Aggregation-induced circularly polarized luminescence and delayed fluorescence enabled by activating high-level reverse intersystem crossing
Ke Wang and Xinwen Ou contributed equally to this work.
Abstract
Circularly polarized luminescence (CPL) materials with delayed fluorescence have attracted much attention due to their ability to efficiently trap triplet state excitons, thereby improving the photoluminescence quantum yields of CPL materials. However, much effort has been normally focused on the utilization of T1 excitons but seldom on the utilization of higher excited triplet state Tn (n > 1) excitons. Rational manipulation of higher excited triplet state Tn (n > 1) excitons and suppression of Kasha's rule of CPL materials remains a major challenge. Herein, two gold complex enantiomers ((R/S)-BPAuBC) based on axially chiral binaphthyls and 3,6-Di-tert-butylcarbazole groups are synthesized and systematically investigated. These materials exhibit aggregation-induced circularly polarized delayed fluorescence. Circularly polarized delayed fluorescence was found to be enabled by activating high-level reverse intersystem crossing (hRISC). The anti-Kasha phosphorescence at 77 K proves that the exciton has a large population in the high-lying triplet state T2, which allows the effective hRISC process to cross back to the singlet state S1 and emit delayed fluorescence. In addition, CPL “on–off” switching is further achieved in nanoparticles by acid–base stimulus, showing its potential as an acid–base responsive material.
1 INTRODUCTION
Chiral functional materials with circularly polarized luminescence (CPL)[1-5] have been widely applied in novel photonic devices,[6-9] information encryption,[10, 11] asymmetric synthesis,[12, 13] diagnosis and treatment.[14] In particular, CPL materials with delayed fluorescence are promising candidates for fabricating circularly polarized organic light-emitting diodes due to their high external quantum efficiency.[6, 9, 15] In these delayed fluorescence molecular systems, the electron-rich donor and electron-deficient acceptor are normally twisted and enable efficient separation of the highest occupied molecular orbital and the lowest unoccupied molecular orbital, thus resulting in small ΔEST and accelerating the reverse intersystem crossing (RISC) process from the lowest excited triplet state (T1) to lowest excited singlet states (S1).[16, 17] Therefore, CPL materials with delayed fluorescence can efficiently trap the triplet state excitons to improve photoluminescence quantum yields (PLQYs).[18-21] However, much effort has been normally focused on the utilization of T1 excitons but seldom on the utilization of higher excited triplet state Tn (n > 1) excitons.[22-25] Therefore, rational manipulation of higher excited triplet state Tn (n > 1) excitons and suppression of Kasha's rule in CPL materials remains a major challenge.
In luminescent materials, most excitons are unstable in higher excited state (Sn, Tn, n > 1) and they easily transfer to S1 or T1 through internal conversion (IC). Such principle that photon emission occurs only from the lowest excited state to the ground state is called Kasha's rule (Scheme 1).[26] So far only a few molecules are unambiguously reported to utilize the higher excited state excitons and display anti-Kasha luminescence, anti-Kasha energy transfer, and high-level reverse intersystem crossing (hRISC) (Scheme 1).[22-25, 27-29] If the IC or the exciton–exciton annihilation of higher excited state is efficiently suppressed and excitons in higher excited state can be also rationally controlled, delayed fluorescence enabled hRISC from a high-lying triplet excited state (T2) to S1 will be achieved in CPL materials. In addition, luminogens with utilization of Tn excitons can be used as smart stimulus materials with intriguing properties.[30] For example, Chen et al. used the anti-Kasha rule in AIEgens to achieve two-color emission, which provides an effective strategy for the realization of two-organelle imaging and photodynamic therapy.[31] Shen et al. designed and synthesized anti-Kasha metal-organic halides that achieve dual emission of both fluorescence and phosphorescence, which can be applied to information encryption.[32] Xiong et al. also utilized the hRISC of the dopant and the RISC of the host to achieve a high external quantum efficiency (EQE) up to 16.1% in rubrene-doped organic light-emitting diodes (OLEDs).[28] All these studies highlight the promising application prospects of materials with utilization of high-lying excited states.
