Luminescent nano-bioprobes based on NIR dye/lanthanide nanoparticle composites
Abstract
Near-infrared (NIR) light, which has ignorable tissue scattering/absorption, minimal photodamage, and no autofluorescence interference, is highly favorable for bioapplications. NIR dye and lanthanide-doped nanoparticle (LnNP), as representative NIR-excited luminescence probes, have attracted increasing interest due to their unique optical property and low biological toxicity. Design of luminescence probes based on NIR dye/LnNP nanocomposites cannot only integrate the advantages but also achieve additional functions via regulating internal energy transfer pathways. In this review, we focus on the most recent advances in the development of NIR dye/LnNP nanocomposites as potential bioprobes, which cover from their fundamental photophysics to bioapplications, including energy transfer mechanisms, interface engineering (involving binding interaction, distance, and aggregation as key factors), and their applications for dye-sensitized upconversion/downshifting luminescent bioimaging, detection of biomolecules, and NIR-triggered diagnosis and therapy. Some future prospects and efforts toward this active research field are also envisioned.
INTRODUCTION
Light-mediated techniques are essential tools in biological research and clinical practice due to the merits of convenience, remote controllability, high sensitivity, and spatiotemporal accuracy.[1] However, the problems of in-tissue absorption/scattering, autofluorescence, and photodamage under light radiation need to be considered.[2] Tissues are composed of many organic molecules such as DNA, proteins, and collagen that typically absorb in the ultraviolet (UV) and visible regions.[3] Moreover, the scattering is more efficient for shorter wavelengths, which significantly limits the tissue penetration depth.[2] Besides, a number of pigmented cellular structures and endogenous chromophores with visible fluorescence emission cause strong autofluorescence.[2] To this end, light in the near-infrared (NIR) region of 700–1700 nm, which is considered to be biological transparency window, has been increasingly utilized.[2, 4, 5] Meanwhile, various luminescent bioprobes under NIR excitation have also been developed, ranging from small molecular dyes, conjugated polymers, to inorganic nanomaterials.4] Among them, NIR dye and lanthanide-doped nanoparticle (LnNP) are the two important representatives and widely employed in biomedical applications. Compared to other luminescent probes such as Pb/Cd-based quantum dots (QDs) or carbon nanotubes, they possess relatively low toxicity, better biocompatibility, as well as superior optical features.2]
NIR light-excited LnNP, with multicolor emission, ignorable photobleaching, and long luminescence lifetime, is emerging as a new generation of luminescent probes and has evoked considerable interest among biomedical researchers.[6, 7] LnNP can be divided into upconversion nanoparticle (UCNP) and downshifting nanoparticle (DSNP) through the basic generation mechanism of luminescence that arises from the 4f–4f orbital electronic transitions of lanthanide ions.3] In a typical upconversion process, sequential absorption of multiple photons through the ladder-like energy levels of sensitizer (e.g., Yb3+ and Nd3+) in UCNP creates sensitizer-excited states, followed by energy transfer between sensitizers and activators X3+ (X = Er, Ho, or Tm) and, subsequently, shorter NIR/visible/UV-region UCL is produced by activators.8] By contrast, DSNP produces Stokes-shifted luminescence by converting the absorbed NIR photon to NIR emission at longer wavelengths through the nonradiative relaxation process. To be used as bioprobes, LnNP with a size less than 100 nm is preferred for maximizing its permeation ability through tissue, organ, or tumor. However, the small size may lead to more surface defects and serious vibrational deactivation from solvents, resulting in reduced lanthanide luminescence.3] Adjusting dopant spatial distribution by core–interlayer–shell design is usually adopted to avoid the surface-related luminescence quenching and suppress detrimental energy relaxation.9]
Different from the luminescence nanomaterials like LnNP, NIR cyanine dye is a class of small molecule probes characterized by a key chromophoric element with a heptamethine chain that links two nitrogen atoms. These NIR dyes are generally low in toxicity. For instance, the commonly used indocyanine green (ICG) is authorized by the US Food and Drug Administration for a number of clinical applications.10] Along with ICG, a variety of NIR cyanine dyes such as cypate, IR780, IR783, IR808, and IR825 have been developed and some of them are commercially available. NIR cyanine dye typically possesses a high absorbance cross-section with an extinction coefficient larger than 200,000 M−1 cm−1 due to the orbital reorganization upon a singlet ground state (S0) to the singlet state (S1) transition.11] Beside of the fluorescence emission from the singlet-excited state, the nonradiative process in the form of thermal deactivation as well as the dissipation pathways by intersystem crossing to a triplet-excited state (T1) endow the NIR dyes photothermal and photodynamic properties.[12, 13] Moreover, modest nucleophilicity and electrophilicity of the heptamethine chromophore imply that the cyanine dye may undergo specific reaction and is available for biodetection.11] In vivo experiments revealed a rapid tissue clearance for these dye molecules. The majority of ICG was cleared out of the body after 6 h in a mouse model.14]
Based on the great diversity in the properties of absorption cross-section, emission width, luminescence lifetime, chemical reactivity, and circulation time in vivo for LnNP and NIR dye, combination of the two luminophores will realize complementary advantages and multiple functions. As newly developed nanoprobes, NIR dye/LnNP composites have just a history of half-dozen years but have attracted more and more attention.[15-17] Although many reviews have highlighted the bioprobes based on LnNP[2, 18-22] or NIR dye,[11, 14, 23, 24] few of them provide a comprehensive overview of NIR dye/LnNP nanocomposites from fundamental photophysics to bioapplications.[25, 26] In this review, we focus on the NIR dye/LnNP nanocomposites, including their energy transfer mechanisms, construction methods, and related issues in interface engineering. Most recent advances in the NIR dye/LnNP nanoprobes for the applications on upconversion luminescence (UCL)/downshifting luminescence (DSL) bioimaging, detection of biomolecules, and multimodal theranostics have also been summarized (Figure 1). Finally, future efforts toward this research field are put forward, including excitation dynamics study, design of novel composite structures, and improvement of the probe biocompatibility.
ENERGY TRANSFER MECHANISMS
In NIR dye/LnNP composites, LnNP and NIR dye are bound together in a relatively short distance, and energy transfer may occur between the two luminophores. The energy may transfer from LnNP to NIR dye, or conversely, from NIR dye to LnNP. The two processes result in NIR dye-quenched and NIR dye-sensitized lanthanide emission, respectively. Due to the distinctive optical features of LnNP and NIR dye, energy transfer in the two directions involves different mechanisms. As a well-understood phenomenon, energy transfer can be divided into radiative energy transfer and nonradiative energy transfer.27] Radiative energy transfer is also known as reabsorption, in which real photons are emitted by the donor and then reabsorbed by the acceptor within a photon travel distance. The lifetime of the donor is independent of the concentration of the accepter due to the emitted photons.27] Nonradiative energy transfer includes Förster and Dexter mechanisms. Förster resonance energy transfer (FRET) can take place through a columbic dipole–dipole interaction and thereby occur over long distance (1–10 nm).8] The Dexter energy transfer (DET) can occur through electron exchange between the donor and acceptor within a short distance (1 nm or less).8] The decay time of the donor emission is shortened during nonradiative energy transfer since the transfer process shortens the lifetime of the excited state. For both nonradiative and radiative energy transfers, the spectral overlap between the excited state of donor and acceptor is required.28] Based on these theories, we summarized the dominant energy transfer between LnNP and NIR dye in Table 1. Detailed processes of energy transfer from LnNP to NIR dye or vice versa are discussed as follows.
| Requirements | ET probability ()27]a | ET from LnNP to NIR dye | ET from NIR dye to LnNP29] | |
|---|---|---|---|---|
| (i) Spectral overlap (J) |  |
 |
 |
|
| (ii) Distance (R) |
Exchange interaction (R < 1 nm)
|
– | – | Dexter energy transfer (DET) |
|
Dipole-dipole interaction (R < 10 nm)
|
Förster resonance energy transfer (FRET) | FRET | FRET | |
|
No interaction
|
Reabsorption | – | – | |
- Abbreviation: ET, energy transfer.
