can be further transformed into new methods for treating diseases, requiring systematic study or introducing the effects of NMs in stimulating biological processes from the perspective of nanotoxicology and therapeutics, thus providing essential research support for the physical effects of NMs-induced biological effects.
Unfortunately, however, NMs-mediated therapy also shows tremendous limitations. First, it is urgently necessary for a breakthrough and improvement to find a more effective way to circumvent the defense systems of tumors in vivo, where the actual phenomenon and circumstances are more intricate and complicated in cancer patients. The tumor heterogeneity is increasingly associated with the limited efficacy of NMs in vivo and is a significant challenge for the clinical translation of NMs-based drugs. The heterogeneity is multifaceted and exists at both macro and micro scales, including diverse cell populations, microenvironment variability, target variation, and so on. Developing personalized NMs-based medicines and combining them with other therapies could be feasible to overcome these barriers. Second, NMs-based medicines are facing several safety challenges. Our understanding of the long-term toxicity of NMs remains limited. Short-term toxicity always causes off-target drug release and acute immune response, while long-term toxicity may lead to severe adverse reactions in late-stage trials. For example, continuous oral administration of nanomedicine may affect the intestinal barrier even if the component of the NMs is an US FDA-approved safe material. Comprehensive preclinical testing, including toxicity, pharmacokinetics, and biodistribution studies, is essential to predict long-term behavior. Future developments, including biodegradable NMs, predictive models, and personalized formulations, are necessary to ensure the safety and biocompatibility of nanomedicines, ultimately facilitating their clinical application. Third, the pharmacokinetics and biodistribution study of NMs shows that precisely targeted NPs accumulation and drug retention in the tumor site is still an unsolved challenge. After NPs enter the blood, the surface chemical modification determines the binding of various serum proteins, which are recognized and internalized by scavenger receptors on the surface of macrophages, resulting in a considerable loss of NPs in the blood circulation. This process is called the reticuloendothelial system or mononuclear phagocyte system (MPS). Since spleen, liver, and bone marrow are rich in macrophages, the NPs prefer to accumulate in these tissues, typically leaving 2–10% of the injected doses being distributed to the target tissue. Also, uptake by MPS results in off-targeted side effects. Although investigators have dedicated themselves to addressing this challenge by optimizing the size, charge, and surface chemistry, the outcome has not fully met expectations. For instance, NPs larger than 50 nm are expected to have less efficient tissue penetration. However, NPs smaller than 50 nm tend to enhance liver uptake, potentially leading to liver toxicity. Furthermore, high tumor accumulation of NPs in the tumor site is not directly correlated to the bioavailability of the drug, which is primarily controlled by the drug release rate [283, 284]. Last but not least, although NMs have become up-and-coming candidates for medical applications, relatively few nanomedicines have been approved for clinical cancer treatment. Most of them are liposomal chemo drugs, such as Doxil (liposomal doxorubicin), Onivyde (liposomal irinotecan), and Vyxeos (liposomal daunorubicin cytarabine) [25, 285]. These medicines serve nanoformulation as a shield to reduce adverse immune responses and clearance, thereby increasing blood circulation time and reducing severe systemic toxicity.
However, the components of NMs are robust weapons to fight against cancer by interacting with biological processes. Their chemical nature can trigger diverse biological responses. Some metallic-based NMs may act as immune modulators due to the composition of some metal ions, such as Fe2+, Fe3+, Zn2+, Mn2+, Ca2+, Mg2+, and so on [202]. Others can strongly regulate redox homeostasis by releasing or doping metal elements with variable valence states, like Fe, Mn, Cu, Ce, and so on [33, 286]. Also, certain NMs with specific structure or catalytic ability can induce potent PCD in tumor cells, for instance, intervention on redox balance by nanozymes or NMs with oxygen vacancy [287]. Therefore, the abovementioned factors, determined by the chemistry of NMs, are worth considering when designing and developing specific NMs to elicit significant cancer therapy. Fully exploiting the intrinsic effect between NMs and biological systems may be a future solution for improving the in vivo performance of NMs. The relevant research is still in the early stages; therefore, exploring the molecular mechanism of different biological effects induced by NMs in more detail is necessary. This NMs-induced regulation is further transformed into new methods for treating diseases, providing essential research support for the physical effects of NMs-induced biological effects in vivo.
Acknowledgments
The authors greatly acknowledge the financial support from the National Natural Science Foundation of China (Grant Nos. 52302359, 82172736), the Science and Technology Projects in Guangzhou (Yat-Sen Excellent Young Scientists Fund, No. 2025A03J4278), the Science and Technology Planning Project of Guangdong Province (No. 2023B1212060013), and the State Key Laboratory of Systems Medicine for Cancer (Nos. ZZ-94-2306 and zz-RCPY-24-41).
Conflicts of Interest
The authors declare no conflicts of interest.




