NIR-II AIEgens nanosystem for fluorescence and chemiluminescence synergistic imaging-guided precise resection in osteosarcoma surgery
Ruotong Li and Kaiyuan Liu contributed equally to this study.
Abstract
Osteosarcoma (OS) is characterized by an unfavorable prognosis and high mortality rates, with the local recurrence attributed to residual lesions post-surgery being a major reason for treatment failure. Precise and tumor-specific resection guidance to minimize recurrence remains a significant challenge. In the present study, a nanosystem based on aggregation-induced emission (AIE) molecules with emission in the second near-infrared window is proposed for the synergistic fluorescence (FL) and chemiluminescence (CL) imaging-guided surgical resection for the elimination of tumor foci. The designed AIE molecule, BBTD14, exhibits stable FL with a high quantum yield of up to 3.95%, which effectively matches the energy levels of CL high-energy states, generating the longest emission wavelength of CL reported to date. Targeted tumor imaging-guided surgery (IGS) is facilitated by FL and CL nanoprobes (FLNP and CLNP) constructed based on BBTD14. During OS surgery, the FLNP, with the stability of FL and a high targeting capability, was first intravenously used to guide the surgical removal of the main tumor. Subsequently, CLNP was locally incubated to facilitate rapid and accurate evaluation of residual tumors at the operative border. High signal-to-noise ratio CL imaging was achieved after spraying with hydrogen peroxide, thereby overcoming the limitations of intraoperative frozen sections. The proposed technique also significantly reduced the recurrence rates in OS mouse models and exhibited high marker specificity in ex vivo OS patient pathology samples, confirming its potential in clinical applications and providing a unique perspective for developing IGS.
1 INTRODUCTION
Osteosarcoma (OS) is the predominant primary malignant bone tumor, characterized by an unfavorable prognosis and high mortality rates, which remains a challenge in clinical medicine.[1-3] Currently, numerous novel therapy models have been proposed to address the challenges of metastasis, recurrence, and drug resistance in OS. However, they are still in the preclinical stage of development.[4, 5] Surgical intervention remains the primary treatment modality for OS, offering superior local control and treatment efficacy when compared to radiotherapy and chemotherapy, which exhibit limited sensitivity in OS.[6, 7] However, the uncertainty of tumor margins remains a notable challenge during clinical surgical resections, often leading to imprecise tumor localization.[8] Surgeons conventionally have relied primarily on visual and tactile cues to ascertain the boundaries of tumors, which is significantly limited by the resemblance of tissue structures and the intricacy of surgical regions.[9] Even widespread utilization of advanced techniques such as frozen section analysis has been unable to significantly reduce the postoperative tumor-positive surgical margin rate.[10] The visual similarity between the tumor and paracancerous tissues, in addition to the small sizes of local lesions, favor the persistence of microscopic lesions and local metastases post-surgery, resulting in an increased recurrence rate and an unfavorable prognosis after OS surgery. Consequently, precise surgical localization techniques and molecular biomarkers have drawn increasing attention in recent years[11, 12] as strategies to improve the accuracy and therapeutic efficacy of surgical intervention for OS.
Imaging-guided surgery (IGS) based on fluorescence (FL) imaging strategy for surgical treatment offers the advantages of effective visualization, high sensitivity, and real-time monitoring.[13, 14] The discernible FL highlights diseased tissue within the surgeon's visual field, enhances the specificity of tumor boundaries and facilitates accurate resection throughout the surgical intervention. Several surgical auxiliary FL agents, such as 5-aminolevulinic acid (5-ALA),[15] indocyanine green (ICG),[9] methylene blue, and Cytalux[16] have received FDA approval for use in cancerous lesions excision.[17, 18] A variety of FL dyes including aggregation-induced emission (AIE) dyes have been reported for application in OS imaging or surgical operations.[19, 20] The emission wavelengths of these agents are primarily within the visible and first near-infrared (NIR-I) regions of the spectrum, resulting in limited tissue penetration depth and pronounced autofluorescence signal. Particularly under complex surgical conditions, the resulting low signal-to-noise ratio (SNR) during imaging significantly affects the delineation of tumor margins.