- a fD(λ) is the emission spectrum of the donor, εA(λ) is the absorption spectrum of the acceptor, τD is the excited-state lifetime of donor, ФD is the fluorescence quantum yield of donor, n is the index of refraction, NA is Avogadro's number, L is the sum of the van der Waals radii, D is the Dexter parameter, κ2 is the orientation factor, and δA is the absorption cross-section of the acceptor.[27, 29]
Energy transfer from LnNP to NIR dye
Multi-wavelength emissions, long-live lifetime, and good chemical stability make LnNP ideal energy donors in energy transfer-based system.30] The energy acceptor can range from organic dye molecules to many nanomaterials such as QDs, graphenes, gold, and carbon nanoparticles.30] With regard to NIR dye as acceptor fluorophore, Yb,Tm-doped nanoparticles with efficient UCL at ∼800 nm and Nd-doped nanoparticles with DSL at 1064 nm are commonly used as LnNP donors, which are matched to a set of NIR I and NIR II absorbing dyes, respectively. The schematic illustration of their energy transfer is shown in Figure 2A. FRET from the narrow-line LnNP to the broad-band NIR dye proceeds over relatively long distance (10 nm); thus, a thin inert shell on LnNP for surface passivation is tolerable in such kind of NIR dye/LnNP composites. Moreover, radiative energy transfer from LnNP to NIR dye could co-exist with FRET due to the large absorption coefficient of NIR dye. The overall energy transfer efficiency can be estimated by (I0 – I1)/I0, where I0 and I1 are the integrated intensities of lanthanide emission in the absence and the presence of NIR dye, respectively. The contribution of the FRET mechanism can be calculated from the percent change of decay times instead of intensities.
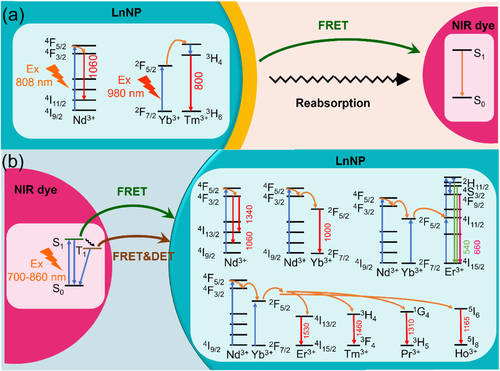
Energy transfer from NIR dye to LnNP
Due to the forbidden nature of the 4f–4f transitions, LnNP suffers from low luminescence efficiency.8] NIR dyes as antennas for LnNP sensitization can significantly enhance the UCL or DSL by increasing the absorption cross-sections and optical bandwidths.[8, 31-38] The first successful utility of NIR dye to empower efficient upconversion was reported by Hummelen and co-workers.39] A total of 3300-fold increase in overall upconversion emission of NaYF4:Yb,Er was achieved through IR806 sensitization upon excitation at 720–1000 nm (Figure 3A). Considering the energy mismatch between the emission of organic dyes and absorption of Yb3+-doped UCNP, Prasad and co-workers designed the energy-cascaded upconversion from the NIR dye IR808 to Nd3+ in the shell, and then to Yb3+ and Tm3+ in the core, resulting in an upconversion quantum yield as high as 4.8% (Figure 3B).40] Subsequently, a set of NIR dye-sensitized NaYF4:Yb,X@NaYbF4@NaYF4:Nd (X = null, Er, Ho, Tm, or Pr) DSL nanocomposites were fabricated, in which energy transfer from light-harvesting ICG, via Nd3+ and Yb3+ ions in the shells, to the emitter X3+ in the core, yielding remarkably enhanced emissions in the NIR-II range (Figure 3C).41]
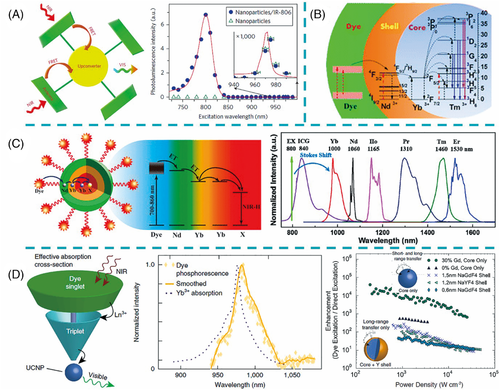
Since dye sensitization produces significant enhancement of LnNP luminescence, its mechanism has also attracted much interest. It is considered that the energy transfer may occur via FRET directly from S1 of NIR dye to the lanthanide emitters or through triplet energy transfer (TET) following an S1–T1 intersystem crossing, as shown in Figure 2B. Recent studies provided evidence that the proximity of the lanthanide ion could enhance the S1–T1 intersystem crossing within NIR dye through the heavy atom effect as well as the paramagnetic property (due to the unpaired 4f electrons).[42, 43] The long-lived triplet state of NIR dye might match well with the absorption band of the Yb3+ transition 2F7/2→2F5/2 and serve as an energy bridge to connect the dye singlet state and the Yb3+-excited state (Figure 3D).42] The origin for TET can be the FRET mechanism and/or the Dexter mechanism. In fact, the UCL enhancement of NIR dye-sensitized core–shell UCNP with 0.6 nm inert shell was two orders of magnitude less than that of core-only UCNP, which implied the contribution of short-range DET (Figure 3D).42]
Even in the FRET process, the critical radius (the distance at which the transfer efficiency is equal to 50%) from NIR dye to a line absorber of LnNP should be quite short as compared with the reverse process. For example, the FRET radius in IR808 sensitized Nd,Yb,Tm-doped UCNP was calculated to be only 1.9 nm by Förster theory, and the actual value was estimated to be 1.5 nm from the measured energy transfer efficiency.40] Therefore, distance is a critical factor in the construction of dye-sensitized LnNP, which will be discussed in Section 3.2.
In addition, for dye-sensitized UCL, the energy back transfer (EBT) process from LnNP to NIR dye should be carefully considered because of the broadband absorption of dye.44] To minimize the absorption of the emitted upconverted photons, choosing NIR dye with weak visible absorption and optimization of dye numbers can be adopted.
INTERFACE ENGINEERING
In this section, we mainly focus on the interface engineering in NIR dye/LnNP nanocomposites as it is an important issue that not only is related to the energy transfer efficiency but also concerns the stability of the bioprobes. The engineering of interface structure in NIR dye/LnNP can be performed through the core–shell design of LnNP and elaborate construction of nanocomposites (Figure 4). In essence, three primary factors should be considered: (i) the binding interaction between NIR dye and LnNP, (ii) the distance between them, and (iii) the distribution state of NIR dye molecules (aggregation or not). Table 2 list typical NIR dye/LnNP nanoprobes constructed by different methods with their luminescent efficiency, stability, and biocompatibility.