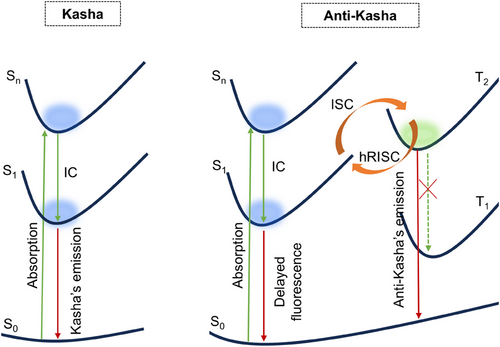
In a recent report, Wang et al. developed the chiral gold complexes that exhibit aggregation- and crystallization-induced CPL, and they attributed the crystallization-induced CPL phenomena to the strong multiple intermolecular C─H⋯π interactions in the crystals.[33] However, the mechanism of the CPL activity needs to be further elaborated. Especially, such circularly polarized delayed fluorescence may be facilitated by the hRISC, which needs to be further elucidated in this system. Herein, two gold complex enantiomers ((R/S)-BPAuBC) based on axially chiral binaphthyls and 3,6-Di-tert-butylcarbazole groups are also synthesized and systematically investigated. These materials exhibit aggregation-induced circularly polarized delayed fluorescence. (R/S)-BPAuBC show obvious CPL signals in crystalline state with much higher glum than that in tetrahydrofuran (THF) solution. Double helical packing structures are found in single crystals with helical pitch of 27.189 Å for (R)-BPAuBC and 27.203 Å for (S)-BPAuBC, respectively. Moreover, the anti-Kasha phosphorescence at 77 K proves that the exciton has a large population in the high-lying triplet state T2, which allows the effective hRISC process to cross back to the singlet state S1 and emit circularly polarized delayed fluorescence. CPL “on–off” switching is further achieved in nanoparticles (NPs) by acid–base stimulus.
2 RESULTS AND DISCUSSIONS
2.1 Synthesis and structural analysis
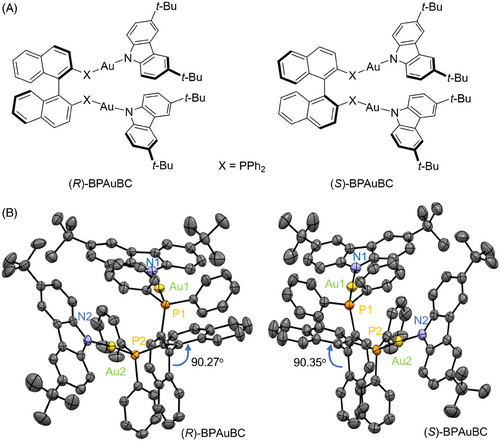
(R/S)-BPAuBC are synthesized according to the previous reports using BINAP, AuCl(tht), and 3,6-Di-tert-butylcarbazole as the starting materials (Scheme S1).[33] The chemical structures of (R/S)-BPAuBC are presented in Figure 1A. Figure 1B shows the single crystal structures of (R/S)-BPAuBC. It is obvious that the single crystal structures of (R/S)-BPAuBC exhibit mirror-image stereochemical configurations. The dihedral angles of binaphthyls in crystal are 90.27° for (R)-BPAuBC and 90.35° for (S)-BPAuBC, respectively. In addition, single-crystal X-ray diffraction (SXRD) analysis revealed that both the enantiomers crystalized in the P212121 chiral space group with orthorhombic system (Table S1).