[21] The FL agents with emissions at the second NIR window (NIR-II) appear to be a solution to this issue, as these agents exhibit high imaging resolution.[22] Moreover, the chemiluminescence (CL) technique presents a promising solution,[23-27] having garnered considerable interest in biosensing and bioimaging.[28, 29] CL utilizes chemical energy to trigger luminescence, offering enhanced SNR and detection sensitivity compared to those achieved using photoluminescence techniques due to its independence from an excitation light source,[30] which enables heightened precision during surgery, especially in discerning minute lesions within tumor boundaries.[31] The peroxy-oxalate-based CL (POCL) system involves the reaction of oxalate esters with hydrogen peroxide (H2O2) to produce the high-energy intermediate 1,2-di-oxetane-dione (DOD), which then transfers its energy to the luminescent substances through the Chemiluminescent Resonance Energy Transfer (CRET) process.[25, 32-38] It is noteworthy that the CRET process necessitates a suitable energy level alignment between the luminescent substance and DOD. Moreover, in the existing POCL systems, the luminescence wavelengths primarily fall within the visible and NIR-I regions (650–900 nm),[29, 32, 39-41] limiting deep-tissue imaging in vivo and SNR enhancement in targeted regions. Therefore, it is imperative to extend the CL emission wavelength to the NIR-II region (900-1400 nm) to achieve improved tissue penetration and SNR.[42-44]
Herein, an NIR-II emitting nanosystem based on AIE dye (named BBTD14) was developed for the FL and CL synergistic imaging-guided surgical resection of OS. The nanosystem comprised FL and CL nanoparticles (abbreviated as FLNP and CLNP, respectively), where BBTD14 aggregated with carboxylated F127 to form the nanoparticles. The surface of the nanoparticles was functionalized with anti-programmed death-ligand 1 antibodies (anti-PD-L1 antibody) to target OS cells. Under 808 nm laser excitation, the FLNP generated NIR-II FL centered at 1083 nm, which facilitated preoperative tumor localization and intraoperative imaging-guided resection of the primary lesion. The small energy gap (1.44 eV) between the HOMO level of BBTD14 and the LUMO level of DOD allowed for the CLNPs formed by the binding of BBTD14 with bis(3,4,6-trichloro-2-(pentoxycarbonyl)phenyl)oxalate (CPPO) to effectively produce NIR-II CL through the CRET process. Spraying the CLNP dispersion on the surgical wound region delineated the tiny residual OS tissues after the initial resection (Figure 1). Overall, the integration of FL and CL for surgical guidance through multiple imaging modalities assisted surgeons in the preoperative assessment of the surgical field while providing intuitive and precise guidance during OS surgery. The proposed approach maximized the preservation of healthy tissues and minimized the risk of inadvertent excision, enhancing surgical precision and therapeutic outcomes, and demonstrating the potential for facilitating noteworthy advancements in improving OS surgical efficacy.
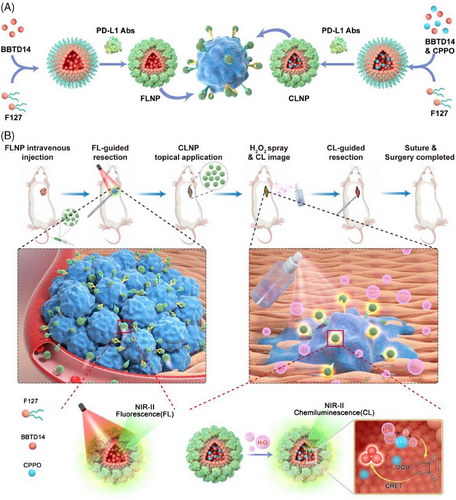
2 RESULTS AND DISCUSSION
2.1 Synthesis and characterization
BBTD14 was synthesized using triphenylamine and two thiophenes as electron donors and benzobisthiadiazole (BBTD) as a strong electron acceptor (Figure 2A), which was well characterized by 1H, 13C nuclear magnetic resonance (NMR) spectroscopy and high-resolution mass spectrometry (Figures S1–S3). The formation of micelles was achieved using the traditional reverse microemulsion method. The required molecules were initially dissolved in an organic phase and dried using rotary evaporation. Phosphate-buffered saline (PBS) was then added, followed by ultrasonication to disperse the micelles. The targeting anti-PD-L1 antibody was conjugated to the micelles through an amidation reaction, and fluorescent secondary antibodies used for labeling FLNP confirmed the successful linking of the anti-PD-L1 antibody (Figure S4). Characterization of nanoprobes was conducted using dynamic light scattering (DLS) and transmission electron microscopy (TEM), which revealed the particle sizes of 19 and 21 nm for FLNP and CLNP, respectively (Figure 2C,E). Such sizes of nanoparticles enabled excellent tumor penetration and retention compared to particles larger than 100 nm, as they typically exhibited limited endothelial permeability, slow transport rates, prolonged retention times, and low susceptibility to clearance by the reticuloendothelial system (RES) in the liver and spleen. Energy dispersive X-ray spectroscopy (EDS) elemental mapping and Fourier-transform infrared spectroscopy (FTIR) confirmed the successful preparation of the micelles (Figures S5–S7).