| Construction method | NIR dye/LnNP | ET | Luminescent efficiency | Stability | Toxicity assessment | Application | Ref. |
|---|---|---|---|---|---|---|---|
| Direct coordination | ADA-800CW/NaYF4:Yb,Er@NaYF4:Nd,Yb | Dye→UCNP | ET efficiency of 81.6% | Stable in PBS | – | In vitro detection of Hg2+ and PDT | [45] |
| Direct coordination | PEI-800CW/NaYF4:Yb,Er@NaYF4:Nd,Yb | Dye→UCNP | ET efficiency of 14.2% | Not stable in PBS | – | – | [45] |
| Silica coating | 800CW/NaYF4:Yb,Er@NaYF4:Nd,Yb | Dye→UCNP | ET efficiency of 2.9% | – | – | – | [45] |
| Mesoporous silica coating | IR806/NaYF4:Yb,Er@NaYF4:Yb@NaYF4:Yb,Nd | No ET | UCL reduced by ∼6/7 in absorption competition with IR806 | <5% of IR806 released in PBS after 24 h | >80% of cell viability with dose of 62.5 μg/ml for 24 h | In vitro imaging and PTT | [52] |
| Protein coating | IR825/NaGdF4:Yb:Er | No ET | UCL was not affected by IR825 | 25% of IR825 leached in PBS after 24 h | No obvious cytotoxicity with a dose of 0.4 mg/ml | In vivo imaging and PDT/PTT | [51] |
| Click reaction | Cy7.5 in DSPE-PEG-N3/NaErYF4 @NaYF4 in DSPE-PEG-DBCO | No ET | Absorption competition between Cy7.5 and DSNP | Better photostability of Cy7.5 in DSPE-PEG than in CHCl3 | – | In vivo detection of HOCl | [53] |
| Encapsulation by polymer of PEG | MY-1057/NaYF4@NaYF4:Nd | DSNP→dye | FRET efficiency of 33% | Stable in cell culture, 10% FBS and the whole blood | >95% of cell viability with dose of 0.5 mg/ml for 24 h | Detection of ONOO− | [17] |
| Encapsulation by polymer of P-PEG | hCy7/NaYF4:Yb,Er,Tm | UCNP→dye | ET efficiency of 90.0% | 8.9% of decrease in absorbance under 980 nm radiation for 10 h (12.6 Mw/cm2) | >80% of cell viability with dose of 0.5 mg/ml for 24 h | In vivo detection of MeHg+ | [15] |
| Encapsulation by polymer of DSPE-PEG | Alk-pi/NaYF4:Yb,Er@NaYF4:Nd | Dye→UCNP | 638-fold increase in UCL | Stable UCL in an aqueous solution within 24 h | >90% of cell viability with dose of 0.6 mg/ml for 24 h; no obvious damage to the tissues in the treated mice | In vivo imaging | [54] |
| Encapsulation by polymer of DSPE-PEG | ICG/NaYF4:Yb,Er@NaYbF4@NaYF4:Nd | Dye→DSNP | Fourfold increase in DSL | No aggregation for 7 days but a decreased DSL | Negligible cytotoxicity with dose of 1 mg/ml for 48 h | In vivo imaging | [41] |
| Encapsulation by polymer of DSPE-PEG | IR808/NaNdF4@NaLuF4 | Dye→DSNP | 10-fold increase in DSL as compared to NaNdF4 | >90% absorption under 808 nm radiation for 18 min (0.080 W/cm2) | >80% of cell viability with dose of <200 μg/ml DSNP and < 80 μg/ml IR808 | In vivo imaging | [60] |
| Encapsulation by polymer of DSPE-PEG | IR806/NaErF4:Ce@NaYbF4@NaLuF4 | Dye→DSNP | 675-fold increase in DSL as compared to NaErF4 | Colloidal stability over 1 week of storage | No clear pathological tissue in the treated mice | In vivo imaging | [64] |
| Encapsulation by polymer of DSPE-PEG | ICG/NaYF4:Er | Dye→UCNP/DSNP | Fivefold increase in DSL and 32-fold increase in UCL | Twofold decrease in DSL after 6 h | 85% of cell viability with dose of 1 mg/ml for 48 h; no inflammatory response nor any tissue injury in the treated mice | In vivo imaging and PAI/PTI | [63] |
| Encapsulation by polymer of PC | Cy787/NaYF4:Yb,Nd,Er@NaYF4:Nd | Dye→UCNP | 17-fold increase in UCL | Stable UCL in PBS, HEPES, DMEM for 24 h | >85% of cell viability with dose of 0.8 mg/ml for 24 h | In vivo imaging and detection of ClO– | [46, 61] |
| Encapsulation by polymer of F127 | IR808/NaGdF4:Yb,Er@NaGdF4:Yb | Dye→UCNP | 178-fold increase in UCL | Stable UCL in PBS and the cell lysates | >95% of cell viability with dose of 0.5 mg/ml for 24 h | Intracellular detection of ClO– | [7] |
| Encapsulation by polymer of F127 | HC-SG/NaYF4:Yb,Er@NaYF4:Nd | Dye→DSNP | ET efficiency of 20% | Stable DSL in HEPES, DMEM and FBS for 2 h | >90% of cell viability with dose of 0.6 mg/ml; not obvious damage to major organs in the treated mice | In vivo detection of GSH | [62] |
- Abbreviations: ADA, alendronic acid; DBCO, dibenzocyclooctyne; DMEM, Dulbecco modified essential medium; DSL, downshifting luminescence; DSNP, downshifting nanoparticle; ET, energy transfer; FBS, fetal bovine serum; GSH, glutathione; HEPES, N-2-hydroxyethyl piperazine-N'-2-ethanesulfonic acid; ICG, indocyanine green; PC, phosphatidylcholine; PBS, phosphate-buffered saline; PDT, photodynamic therapy; PDT/PTT, photodynamic therapy/ photothermal therapy; UCL, upconversion luminescence; UCNP, upconversion nanoparticle.
Binding interaction
The as-prepared LnNP synthesized by the common methods such as thermal decomposition or high-temperature co-precipitation is hydrophobic due to the capping ligands of oleic acid (OA) and/or oleylamine (OM). Surface modification is thus needed to assemble NIR dye molecules onto LnNP and meanwhile endow the composites with water solubility. As shown in Figure 4B1, the bare LnNP can be coordinated with NIR dye directly after the removal of OA/OM ligands. The NIR dyes are usually structurally modified to possess sulfonic and/or carboxyl groups, allowing their binding to the surface metallic ions of LnNP. This method is experimentally simple and often employed to study the energy transfer mechanism and other optical properties. However, most of the nanocomposites have not enough stability as bioprobes due to the serious detachment of NIR dyes in the aqueous environment. It is worth mentioning that Liu and co-workers recently linked NIR dye 800CW with alendronic acid (ADA) to anchor on NaYF4:Yb,Er@NaYF4:Nd,Yb and demonstrated the high stability of UCNP-ADA in cytoplasm.45] It was found that phosphate groups in phosphate-buffered saline (PBS) could replace o-phosphorylethanolamine (AEP) and carboxyl groups rather than ADA on the UCNP surface. Density functional theory calculations further confirmed that ADA with a bisphosphonate ligand possessed larger binding energies on (100) and (001) NaYF4 surfaces than those of AEP and CH3COO– (Figure 5A).