2.2 Photophysical property
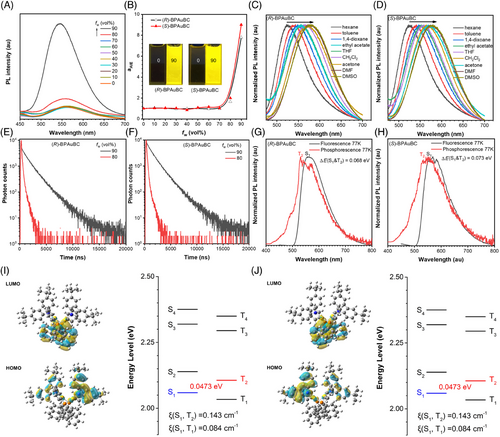
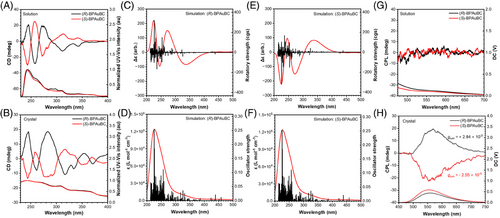
(R)-BPAuBC exhibits weak photoluminescence (PL) with an emission peak of 558 nm and PLQY of nearly 0% in pure THF (Figure 2A, Table 1). A yellow PL enhancement at 545 nm is observed upon the addition of water with fw = 90% (Figure 2A,B). The corresponding αAIE (αAIE = I/I0, where I and I0 are the emission peak intensities in the THF/water mixture with a specific fw and in pure THF solution, respectively) of (R)-BPAuBC is calculated as 7.80 (Figure 2B), suggesting that it is AIE-active.[34] (S)-BPAuBC also shows identical AIE properties (Figure 2B and Figure S1). The PL emissions of (R/S)-BPAuBC exhibit obvious redshift as increasing the polarity of solvent (Figure 2C,D), indicating that the PL emissions of the enantiomers may involve a twisted intramolecular charge transfer process. In addition, the lifetimes of (R/S)-BPAuBC in a mixture solution of THF and H2O with a water fraction of 90% are 1.39 and 1.41 µs, which are higher than that in an 80% water fraction solution of 216.6 and 209.3 ns, respectively (Figure 2E,F, Table 1). These results imply that these AIEgens possess the aggregation-induced delayed fluorescence (AIDF) properties. In addition, both (R)-BPAuBC and (S)-BPAuBC exhibit PL emission with emission peak at 555 nm in crystalline state (Figure S2) and show lifetime of 2.7 μs (R enantiomer) and 2.4 µs (S enantiomer) in crystalline state, respectively (Figure S2, Table 1). To further investigate the AIDF of chiral AIEgens, the fluorescence spectra of (R)-BPAuBC and (S)-BPAuBC single crystals are measured at 77 K, with a delay time of 3 ms set for the phosphorescence measurements. Interestingly, phosphorescence spectra of (R)-BPAuBC exhibit obvious blueshift compared with the fluorescence spectra of (R)-BPAuBC at 77 K (Figure 2G,H). This phenomenon may be attributed to the anti-Kasha emission from the high-level excited state T2. The experimental ΔE(S1&T2) values are 0.068 eV for (R)-BPAuBC and 0.073 eV for (S)-BPAuBC, respectively, which are calculated from the onset energies of fluorescence and phosphorescence spectra. In addition, time-dependent density functional theory (TD-DFT) calculations also reveal that both ΔE(S1&T2) values of (R/S)-BPAuBC are 0.0473 eV (Figure 2I,J). These values are compatible with the experimentally measured values. Therefore, the delayed fluorescence enabled by the hRISC process from T2 to S1 was observed in these (R/S)-BPAuBC crystals at 77 K. More importantly, the spin-orbit coupling constant between S1 and T2 is 0.143 cm−1, which is higher than that (0.084 cm−1) between S1 and T1 (Figure 2I,J). This further suggests that the process of hRISC from T2 to S1 is more likely to occur. Therefore, the anti-Kasha phosphorescence at 77 K proves that the exciton has a large population in the high-lying triplet state T2, which allows the effective hRISC process to cross back to the singlet state S1 and emit delayed fluorescence.