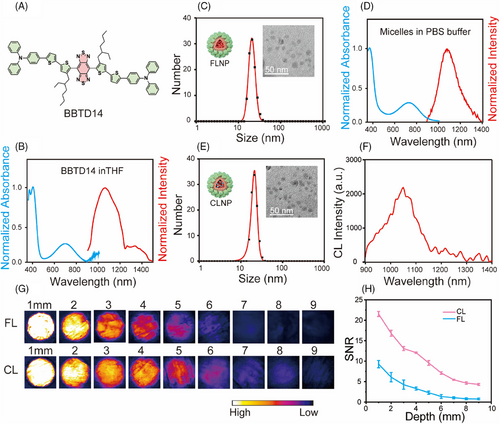
The absorption spectrum of BBTD14 in the organic phase presented a near-infrared absorption peak at 702 nm, with maximal absorption extending up to 900 nm (Figure 2B). Considering that the 808 nm wavelength offered favorable tissue penetration with reduced absorption and scattering effects, it was chosen as the excitation light source for subsequent experiments. Additionally, BBTD14 emitted light within the NIR-II region, with a main peak at 1026 nm and a tail extending up to 1400 nm, promising a higher SNR for biological imaging. The absorption spectrum of the micelles was similar to that in the organic phase, demonstrating the capability to be excited by the NIR light. Notably, the main emission peak of FLNP exhibited a slight red shift to 1083 nm, suggesting increased molecular aggregation within the micelles (Figure 2D). Meanwhile, the chemiluminescence emission spectrum of the CLNP micelles, activated by the oxidant H2O2, resembled that of FLNP (Figure 2F). The result confirmed the successful occurrence of the CRET process, with the emitted wavelength being the longest for CRET systems reported to date.[45]
The micelles dispersed in PBS, Dulbecco's Modified Eagle Medium (DMEM), physiological saline, and simulated body fluid exhibited minimal changes in status and particle size for 7 days, which confirmed the outstanding stability of micelles in these fluids (Figures S8 and S9). Meanwhile, under near-infrared light irradiation for imaging (2 mW/cm2), FLNP did not show obvious reactive oxygen species (ROS) production. Type I and Type II ROS generations were detected only at a high power density of 150 mW/cm2[46] (Figure S10), indicating good biocompatibility of FLNP during imaging. The advantages of FLNP and CLNP with emissions in the NIR-II region for bioimaging were further assessed through tissue depth simulation using chicken breast samples of varying thicknesses. The assessment results indicated that the luminescence signal remained observable under both modes up to the tissue depth of 9 mm (Figure 2G). The subsequent quantitative analysis revealed a gradual reduction in FL and CL intensities with increasing tissue depth, establishing 9 mm as the maximum discernible tissue depth (Figure S11). When FL and CL collected signals of similar intensity, CL imaging exhibited a higher SNR (Figure 2H). Concurrently, the relative quantum yield (QY) of FLNP was 3.95%, which was conducive to the effective supervision and analysis of deep tissue structures and functions in vivo.
2.2 Theoretical calculation
BBTD14 was dispersed in different proportions of the organic phase and aqueous phase to verify the AIE properties. The results showed that with the elevation of water content in tetrahydrofuran (THF), the extinction coefficient of BBTD14 at 808 nm gradually increased, and the emission enhanced in solutions with 60% and 80% water contents. The emission peak of BBTD14 shifted from 1030 nm in THF to 1066 nm in THF solution with 80% water content, with an even greater red shift observed in the emission of FLNP at 1083 nm. The QY increased from 0.56% in THF to 3.15% in the aggregated state (80% water in THF) and further to 3.95% in the micelle, demonstrating pronounced AIE properties. The absorption and emission spectra of FLNP micelle dispersion at the same concentration were slightly stronger than those of THF with 80% water. Moreover, micelles exhibited a longer FL lifetime,[47] indicating a good aggregation state of the BBTD14 in micelles, which was advantageous for enhancing FL intensity and achieving efficient energy transfer (Figure 3A, Figures S12 and S13, and Table S1). Subsequently, a molecular dynamics simulation was conducted to investigate the microscopic aggregation behavior of the molecules. Firstly, twenty BBTD14 molecules were randomly inserted into a cubic box with a side length of 8 nm, followed by equilibrium simulations for 100 ns under vacuum conditions to obtain the initial aggregate.[48] In subsequent observations, no evident changes were noted after the addition of water molecules to solvate the aggregates in the equilibrium simulation box (Figure 3C). The strong luminescence generated by the AIE molecules was attributed to the restriction of intramolecular motion (RIM), which restrained the internal free movement and reduced the possibility of non-radiative decay when the molecules aggregated. Therefore, it was essential to evaluate the changes in the donor-acceptor dihedral angle of BBTD14 (Figure 3B).