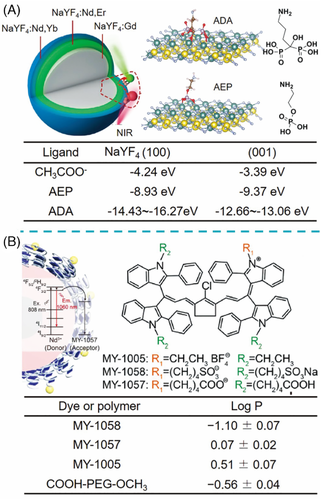
The most efficient and frequently used approach for the construction of NIR dye/LnNP nano-bioprobes should be encapsulation by an amphiphilic polymer such as distearoylphosphatidylethanolamine-poly(ethylene glycol) (DSPE-PEG), Pluronic F127, phosphatidylcholine (PC), and Tween 80.[8, 46-49] Generally, the hydrophobic segments of the amphiphilic polymer are embedded into the capping ligands of LnNP through hydrophobic–hydrophobic interaction while the hydrophilic segments face the outside surface to render the nanocomposites water-dispersible (Figure 4B2). Various hydrophobic or hydrophilic cyanine dyes have been reported to encapsulate inside the ligand-polymer layer.[15, 17, 35, 50] To investigate the encapsulating ability of NIR dyes, Zhang and co-workers compared three cyanine dyes MY-1005, MY-1057, and MY-1058 with different terminal groups of −BF4–, −COO–, and −SO3–, respectively.17] Only MY-1057 and MY-1058 were successfully encapsulated into the polymer layer on the LnNP surface, which could be explained by the close partition coefficient for NIR dye and amphiphilic polymer (Figure 5B). Furthermore, they observed that MY-1058 tended to bind with bovine fibrinogen and be extracted out from nanoprobe in the presence of blood, while MY-1057-encapsulated nanoprobe was stable in various physical media including cell culture, 10% fetal bovine serum (FBS), and the whole blood.
Silica or mesoporous silica coating is a common method for LnNP modification. NIR dye can be further embraced into the nanocomposite by chemical coupling or physical adsorption (Figure 4B3 and B4).2] Strong interaction such as covalent coupling and high hydrophobicity of NIR dye favor the retention of NIR dye. As shown in Table 2, less than 5% of IR806 were leached from the coating shell of mesoporous silica after 24 h in PBS. Besides inorganic silica, proteins can also be employed as a coating layer of LnNP and in the meantime as a carrier for NIR dye (Figure 4B5). In the procedure reported by Liu and co-workers, UCNP was first modified with polyacrylic acid, then covalently conjugated with bovine serum albumin (BSA) protein by typical N-ethyl-N’-(3-(dimethylamino)propyl)carbodiimide-N-hydroxysuccinimide chemistry, and finally loaded with IR825 via hydrophobic interaction.51] In this system, a high loading of IR825 (22%wt) was achieved but leaching might be a problem. About 25% of NIR dyes were leached from the nanoprobe after 24 h incubation in PBS.
Distance
Synthetic design of LnNP with small particle size, thin inert-shell or inert-core may shorten the distance between LnNP and surface NIR dye, but it may also affect its intrinsic luminescence (Figure 4A). Manipulating the distance is more practicable through the construction methods of nanocomposite (Figure 4B). In general, direct and tight coordination can provide the shortest distance between NIR dye and LnNP, which yields highly efficient energy transfer. For example, NIR dye 800CW was coordinated with UCNP by an ADA ligand, resulting in the transfer efficiency from NIR dye to UCNP as high as 81.1%, impressively higher than that of 2.9% by silica coating approach.45] Coating a shell of silica or protein may extend the distance and cause energy depletion during migration, which is suitable for applications without energy transfer.[51, 52]
For the encapsulation method by amphiphilic polymer, the NIR dye molecules can be trapped inside the hydrophobic layer on the surface of LnNP within the distance close enough for energy transfer. It should be noted that amphiphilic polymer with different sizes of hydrophobic segments may affect the distance and consequently the efficiency of energy transfer. In the case of NIR dye 1859 SL-sensitized NaYF4:Yb,Er nanoprobes, two amphiphilic polymers Tween 80 (Mr 1300) and F127 (Mr 13000) were, respectively, wrapped around the nanoparticles for comparison. The former showed narrower size distribution and much higher dye-sensitized UCL intensity.49] In a few cases, dyes and LnNP are encapsulated in separated micelles and then conjugated by click reaction (Figure 4B6). Zhang and co-workers designed [email protected] nanoprobes through a click conjugation of azide (N3)-bearing DSPE-PEG-coated DSNP and dibenzocyclooctyne (DBCO)-bearing DSPE-PEG nanomicelles containing Cy7.5.53] According to the size of nanomicelles, the shortest distance between Cy7.5 and LnNP was estimated to be ∼14 nm. Lifetime measurements indicated there was no energy transfer interaction between DSNP and Cy7.5.
Aggregation of NIR dye
As most other organic fluorophores, NIR cyanine dyes undergo self-quenching at high concentrations, which is known as aggregation-caused quenching (ACQ). ACQ effect of NIR dyes on the surface of LnNP is the most important factor limiting the sensitization efficiency.54] In NIR dye/LnNP nanocomposites, the amount of NIR dye should be optimized to enhance the overall absorption with enough dyes and avoid the undesired self-quenching. Encapsulation by an amphiphilic polymer can provide a local hydrophobic environment in aqueous solution, which is often used to reduce the degree of dye aggregation and increase the optimal concentration of NIR dye in the system. In our previous report, the optimal ratio of IR808 to UCNP in dye-sensitized nanocomposites prepared by F127-encapsulation was significantly increased to 90:1 as compared to 5:1 by direct coordination.7] Liu and co-workers found that the distribution of NIR dye may also affect the aggregation degree.54] Despite the protection of the same amphiphilic polymer, NIR dye Car-Cl with carboxyl groups tended to tightly bond on the surface of UCNP while another dye Alk-Cl with long hydrophobic alkyl moieties could freely spread in a hydrophobic layer (Figure 6A). It was estimated that the distance of adjacent Alk-Cl was close to Car-Cl (8.7 vs. 7.6 nm) at a low concentration of 1 nmol/mg but was twice that of Car-Cl (4.8 vs. 2.4 nm) at 10 nmol/mg. Due to the longer distance between Alk-Cl molecules, the aggregation was effectively inhibited; thus, resulting in high sensitization efficiency.
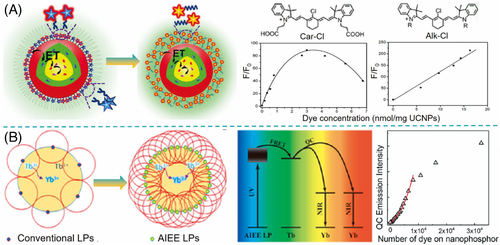
The phenomenon of aggregation-induced emission (AIE), opposite to the ACQ effect, was first reported by Tang and co-workers.[55, 56] AIE dyes do not suffer from concentration quenching and feature enhanced luminescence in their aggregated state as the result of the restriction of intramolecular rotations (e.g., in tetraphenylethene), head-to-tail molecular arrangement (e.g., in cyanostilbene derivatives), or other principles.57] Therefore, the AIE effect offers an opportunity to prepare dye-concentrated materials with both maximized absorbance and effective fluorescing capability (Figure 4C). Recently, Prasad and co-workers developed a dicyanostilbene derivative with AIE behavior and employed it as an antenna to sensitize quantum cutting in NaYF4:Yb,Tb under 405 nm excitation (Figure 6B).57] Since the AIE dyes were immune to concentration quenching, they could fully cover the LnNP surface at high density to harvest the excitation energy, producing 2260-fold enhancement of quantum cutting emission relative to the dye-free LnNP. So far, sensitization of LnNP by NIR dye with AIE behavior has not been demonstrated, and its feasibility remains to be explored.