| λem | Фfa | τb | τp/Rpc | τd/Rdd | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (nm) | (%) | (ns) | (ns)/(%) | (ns)/(%) | ||||||
| R | S | R | S | R | S | R | S | R | S | |
| THF | 558 | 558 | 0 | 0 | None | |||||
| 80%e | 559 | 559 | None | 216.6 | 209.3 | 113.8/78.1 | 129.8/83.2 | 582.2/21.9 | 602.5/16.8 | |
| 90%e | 545 | 545 | 1.10 | 1.79 | 1391.8 | 1410.3 | 75.85/6.5 | 108.8/2.8 | 1483/93.5 | 1447/97.2 |
| BPAuBC | 555 | 555 | 50.4 | 49.4 | 2698.3 | 2364.4 | 83.15/1.1 | 68.83/0.5 | 2728/98.9 | 2375/99.5 |
- a Measured by absolute method using an integrating sphere, excitation wavelength 365 nm.
- b τ = ΣAiτi2/ΣAiτi, where Ai is the pre-exponential for lifetime τi.
- c Rp is individual component ratio for prompt fluorescence.
- d Rd is individual component ratio for delayed fluorescence. Rp = A1τ1 /ΣAiτi, Rd = 1 − Rp.
- e Mixture solution of THF and water with water fraction of 80% or 90%.
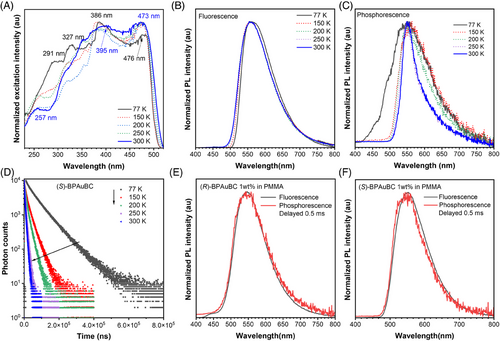
We also carried out temperature-dependent excitation, steady-state, and delayed emission spectra of (S)-BPAuBC crystallite to gain deep insights into the excited-state dynamics (Figure 3). With the increase in temperature, the excitation peaks at 291 and 327 nm gradually disappear, and the peaks at 257, 395, and 473 nm appear, indicating that the ground-state origin of emissions of (S)-BPAuBC crystal at 300 and 77 K is different. Additionally, obviously different excitation profiles in the short wavelength region at 300 and 77 K are observed, which can also be considered as indications of anti-Kasha behavior.[35, 36] As shown in Figure 3B, the fluorescence emission spectrum of (S)-BPAuBC crystal powder exhibits a slightly blue shift as the temperature increases. Additionally, the phosphorescence emission peak of T2 gradually weakens and even disappears at 300 K (Figure 3C). This may be attributed to the higher triplet state T2 excitons that are unstable and easy for annihilation at high temperatures. Moreover, the full width at half maxima of phosphorescence curve becomes narrower with the increase in temperature due to the low-temperature atmosphere, which can significantly stabilize the vibrational energy states of excited molecules. Normally, the typical thermally activated delayed fluorescence materials show an enhanced delayed lifetime component relative to the prompt lifetime component in PL decay profiles upon increasing temperature due to RISC process.[15, 37] However, the delayed lifetime component of (S)-BPAuBC crystal decreases with increasing temperature (Figure 3D), further suggesting the existence of a process of hRISC from T2 to S1 state. Herein, T2 excitons are easy to cross back to S1 at low temperatures because the energy level of T2 is higher than S1 (Figure 2I,J), resulting in an enhanced lifetime component at 77 K. The delayed spectrum of doped films exhibits almost negligible blue-shifted emission compared to the fluorescence of (R/S)-BPAuBC doped films at RT (Figure 3E,F). This also demonstrates that trace T2 excitons can be detected at RT and most T2 excitons can cross back to S1 state through hRISC process. In addition, fluorescence of (R/S)-BPAuBC in a solution of 2-methyltetrahydrofuran at 77 K can be observed but delayed spectra of (R/S)-BPAuBC solution at 77 K are not monitored (Figure S3), indicating that the anti-Kasha emission and hRISC from T2 to S1 process arise from aggregate rather than single molecular behavior.