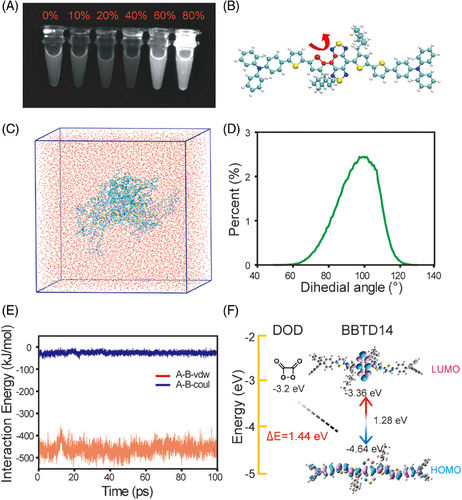
The individual BBTD14 molecules exhibited a broad dihedral angle distribution in vacuum, organic phase, and aqueous phase, with little variation among the three phases. However, after the aggregation and solvation of the aggregate in water, the dihedral angle distribution became concentrated (Figure 3D and Figure S14), with no water molecules observed within the BBTD14 aggregate (Figure S15), which explained the narrow dihedral angle distribution and the strengthened intermolecular interactions within the aggregate. Without the interference of water molecules, the BBTD14 aggregate tended to have a more orderly arrangement, which enhanced the RIM effect,[49] thereby providing a further stable environment. Quantitative evaluation of intermolecular interactions was achieved by calculating the interaction energy between the innermost molecules and the surrounding dyes. It was revealed that both electrostatic and van der Waals interactions were negative, with the corresponding values of –26.4634 and –459.72 kJ/mol, respectively, which indicated a mutual attraction between the innermost BBTD14 molecules and the surrounding dyes, further demonstrating a higher degree of RIM for the BBTD14 aggregate in water (Figure 3E).
The CRET process occurring within the CLNP micelles required good matching between the highest occupied molecular orbital (HOMO) energy level of BBTD14 and the lowest unoccupied molecular orbital (LUMO) energy level of DOD generated by CPPO. Previous literature has summarized the common CRET acceptor dye molecules, providing a feasible range of the energy difference between the LUMO energy level of DOD and HOMO energy level of dye to achieve chemiluminescence, which is from 1.20 to 5.01 eV.[32] The molecular structure was optimized using Gaussian 09 at the B3LYP/6-31G(d) level of theory (Figure S16), followed by calculating the frontier orbitals of BBTD14 using time-dependent density functional theory (Figure 3F). It was known that if the HOMO energy level of dyes was closer to the LUMO energy level of DOD, it would be easier for the dyes to accept the energy from DOD.[50] Compared with similar D-A structured molecules,[51-54] the energy difference between the HOMO energy level of BBTD14 (−4.64 eV) and the LUMO energy level of DOD (−3.2 eV) was 1.44 eV, which was less than that of most fluorophores reported to date,[25, 55] and therefore BBTD14 is suitable for achieving DOD intermediate based chemiluminescence. Additionally, the HOMO-LUMO energy gap of BBTD14 was 1.28 eV, facilitating electron excitation and making it suitable as an ideal CRET acceptor. Moreover, in comparison to reported NIR-II POCL systems,[45] BBTD14 had a smaller energy gap, indicating that the molecule more readily underwent electron transition and emitted at longer wavelengths. Thus, a rational design to reduce the energy gap was a key condition for extending the POCL system into the NIR-II region. The electron distribution in the HOMO was primarily concentrated on the donor (thiophene) unit, while the electron density in the LUMO was concentrated on the strong electron-accepting BBTD unit. When the donor-acceptor components combined into molecular orbitals, the bandgap difference decreased, causing a red shift in the emission wavelength. The bis-thiophene donors enhanced the degree of conjugation and further reduced the bandgap difference. The first thiophene donor, equipped with two long alkyl chains closed to the acceptor, could reduce intermolecular interactions and ensure a certain hydrophobicity around the acceptor unit. This lowered energy transfer from the excited state to the surrounding water molecules, improved the overall fluorescence QY of the molecules ultimately.