Another way to make use of the aggregation of NIR dye is the potential photothermal property. The ACQ of luminescence may enhance the photothermal conversion efficiency of NIR dyes, which provides a straightforward strategy for the design of more efficient photothermal nanoagents without structural modulation.[58, 59] For instance, Liu and co-workers utilized the surfactant-stripped frozen micelles method by using F127 to noncovalently confer a hydrophobic NIR dye, β-thiophene-fused BF2-azadipyrromethene (aza-BDTP) with excellent aqueous solubility. The resulting aza-BDTP-containing micelles exhibited high photothermal conversion efficiency at 33.9%, significantly higher than 16.9% for bare aza-BDTP molecules, owing to ACQ of aza-BDTP fluorescence.58]
Biocompatibility of nanoprobes
Biocompatibility should be considered during the application of NIR dye/LnNP nanoprobes, especially for in vivo application. The biosafety assessments for typical NIR dye/LnNP nanoprobes are presented in Table 2. All the cell viabilities could remain over 80% after incubation of the nanoprobes for 24 or 48 h by a standard methyl thiazolyl tetrazolium assay, indicating negligible cytotoxicity for these nanoprobes.[7, 15, 17, 41, 46, 50-52, 60, 61] In vivo toxicity of NIR dye/LnNP nanoprobes has also been evaluated by hematology analysis, blood biochemical assay, and histological analysis on 7 or 15 days after intravenous injection of the nanoprobes into the nude mice.[54, 62-64] All the results showed no inflammatory response, nor any tissue injury in the treated mice as compared to the control group. In contrast, Pb/Cd-based QDs probes have Pb2+/Cd2+-induced cytotoxic effect and influence the hematopoiesis through oxidative stress.[65-67] Carbon nanotubes may cause adverse side effects at the state of agglomeration.[68, 69] Therefore, these NIR dye/LnNP nanoprobes exhibit superior biocompatibility and have great potentials in the fields of bioscience and biomedicine.
LUMINESCENT BIOIMAGING
Both NIR dye and LnNP are potential candidates for luminescent bioimaging, which results in high imaging depth and low autofluorescence due to the use of NIR light for excitation. However, as a small molecular probe, NIR dye is often limited by its poor targeting and low accumulation in vivo. LnNP also suffers from weak luminescence under NIR excitation at a low power density. According to the American National Standard, a conservative limit for human skin exposure at 980 nm is around 730 mW/cm2.70] Therefore, a set of NIR dye/LnNP nanoprobes were designed for bioimaging (Table 3), in which NIR dyes served as an antenna to sensitize LnNP to tailor the excitation wavelengths or enhance the UCL or DSL NIR emission.
| Dye-sensitized UCL/DSL | NIR dye/LnNP nanoprobe | Laser parameter | Detected signal | Administration route | Small animal imaging | Ref. |
|---|---|---|---|---|---|---|
| UCL (visible) | IR806/NaYF4:Yb,Er@NaYF4:Yb | 808 nm, 1.0 W/cm2 | 540 nm/660 nm (Er3+) | Subcutaneous injection | In vivo imaging | [47] |
| UCL (visible) | Cy7/NaYF4:Yb,Nd,Er@NaYF4:Nd | 808 nm/785 nm/760 nm | 540 nm (Er3+) | Subcutaneous injection | Lymphatic imaging (Figure 7A) | [46] |
| DSL (NIR IIb) | ICG/NaYF4:Yb,Er@NaYbF4@NaYF4:Nd | 800 nm, 0.2 W/cm2 | 1530 nm (Er3+) | Subcutaneous injection | In vivo imaging | [41] |
| DSL (NIR I) | Cy7/NaYbF4@NaYF4:Nd | 808 nm, 8.5 mW/cm2 | 980 nm (Yb3+) | Subcutaneous injection | Imaging of blood vessel (Figure 7B) | [71] |
| DSL (NIR IIa) | IR808/NaNdF4@NaLuF4 | 808 nm, 0.16 W/cm2 | 1340 nm (Nd3+) | Tail vein injection | Imaging of orthotopic glioblastomas (Figure 7C) | [60] |
| DSL (NIR IIb) | IR806/NaErF4:Ce@NaYbF4@NaLuF4 | 808 nm, 0.12 W/cm2 | 1525 nm (Er3+) | Tail vein injection | Imaging of brain vasculature and orthotopic gliomas (Figure 7D) | [64] |
Dye-sensitized UCL bioimaging
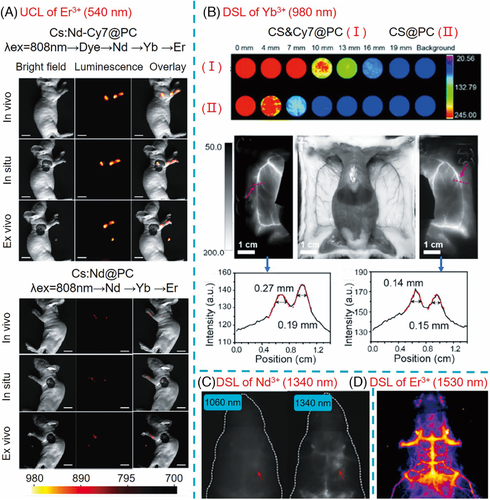
In 2016, Han and co-workers prepared water-dispersible IR806-sensitized NaYF4:Yb,Er@NaYF4:Yb by Pluronic F127 encapsulation and employed such dye-sensitized UCNP for in vivo animal imaging.47] Through subcutaneous administration of the bioprobes (50 μl, 10 mg/ml), in vivo UCL signals under irradiation at 808 nm (1.0 W/cm2) were observed, suggesting the feasibility of biophotonic application for dye-sensitized UCNP. Further, Li and co-workers. demonstrated that the dye-sensitized upconversion nanoprobe resulted in images with improved UCL intensity and broader NIR excitation responses.46] In this work, Cy7-sensitized NaYF4:Yb,Nd,Er@NaYF4:Nd with PC modification (CS:Nd-Cy7@PC) was prepared and injected intradermally (20 μl, 2 mg/ml) into the claw of mice with the expected drainage pattern to nodes within the oxter. An intense UCL signal appeared in the lymphatic drainage basins under 800 nm excitation, whereas a very weak signal was detected for the mice after injection with the CS:Nd@PC counterpart (Figure 7A).
Dye-sensitized DSL bioimaging
UCL imaging in the visible region of 400−700 nm or NIR-I of 700−950 nm still suffers from limited imaging depth, restricting the ability to unravel biological complexities in deep tissue. Therefore, dye-sensitized DSL bioimaging with enhanced NIR-II emission (1000–1700 nm) is developed. The imaging cameras are switched from silicon-based detectors to indium gallium arsenide (InGaAs)-based detectors accordingly.