The electronic circular dichroism (ECD) and CPL spectra of (R/S)-BPAuBC are evaluated in solution and crystalline states. Mirror-symmetrical CD signals are observed at 275, 314, and 342 nm in pure THF solution (Figure 4A). Figure 4B also shows a mirror-image Cotton effect in crystalline state for (R/S)-BPAuBC. The true CD signals are verified through multiple measurements by rotating the sample at different angles around the optical axis (Figure S4). These results suggest that CD signals do not originate from the chiroptical artifacts of linear dichroism and linear birefringence.[38, 39] Herein, we further calculated the ECD and UV–Vis spectra of single (R/S)-BPAuBC molecule, which are obtained based on the optimized ground-state geometry in gas phase and single-point TD-DFT calculations. As in the single-molecular state, the simulated ECD and UV–Vis spectra of (R/S)-BPAuBC are similar to the experimental spectra in solution (Figure 4C–F). (R/S)-BPAuBC crystallites exhibit obviously different CD signals from those in solution at the wavelength from 350 to 400 nm (Figure 4A,B). Such different CD signals at long wavelength region in the crystalline state from the solution state may be attributed to the double helical structures formed in crystals (Figure 5). As depicted in Figure 4G, it is noteworthy that no CPL signals are observed in pure THF. This can be attributed to the fact that the measured PLQY of the solution is nearly zero (Table 1). By contrast, (R/S)-BPAuBC show obvious CPL signals in the crystalline state with glum (555 nm) of +2.84 × 10−3 for (R)-BPAuBC crystal and −2.55 × 10−3 for (S)-BPAuBC crystal, respectively (Figure 4H). The CPL signals of (R/S)-BPAuBC crystallites are further confirmed through eight measurements by rotating and flipping the sample at different angles and front/back sides around the optical axis. The glum values of (R/S)-BPAuBC crystallites considering the azimuthal rotation and flipping of samples are illustrated in Figure S5. The corresponding CPL spectra are illustrated in Figures S6 and S7. Such measurements verified that the real and intrinsical CPL signals were obtained in crystalline state. We also refer to this phenomenon of enhanced CPL signals due to aggregation in crystal as aggregation-induced CPL (AICPL).[40]
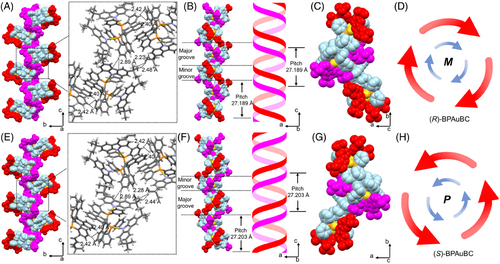
As illustrated in Figure 5, the crystal packing of (R/S)-BPAuBC exhibits double helical structures with a “major groove” and a “minor groove”. The internal helical structures are formed by the nonbonding C─H⋯π interaction[41-43] (Figure 5A,E). The external chiral luminescent AIEgens surround the internal helical structures through the nonbonding C─H⋯H─C interaction[44] (Figure 5A,E). These external chiral AIEgens also formed the helical structure with a larger radius than the internal helix (Figure 5D,H). (R)-BPAuBC formed M-helical structures with a helical pitch of 27.189 Å (Figure 5B,C), while (S)-BPAuBC adopted a P-helix with a helical pitch of 27.203 Å (Figure 5F,G). The double helical structures of the crystals can promote the CPL generation, thus resulting in the AICPL phenomenon in the crystalline state.