2.3 NIR-II CL performance of CLNP
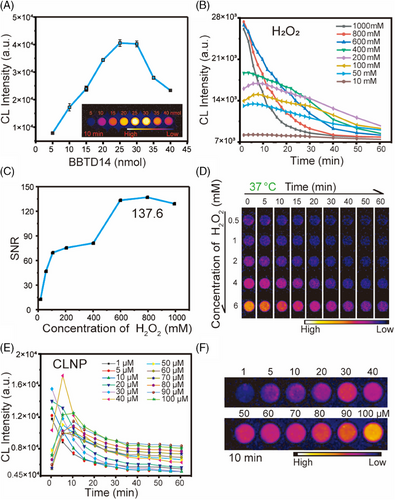
The CL properties of CLNP were explored. Firstly, the optimal amount of BBTD14 coating in the micelles was determined by fixing the amounts of carboxylated F127 and CPPO and varying the dye concentration, observing the CL intensity to ascertain the optimal ratio (Figure 4A). The results indicated that the group with 25 nmol of BBTD14 encapsulation exhibited the most outstanding performance after the addition of H2O2 for 10 min. According to clinical medical standards, the maximum acceptable concentration of H2O2 in the human body is 1 M. Peroxyoxalate systems have been proven to selectively oxidize the reaction substrates by H2O2 only. Therefore, to determine whether the concentration of H2O2 required by the system met the requirements, the CL signal was detected under different H2O2 concentrations at a fixed micelle concentration (Figure 4B), which showed that the CL intensity could be sustained for more than 1 hour in all groups. The change in the CL intensity at 50, 100, and 200 mM with time exhibited an initial increase followed by a gradual decline, with the corresponding half-lives of 83, 77, and 64 min, which was conducive to surgical localization. Furthermore, the SNR at the strongest signal time point was calculated for each curve, and the value was observed to reach a maximum of 137.6, which was particularly essential for the surgical removal of small tumors (Figure 4C). Remarkably, the CL signal of CLNP remained distinctly observable at an H2O2 concentration as low as 0.5 mM under NIR-II imaging (Figure 4D). To ensure long-term stability and strong luminescence of CLNP changes in CL intensity over time were observed under different concentrations of CLNP, quantified by the BBTD14 concentration (Figure 4E,F). The CL intensity presented a continuous decrease as the CLNP concentration increased from 1 to 30 µM, indicating an excess of H2O2. However, in the range of 40–100 µM, the CL intensity initially increased and then declined, with half-lives exceeding 60 min for concentrations above 50 µM. Therefore, it was inferred that CLNP concentrations between 50 and 100 µM could provide the necessary duration and intensity for intraoperative guidance.
2.4 The in vitro verification of affinity
Firstly, the cytotoxicity of FLNP and CLNP against U2OS cells was evaluated using the CCK8 assay. The results revealed no significant cytotoxic effects (Figures S17 and S18), with the micelles showing negligible toxicity and over 80% cell viability maintained at 0.36 µM micelle concentration. Existing research has advanced our understanding of the molecular pathogenesis of OS, although identifying its specific antigens remained a formidable challenge to date.[56, 57] To expedite the clinical translation of FL probes while maintaining safety standards, candidate biomarkers for OS were identified using clinically routine humanized monoclonal antibodies targeting CD40, IGF1R, PDGFR, TEM1, PD-L1, CTLA-4, VEGFR-1, and EGFR.[20] These antibodies were selected as the only antigens widely expressed in OS, making them specific targets for FL tracers. It was observed that CD40, IGF1R, TEM1, PD-L1, and EGFR were generally upregulated in various human OS cell lines, including MG-63, 143B, U2OS, and Saos-2 cells (Figure S19). Candidate biomarkers were further screened based on their expression profiles in tumors and normal tissues (Figure 5A). TEM1 showed the highest expression in OS, but its widespread expression in other normal tissues compromised its specificity, which was crucial for diagnostic probes. Conversely, PD-L1 exhibited significant differential expression between tumor and normal tissues, making it a more promising target for further exploration. In previous studies, therapeutic antibodies targeting PD-L1 have shown promising outcomes in IGS across various types of tumors.[58-60] The targeting efficacy of anti-PD-L1 antibody-modified FLNP was evaluated in cultured mouse OS cells (LM8) and human OS cells (U2OS), with mouse fibroblasts (3T3-L1), myoblasts (C2C12), and human skin fibroblasts (HSF) serving as controls (Figure 5B). The results demonstrated enhanced affinity of FLNP and CLNP for both mouse and human OS cells, confirming excellent targeting specificity at the cellular level (Figure 5C and Figures S20 and S21).