Prasad and co-workers designed ICG-sensitized NaYF4:Yb,Er@NaYbF4@NaYF4:Nd DSL nanoprobes, from which NIR-II image can be observed through chicken breast tissue with a depth of 9 mm and signal can be detected at a depth of 23 mm.41] The achieved image based on NIR II emission at 1530 nm is more contrast than those from the green upconversion emission at 540 nm of the same nanoprobe and the NIR-I upconversion emission at 800 nm of Yb,Tm-doped counterpart. After encapsulation with DSPE-PEG, the ICG-sensitized Er-doped nanoprobe (0.2 ml, 2 mg/ml) was subcutaneously injected into a mouse with a depth of ∼3 mm, resulting in a clear NIR-II image of the injection point (excited at 800 nm, ∼0.2 W/cm2). Similarly, an efficient dye-sensitized NIR emissive nanoprobe, termed as CS&Cy7@PC, for deep tissue imaging was reported.71] Cy7, with a higher quantum yield (19.7%) than ICG (12.9%), was used as a sensitizer to transfer the excitation energy to Nd3+ ions, and further to the emitter Yb3+ ions in-NaYbF4@NaYF4:60%Nd. Under the excitation of 808 nm (8.5 mW/cm2), the NIR emissive image can be distinguished by the camera with a tissue depth of 13 mm and the signal can be detected with a depth of up to 16 mm. Furthermore, the dye-sensitized NIR signal was successfully used in blood vessel imaging and luminescence-guided peritumoral lymph node dissection in a mouse model (Figure 7B).
Nd3+ ions, often used as sensitizers, can also emit NIR-II luminescence at 1060 and 1340 nm. Due to the much stronger emission at 1060 nm than that at 1340 nm (∼20 times), the latter is mostly neglected and has not been applied for bioimaging. In this regard, Li and co-workers. boosted the 1340 nm emission of NaNdF4@NaLuF4@DSPE-PEG through IR808 sensitization and applied the nanoprobes for imaging of orthotopic glioblastomas.60] The image obtained from 1340 nm emission exhibited better resolution and contrast compared to that from 1060 emission (Figure 7C). Very recently, they further made use of the strong NIR IIb (1500−1700 nm) luminescence from dye-sensitized Er-based LnNP for in vivo imaging64] They optimized the core–shell structure of NaErF4:Ce@NaYbF4@NaLuF4 and the amount of IR806 to facilitate 4I13/2→4I15/2 transition, which led to an impressive 675-fold enhancement of 1525 nm emission in comparison with NaErF4 nanoparticles. Due to the strong NIR IIb luminescence and the deep penetration through intact scalp and skull, NIR IIb imaging of brain vasculature in a healthy nude mouse was clearly observed under 808 nm excitation (120 mW/cm2) after tail vein injection of the nanoprobes (20 mg/kg; Figure 7D).
LUMINESCENT BIODETECTION
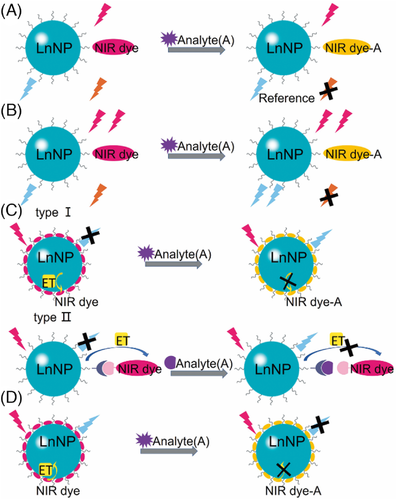
For biodetection application, NIR dye/LnNP nanoprobes are excited by NIR radiation, which is located within a “biological transparency window,” providing a background-free signal and thus a remarkable sensitivity.[2, 72] In most cases, the detection is based on energy transfer. The appearance of the analyte may affect the energy transfer process between NIR dye and LnNP by altering their spectral overlap [17, 73, 74] or distance.[61, 62, 75] During the detection, NIR dye and LnNP may serve as analyte-responder, luminescence reference, absorption competitor, or energy donor/acceptor. Herein, we classify the detection strategies of these NIR dye/LnNP nanoprobes into four modes according to the roles of the two luminophores (Figure 8). The design principle, feature, application scope, and recently reported examples are discussed in detail.
Independent emissions
In some NIR dye/LnNP composites, there is no energy transfer or signal crosstalk between NIR dye and LnNP, resulting in two independent emissive luminescence. Such nanocomposites can be used for ratiometric detection in which the sensitive emission of NIR dye responds to the analyte while the constant emission of lanthanide serves as a reference (Figure 8A). For example, Li and co-workers reported an 808 nm excited ratiometric nanoprobe with NIR I (925 nm) and NIR II (1525 nm) dual emission originating from NIR dye Cy925 and NaYbF4:Er@NaYF4:Yb@NaYF4:Nd DSNP, respectively.76] Due to the high selectivity of Cy925 with hypochlorite (HClO/ClO−), one of the most important reactive oxygen species (ROS) associated with various diseases, this nanoprobe was applied for ratiometric detection of ClO– in vivo, which proved the content variation of ClO− during inflammation formation of mice hind limbs. Such a detection mode with independent ratiometric luminescence is very simple but remains several problems. First, the light attenuation in two NIR windows is different; thus, working curves of ratio change under tissues are required to reduce the deviation of detection in vivo. In addition, absorption competition may exist due to the same excitation for NIR dye and LnNP, which we will discuss in the following detection mode.
Absorption competition
The second detection mode with NIR dye/LnNP composites is also energy transfer-independent but involves an absorption competition effect between LnNP and NIR dye (Figure 8B). Zhang and co-workers designed such a nanoprobe for HClO detection by employing NIR dye Cy7.5 as a competition absorber of NaEr0.5Y0.5F4@NaYF4.53] Cy7.5 with a large molar extinction coefficient (six orders of magnitude larger than that of Er3+ ion at 808 nm) could attenuate the excitation energy on DSNP through a photon filtration effect. Under 808 nm excitation, the emission at 1550 nm of Er3+ increased with HClO due to the degradation of Cy7.5 while the same emission under 980 nm excitation remained constant for self-calibration. Benefiting from the features of sensitivity, dual-wavelength excitation, and NIR-IIb emission, this nanoprobe was successfully used for high-resolution (μm-scale) sensing of lymphatic inflammation in vivo.
Detection based on independent emission or absorption competition can be applied for sensing of various NIR dye-sensitive ions and small molecules.[53, 76] Because no energy transfer process is involved in the nanocomposites, the proximity of LnNP and NIR dye is unnecessary. NIR dye and LnNP can either be enveloped together in one amphiphilic polymer or be linked by a click conjugation.
NIR dye-quenched lanthanide emission
Organic visible dye as an energy acceptor is very common in UCNP-based detection platform.77] A lot of such studies have been reported and discussed in previous reviews.78] In contrast, nanoprobes with NIR dye as acceptors are much fewer due to fewer matching pairs and poorer stability of NIR dye.8] Nonetheless, the absorption of NIR dye may match the NIR emission of UCNP or DSNP to construct an efficient energy transfer.38] Moreover, such nanoprobes feature NIR region for both excitation and emission, which are able to give more exquisite signal in vivo detection.