2.3 Acid–base stimulus response
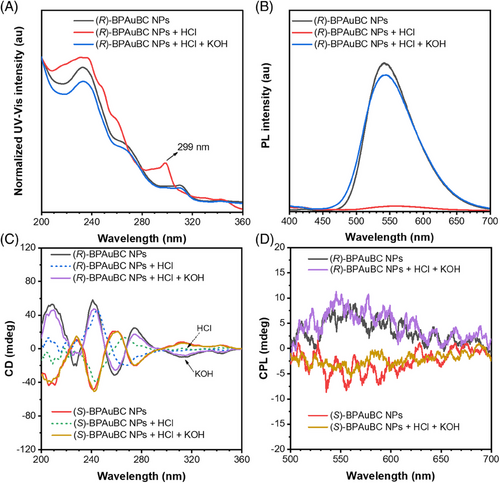
Based on the intrinsic AICPL properties, CPL switching in an aggregation state is also investigated. Herein, water-dispersible NPs are fabricated by chiral AIEgens and amphiphilic polymers called 1,2-distearoylsn-glycero-3-phosphoethanolamine-N-[amino(polyethyleneglycol)-2000] (DSPE-PEG2000) in a mixture solution of THF and H2O (v:v, 10:90) under ultrasound conditions. Then the mixture was placed in a fume hood for 12 h to remove the THF to obtain (R/S)-BPAuBC NPs in water. As illustrated in Figure 6, (R/S)-BPAuBC NPs show CD responses and CPL “on–off” responses enabled by acid–base stimulus. A new absorption peak at 299 nm is observed in UV–Vis absorption spectra upon the addition of excess HCl (Figure 6A and Figure S8A). (R/S)-BPAuBC NPs exhibit a bright emission at 542 nm and CPL signals with glum (542 nm) of +1.32 × 10−3 and −9.99 × 10−4 due to AICPL characteristics (Figure 6B,D and Figure S8B). Moreover, fluorescence quenching is observed for (R/S)-BPAuBC NPs after the addition of excess HCl (Figure 6B and Figure S8B). The CD signals at the wavelength from 300 to 350 nm and the CPL signals from 500 to 650 nm disappear due to the cleavage of Au─N bond under the acid conditions (Figure 6C and Figure S9). More interestingly, the CD and CPL signals can be recovered after KOH treatment (Figure 6C,D). We proposed that NPs provide a confinement environment for the reactions of Au and nitrogen anion under the basic conditions, resulting in the regeneration of the chiral AIEgens. Hence, CPL “on–off” switching can be achieved by acid–base stimulus.
3 CONCLUSION
In this study, we systematically investigated the photophysical properties of a pair of gold complex enantiomers ((R/S)-BPAuBC), which are based on axially chiral binaphthyls and 3,6-Di-tert-butylcarbazole groups. These enantiomers show the intriguing AICPL phenomenon. (R/S)-BPAuBC exhibited distinct CPL features in the crystalline state, with higher glum values compared to that in solution. SXRD analysis further revealed the enhanced CPL responses of (R/S)-BPAuBC in the crystal due to its more stable molecular conformation and orderly helical packing. Additionally, the observation of circularly polarized delayed fluorescence facilitated by hRISC process from T2 to S1 suggests the feasibility of employing the high-lying triplet state T2 to achieve high photophysical performances of CPL materials. And CPL “on–off” switching is achieved in NPs by acid–base stimulus, showing its potential application as acid–base responsive material. Hence, this research introduces a straightforward yet effective method for developing high-performance smart CPL materials.
ACKNOWLEDGMENTS
F. Li is grateful for financial support from the National Natural Science Foundation of China (52003298) and the Natural Science Foundation of Jiangsu Province (BK20200578). F. Song acknowledges the start-up funding from the Beijing University of Technology (049000513202 and 049000514123564). The authors also acknowledge the support of the Research Grants Council of Hong Kong (C6014-20W), the Innovation and Technology Commission (ITC-CNERC14SC01), Shenzhen Key Laboratory of Functional Aggregate Materials (ZDSYS20211021111400001), and the Science Technology Innovation Commission of Shenzhen Municipality (KQTD20210811090142053 and JCYJ2022081810-3007014). The authors thank to the AIE Institute (www.aietech.org.cn) for providing some AIE materials and technical assistance.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
CCDC 2279079 and 2279080 contain the supporting crystal data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing [email protected].