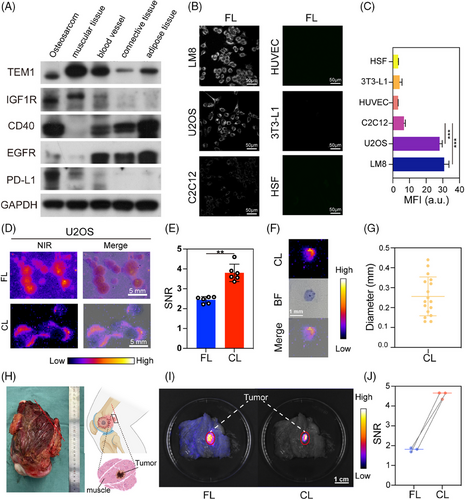
Further, the minimum detectable cell clusters were evaluated in both modes to determine the resection accuracy of the synergistic FL and CL-IGS. U2OS cell clusters were constructed through in vitro clone formation experiments and then labeled with FLNP and CLNP, respectively. Distinct resolution of tumor cell clusters was achieved at the millimeter level in both FL and CL imaging (Figure 5D and Figure S22). The CL based on CLNP could detect tiny tumor cell clusters ranging from 0.1 to 0.4 mm, demonstrating significantly higher SNR and enhanced imaging contrast (Figure 5E–G). Subsequently, the pathological samples obtained from OS patients were utilized to simulate clinical scenarios (Figure 5H). FLNP and CLNP were applied externally to the samples, both demonstrating excellent targeting properties, with the SNR of CL imaging being twice that of FL imaging (Figure 5I,J). This result underscored the clinical feasibility of the proposed nanosystem as a promising technical tool for the surgical resection of OS patients.
2.5 The in vivo NIR-II FL performance of FLNP for IGS
The in vivo safety profiles of FLNP and CLNP were evaluated using animal models. Within 36 h of treatment, blood sampling and histopathological examinations were conducted to detect any potential adverse reactions or toxicological effects associated with the nanoprobes, and near-infrared radiation. Following this, vital organs including kidneys, lungs, spleen, liver, and heart were dissected, and hematoxylin and eosin (H&E) staining was performed to comprehensively assess the toxicity and efficacy of the proposed nanosystem. The results revealed unaltered tissue morphology in the experimental groups, indicating no evident organ damage (Figure S23A). The statistical analysis of the standard blood parameter data of experimental and control cohorts did not reveal any significant differences (p < 0.05) (Figure S23B–G). The post-intervention assessments conducted to determine the levels of alkaline phosphatase (ALP), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) indicated no notable deviations, suggesting minimal hepatic impact on the murine subjects. The blood urea nitrogen (BUN) and creatinine (CREA) concentrations were also maintained within normal ranges, which confirmed the absence of significant nephrotoxicity. Besides, there were no significant red blood cell (RBC) differences between the three groups. Previous studies have also demonstrated that the adverse effects of in vivo NIR radiation could be negligible.[61, 62] Collectively, these findings underscored the remarkable biocompatibility of the proposed nanoprobes.
With advancements in surgical techniques, limb salvage surgery has replaced amputation as the preferred treatment for OS.[61] The NIR imaging technology enabled surgeons to visualize lesions, delineate tumor boundaries, and resect tumors, minimizing tumor recurrence while preserving the affected limb. Initially, tumors were resected using photoluminescence imaging guidance to ensure precise removal of identified tumor regions (Figure 6A). Subsequently, the optimal timing for FL-guided surgery was determined to ensure effective resection. Monitoring FLNP accumulation in an OS mouse model at various time points post-tail vein injection revealed that tumor outlines became increasingly distinct for up to 2 h. FL intensity increased over time, peaking between 36–60 h and maintaining a high level before declining by 72 h (Figure 6B,C). The sustained stability of the FL intensity over 36–60 h provided sufficient time for preoperative planning and intraoperative IGS. NIR-II imaging significantly reduced light scattering and autofluorescence from biological tissues, enabling deeper and higher-fidelity optical tumor imaging. Additionally, the SNR of FLNP far exceeded that of non-specific NIR fluorophores such as ICG reported in previous studies.[62]
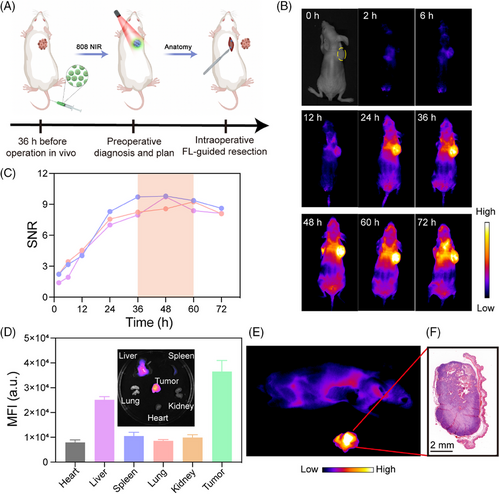
The organs were dissected for ex vivo FL imaging 36 h after the injection of FLNP (Figure 6D). The signals were predominantly concentrated in the tumor region, with relatively low FL intensity observed in the RES organs (liver and spleen), and negligible signals detected in other organs. These observations confirmed the excellent tumor-targeting capability of FLNP. FL-guided surgery was performed in the NIR-II region 36 h post-injection, achieving the highest tumor SNR, which was subsequently validated through H&E staining (Figure 6E,F). However, while most tumors and normal tissues could be distinguished using FL-guided surgery, OS tended to infiltrate the surrounding tissues, which rendered the accurate identification of residual tumors located at the margins quite difficult. Therefore, it was imperative to develop additional synergistic imaging modalities to reduce the incidence of positive tumor margins.