In this detection mode, the analyte in the environment reacts with NIR dye and the quenching process is interrupted resulting in the recovery of lanthanide emission (Figure 8C). In the NIR dye-quenched UCL system, Yb,Er,Tm-codoped UCNP is usually employed. Efficient NIR upconversion emission at ∼800 nm of Tm3+ is absorbed by analyte-sensitive NIR dye. The green or red emission of Er3+ can provide a ratiometric signal or match with another visible dye for multiplexed detection. In 2013, Li and co-workers prepared NIR cyanine dye hCy7-assembled NaYF4:Yb,Er,Tm for UCL monitoring of toxic methylmercury (MeHg+) ex vivo and in vivo.15] The detection principle is based that the absorption peak of hCy7 shifts from 670 to 845 nm in presence of MeHg+, inducing the quenching in NIR UCL emission of the nanoprobe. Gu and co-workers. designed a photodynamic therapy (PDT) nanoplatform with NaYF4:Yb,Tm@NaYF4:Yb,Er as UCNP, merocyanine 540 (MC540) as photosensitizer, and NIR dye IR820 as indicator of produced singlet oxygen.73] Singlet oxygen decomposed IR820 through the break of alkene, resulting in the gradual increase of upconversion emission at 800 nm. This nanoprobe can sensitively monitor the generation of singlet oxygen in living biological systems. Recently, Xing and co-workers developed a nanoprobe for tracking multiple radicals by anchoring the ROS-responsive HCy5 and nitrogen species (RNS) responsive Cy7 fluorophores onto UCNP with their maximum absorptions matching the UCL at 660 and 800 nm, respectively.74] Such effectively spectral overlap facilitated efficient energy transfer for optical imaging of RNS/ROS in one unified system.
In the NIR dye-quenched DSL system, Nd3+-doped LnNP and NIR dye with matched DSL emission and absorption, respectively, are often chosen. In this regard, Li and co-workers assembled NaYF4:5%Nd DSNP and NIR dye Cy860 into one nanocomposite in which the energy could transfer from NaYF4:Nd to Cy860.75] Both of the two luminescent materials had emission at 893 nm but their lifetimes were much different (53 μs and 0.23 ns for NaYF4:Nd and Cy860, respectively). They employed time-gated (TG) technology to distinguish the two luminescence signals. With the addition of HClO, the TG-off signal collecting all the luminescence decreased, while the TG-on signal collecting only the long-lifetime luminescence of NaYF4:Nd increased. The ratios of TG-on signal to TG-off signal with excitation and emission at the same wavelength provided sensitive and accurate detection of HClO in vivo (Figure 9A).

Lifetime measurement, which is non-sensitive to the tissue depth and the excitation power density, has drawn people's attention to FRET-based detection. Recently, Zhang and co-workers designed an ONOO−-responsive NIR-II nanoprobe based on the FRET of NaYF4@NaYF4:1%Nd DSNP to dye MY-1057.17] DSNP exhibited shortened luminescence lifetime at 1060 nm from 305 ± 3 to 203 ± 2 μs after incorporation of MY-1057. The lifetime recovered continuously along with the addition of ONOO−. Through lifetime measurement, the ONOO− amount at tumor lesions could be quantitated (Figure 9B).
NIR dye-quenched lanthanide luminescence system can also be employed for homogeneous competitive immunoassay (Figure 8C). In recent work, NaYF4@NaYF4:Yb,Tm@NaYF4 UCNP with surface anti-progesterone antibody conjugation was used as an energy donor and a compact progesterone–horseradish peroxidase–IRdyeQC-1 complex served as an energy acceptor.79] Based on such a NIR-to-NIR system, progesterone was sensitively detected with a limit of detection as low as 1.36 pg/ml.
NIR dye-sensitized lanthanide emission
The first successful demonstration of dye-sensitized upconversion emission for detection (Figure 8D) was reported in 2018 by Liu and co-workers.16] They designed a nanoprobe comprised of cyanine dye F-SG and NaYF4:Yb,Er@NaYF4:Nd UCNP and exploited the sensitized UCL of Er3+ at 540 nm to monitor the alteration of glutathione (GSH) level in vivo (Figure 10A). The results showed that such dye-sensitized nanoprobe could afford a maximal signal-to-background ratio of 30, which was about three-fold of most existing dye-quenched UCL nanoprobes. Furthermore, Li and co-workers demonstrated dual-excitation UCL ratiometric probes based on dye-sensitized UCNP for semi-quantitative detection in vivo.61] Specifically, the UCL was efficiently enhanced by the Cy787 dyes on the surface of NaYF4:Yb,Nd,Er@NaYF4:Nd and subsequently decreased upon the addition of ClO– under 808 excitation, whereas the UCL excited at 980 nm remained essentially constant, thus providing ratiometric monitoring of ClO– in vivo (Figure 10B).
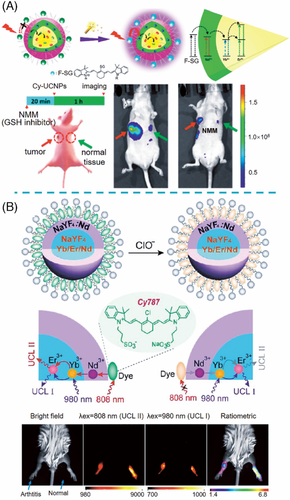
For the nanoprobes with Nd-doped nanoparticles as energy acceptors, the close excitation wavelengths at ≈800 nm between Nd3+ and NIR dye may cause an inevitable high background luminescence. Therefore, we designed the nanoprobes consisting of ClO– recognizer NIR cyanine dye IR808 as an energy donor, and NaGdF4:Yb,Er@NaGdF4:Yb as energy acceptor.7] The efficient analyte-dependent energy transfer and the large absorption discrepancy at 808 nm between NIR dye and lanthanide ions (Yb3+ and Er3+) endow the nanoprobes with ultrahigh detection sensitivity (Figure 11A). Furthermore, UCL spectra under 808 and 980 nm excitation were separately corrected at a fixed position of the cell by a purpose-built NIR dual-laser confocal microscope. The UCL under 980 nm excitation was employed as a self-calibrated signal to alleviate the interference of intracellular fluctuation. With this ratiometric measurement, accurate quantification of intrinsic and exogenous ClO− in the live human breast cancer cell (MCF-7) was achieved (Figure 11B).
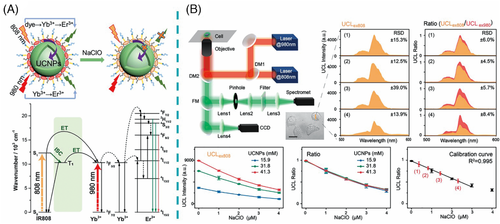
Note that such dye-sensitized upconversion nanoprobes with ultrasensitivity and high accuracy can only be applied for single-analyte detection. In order to achieve multiplexed ratiometric detection, we recently integrated the features of dye-sensitization and dye-quenching of the UCL into a single nanoprobe.50] In particular, a novel dual-excitation/dual-emission upconversion strategy was proposed through the design of UCNP loaded with two dyes for sensitization and quenching of the UCL, respectively. Based on the two independent energy transfer processes of NIR dye IR845 to UCNP and UCNP to visible dye PAPS, intracellular ClO− and Zn2+ were simultaneously quantified.
In principle, dye-sensitized strategy can also apply to DSNP with emission in the NIR region, which displays distinct advantages for in vivo bioassays. Based on NIR dye HC-SG sensitized NaYF4:Yb,Er@NaYF4:Nd process, Liu and co-workers utilized luminescence intensity of Er3+ at 1530 nm to track the fluctuation of GSH level in liver and lymphatic drainage in mice.62]
In general, detection of ions or small molecules based on NIR dye-sensitized luminescence may be more sensitive than that based on NIR dye-quenched luminescence. However, due to the strict distance limit for energy transfer from NIR dye to LnNP, the utility of NIR dye-sensitization luminescence for immunoassay of bio-macromolecules has not been realized up to date.