2.6 The in vivo NIR-II CL performance of CLNP for IGS
In limb-salvage surgeries, accurately assessing surgical margins is challenging, leading to a persistent risk of local recurrence due to positive margins, which ultimately reduces the overall survival rate of OS patients.[63, 64] Intraoperative selective frozen section analysis is considered feasible, although potential challenges such as time constraints and tissue damage are associated with this technique.[65] Therefore, it is crucial to develop a real-time intraoperative technique with high precision for the evaluation of surgical residual margins.
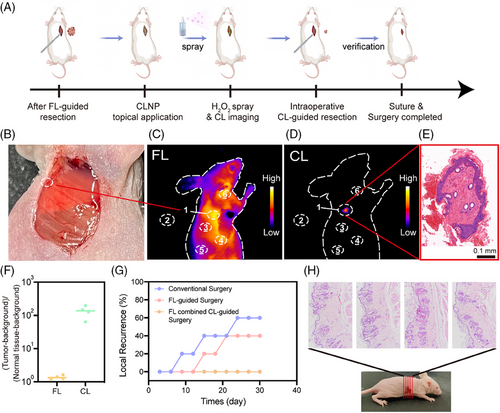
After the FL-guided tumor resection, CL-guided surgery based on CLNP was performed to identify the potential residual local tumors (Figure 7A). No tissue-resembling tumors were observed around the surgical margins during naked-eye observation (Figure 7B), and FL imaging also failed to clearly identify any residual tumors (Figure 7C). CLNP was then incubated in the wound for 10 min, followed by three rinses with physiological saline to remove the non-specifically bound CLNP. Subsequently, H2O2 was applied to activate the internal reaction in CLNP, and the resulting CL signals were detected using NIR-II imaging (Figure 7D). Suspicious residual tumors with a relatively high signal intensity were visible in CL imaging (the circled region in Figure 7D) and further confirmed by H&E staining (Figure 7E). Minimal background interference allowed for high-contrast imaging of any residual tumors, facilitating precise resection of the target tissue. The process was then repeated to confirm the negative margins (Figure S24). Quantitative analysis of SNR in both FL and CL modes revealed that CL imaging provided an SNR that was over 100 times greater than that achieved with FL imaging, using normal tissue as the background (Figure 7F). Additionally, the impact of FL combined with CL-guided surgery on reducing postoperative recurrence rates was evaluated using conventional surgery without imaging guidance and pure FL-guided tumor resection as control groups. Each group consisted of five mice. In the synergistic surgical strategy group, 60% of the mice showed residual tumors after FL-guided resection, necessitating a secondary CL-guided surgery. Subsequent FL combined with CL-guided surgery revealed no tumor recurrence even after 30 days, contrasting with severe recurrence observed in the other two groups (Figure 7G). Continuous sectioning and H&E staining were performed on the original tumor sites resected under FL combined with CL surgery guidance to confirm the absence of local tumor recurrence in mice without evident recurrence (Figure 7H). Overall, this novel synergistic IGS strategy effectively delineated positive tumor margins, significantly reducing the likelihood of local tumor recurrence.
Significantly, the improved capability of rapid tumor localization and precise navigation is promising in terms of enabling surgeons to shorten the operation procedures and reduce anesthesia exposure and the associated risks, which could also increase the patient throughput in hospitals and enhance the utilization of hospital resources. The advantages of this resection technique are expected to facilitate accurate assessment and further clinical trial implementation.
3 CONCLUSION
This synergistic FL and CL imaging-guided surgical approach combined the advantages of both luminescence modes, providing dynamic preoperative localization and precise intraoperative resection of OS. FLNP efficiently accumulated in the tumor regions after intravenous injection, and its stable optical properties ensure sustained signal intensity during prolonged intraoperative excision. The peripheral application of CLNP generated CL with high SNR upon activation with H2O2, facilitating effective identification of suspicious margins after FL-excision and enhancing the intraoperative positive detection rates. The subsequent high-selectivity targeting and prominent SNR in the clinical OS patient sample validated the clinical application potential of the developed nanosystem. This study provided a novel direction for IGS and served as a reference for developing further precise and effective therapeutic strategies for patients with OS.
4 METHODS
4.1 Fabrication of FLNP and CLNP
In a typical procedure, dissolve 100 mg of Carboxylated F127, 0.3 mg of BBTD14 (250 nmol), and 40 mg of CPPO (FLNP production did not require addition) in tetrahydrofuran solution. Then, mix thoroughly and evaporate the organic solvent using a vacuum rotary evaporator. Add 2.5 mL of PBS buffer and sonicate to self-assemble into micelles. Store the solution in the dark at 4°C and dilute according to the specific protocol before use. In determining the optimal dye coating ratio of CLNP, the concentrations of CPPO and carboxylated F127 were held constant while varying the amount of BBTD14 added. The ideal coating ratio of the material was assessed based on the highest signal observed in the imaging at 10 min. The conjugation of anti-PD-L1 antibody to the micelles occurred through an amide reaction between the amino groups on the antibody protein and the carboxylated F127 on the exterior of the micelles. 1-Ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (EDC) and N-hydroxysulfosuccinimide sodium salt (Sulfo-NHS) were dissolved in a 2-N-morpholino ethane sulfonic acid (MES) aqueous solution (10 mM) at a concentration of 200 mM. Materials with a concentration of 50 µM were added to 62.5 µL of EDC solution and 75 µL of NHS solution and incubated at 4°C in the dark for 40 min. Then, the mixture was centrifuged at 6000 rpm for 5 min using a 100,000 molecular weight cutoff ultrafiltration tube, repeated twice. After centrifugation, 50 µg of PD-L1 antibody was added, and the solution was shaken at 4°C for 24 h, ensuring complete avoidance of light throughout the process.
4.2 Clinical samples for FL or CL imaging
Tissue samples from OS patients were obtained at Shanghai General Hospital. Tumor tissue samples were confirmed by two experienced pathologists. This study was approved by the Ethics Committee of Shanghai General Hospital (K-2024-079). The informed consent was obtained from the participant, allowing the use of the sample. No additional compensation was provided to participants. After the tissue samples were resected, a local incision was performed immediately to obtain normal tissue containing OS tissue. Then local incubation of FLNP (35 µM, 1000 µL) or CLNP (50 µM, 700 µL) was given for 10 min, followed by washing with normal saline to remove excess nanoparticles. Finally, the sample was then irradiated under 808 nm irradiation for FL imaging, or sprayed with H2O2 (100 mM) for CL imaging.
4.3 Establishment of OS tumor mice model and FL or CL-guided surgery
The animal experiments in this study were approved by the Shanghai General Hospital Clinical Center Laboratory Animal Welfare & Ethics Committee (IACUC No. 2024AW009). The female Balb/c nude mice (4–6 weeks) were subcutaneously injected with U2OS cells (2 × 106 per mouse) in the right flank. As soon as the tumor volume reached about 200 mm3, the nude mice were used for IGS (2 mW/cm2). The FL intensity of the tumor in situ was monitored from 0 to 72 h after the tail vein injection of FLNP (35 µM, 100 µL). Ex vivo NIR-II FL imaging for different organs was conducted 36 h post-injection. For CL-guided resection, the CLNP nanoprobes (50 µM, 70 µL) were first incubated at the surgical margin for 10 min, and then the nonspecific binding nanoprobes were removed with normal saline three times. Subsequently, the surgical margin was treated with H2O2 (100 mM), and CL signals were captured using NIR imaging technology. Upon detection of the signal, the tissue within the signal region was promptly excised, followed by a repetition of the aforementioned process to confirm a negative margin.
ACKNOWLEDGMENTS
This work was supported by Sponsored by the National Natural Science Foundation of China (82373177 and 82001945), Shanghai Sailing Program (23YF1434400), Shanghai Jiao Tong University “SJTU Star” Medical Engineering Interdisciplinary Research Fund (YG2023LC09), the starting grant of ShanghaiTech University, and Shanghai Clinical Research and Trial Center. The authors thank the Centre for High-resolution Electron Microscopy (ChEM), School of Physical Science and Technology, ShanghaiTech University (No. EM02161943) for the characterization support. The authors thank the Dr. Rong Gao and Analytical Instrumentation Center (#SPST-AIC10112914), School of Physical Science and Technology, ShanghaiTech University for the spectral test support.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.