MULTIMODAL THERANOSTICS
Besides high-contrast deep tissue luminescence imaging, NIR dye/LnNP nanoprobes can also specifically bind to receptor-overexpressed lesion for imaging-guided therapy after modification with targeting biomolecules. For instance, in the system of IR806-sensitized Er-based nanoprobes established by Li and co-workers, angiopep-2 peptide was conjugated with nanoparticles to target glioma cells.64] A tumor-to-background ratio as high as 12.5 was achieved for through-skull NIR IIb imaging of orthotopic glioma. They further demonstrated the potential of the nanoprobes in fluorescence-guided surgery of deep-seated small orthotopic glioma due to the precise visualization of tumor margins (Figure 12A).
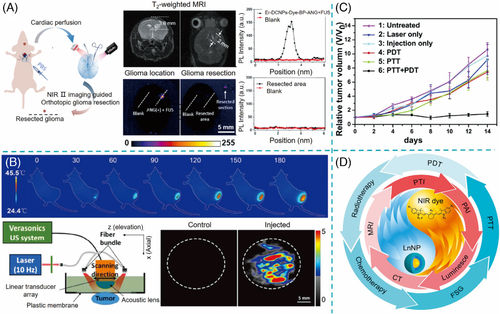
To obtain a reliable and accurate diagnosis, it is very common to design nanoprobes with multiple imaging modalities. Fortunately, LnNP with rich optical, magnetic, radioactive, and X-ray attenuation properties provides an opportunity for X-ray computed tomography (CT), magnetic resonance imaging (MRI), and single-photon emission CT. For instance, Lu or Yb -based LnNP can serve as a CT contrast agent due to its high X-ray absorption capability.[80, 81] Gd-based LnNP with a large number of unpaired electrons and relatively long electronic relaxation has proved for high-efficiency T1-weighted MRI.82] Recently, photoacoustic imaging (PAI) with the feature of high spatial resolution has emerged as a new type of promising imaging technique.83] PAI is based on the ultrasonic signals from thermos-elastic expansion produced by the competing nonradiative relaxation of probes under short-pulsed laser irradiation.83] Benefiting from efficient NIR absorption and photothermal capacity, NIR cyanine dyes are ideal candidates for PAI.12] Therefore, NIR dye/LnNP nanoprobes can make use of CT/MRI/PAI modalities as a supplement to luminescence to accomplish high sensitivity and high resolution simultaneously. For this purpose, Prasad and co-workers designed ICG-sensitized NaYF4:Er nanoprobes in which the enhanced emission of Er3+ provided NIR-II imaging and a part of the ICG excitation decays produced local heating for PAI and photothermal imaging (PTI; Figure12B).63] Moreover, it was found that an efficient energy transfer from ICG to Er3+ prevented overexcitation and intensive bleaching of ICG and thus supplied favorable conditions for localized thermal heating.
Due to high photothermal capacity, NIR dyes are often used as photothermal therapy (PTT) agents and enable the NIR dye/LnNP nanocomposites to apply for theranostics. Chang and co-workers combined Nd, Yb, Er-doped core–shell UCNP with IR806 through a shell of mesoporous silica and demonstrated the use of the nanoprobes for PTT and UCL imaging under 793 nm light irradiation.52] In another theranostic nanomaterial, BSA protein was coated on the surface of NaGdF4:Yb:Er UCNP to accommodate cyanine IR825 and photosensitizer Rose Bengal (RB) simultaneously.51] RB absorbed green UCL from UCNP to induce PDT of cancer cells, while IR825 offered nanoparticles a strong photothermal performed under NIR laser irradiation. Such combined photodynamic and photothermal effects under the guidance of in vivo UCL imaging could result in an improved therapeutic efficacy (Figure 12C).
NIR cyanine dyes are also potential photosensitizers for PDT as their relaxation from an excited state to the ground state is accompanied by the generation of toxic ROS such as singlet oxygen (1O2).23] However, the quantum yield of 1O2 from cyanine-based PDT agents is relatively low. For example, the commonly used ICG and IR783 have the 1O2 quantum yields of only 0.008 and 0.007, respectively.23] NIR cyanine dyes can evolve as better photosensitizers through structural modification. It was found that incorporation of iodine atom, platinum (II) complex or radical 2,2,6,6-tetramethylpiperidinyloxy into the structure of cyanine largely enhanced the production of 1O2.23] As to NIR dye/LnNP nanocomposites, it is possible that employing the lanthanide ions improves the intersystem crossing mechanism in cyanine moieties through the heavy atom effect and/or paramagnetic effect, resulting in better 1O2 generator. To sum up, NIR dye/LnNP composites can serve as versatile nanoplatforms for the combination of multiple imaging and therapeutic modalities to achieve efficient theranostics of disease (Figure 12D).
CONCLUSION AND PERSPECTIVES
In the past decades, by taking advantage of exceptional physicochemical features of NIR dye/LnNP nanocomposites along with the deep light penetration depth and the absence of autofluorescence under NIR excitation, a series of dye-sensitized UCL/DSL bioimaging, biomolecules-related detection, NIR-triggered diagnosis, and therapy techniques have been established based on these novel NIR dye/LnNP nanoprobes. Despite these fantastic studies, there are still several fundamental and practical issues that should be addressed.
First, the excitation dynamics in NIR dye sensitization are not yet fully understood; thus, a more detailed fundamental investigation of energy transfer mechanisms in NIR dye/LnNP nanocomposites is needed by utilizing ultra-fast transient absorption and photoluminescence spectroscopy as well as the quantum calculation. For instance, transient absorption spectroscopy and transient photoluminescence spectroscopy are able to study the NIR dye triplet generation and quenching rate, and further estimate the contribution of triplet states in energy transfer. The calculation of excited-state wavefunctions is also expected to reveal the process of DET.
Second, it remains challenging to construct robust and bright NIR dye/LnNP nanocomposites for applications in aqueous solutions, especially in the biosystem. The immature synthesis technology as well as relevant stability, efficiency, and cost issues strongly hamper the application and commercialization of these nanoprobes. Future efforts should be made in the following aspects: (i) NIR dyes with better photostability and higher efficiency in fluorescent, photodynamic or photothermal abilities to meet various applications, are desired. In addition, developing NIR dye with AIE effect may be a promising direction to enhance the dye sensitization efficiency. (ii) It is critical to further exploit general construction methods to keep the luminescence of NIR dye and LnNP in the composites and promote their energy transfer. The possible approaches may be fixing NIR dye in OA or OM ligand by thiol-ene click reaction, adjusting the length of ligands and amphiphilic polymers, or developing more efficient linkers. (iii) More detection strategies based on NIR dye/LnNP nanoprobes are required both in vitro and in vivo. Multi-wavelength emissions of LnNP from UV to NIR and multi-excitation by different dye antennas can be fully utilized to realize multiplexed detection of various small molecules or ions. Research on dye-sensitized LnNP for immunoassay of bio-macromolecules is still in its infancy. Dye sensitization may enhance the UCL/DSL intensity, increase the signal-to-background ratio, and thus improve the sensitivity in heterogeneous bioassays, which is particularly suited to the detection of disease biomarkers in human saliva and serum.
Last but not the least, biosafety concerns for NIR dye/LnNP nanoprobes remain, especially with respect to the long-term biocompatibility. Low biodegradability of inorganic LnNP increases the likelihood of chronic toxicity to the living organism.[84, 85] This problem is one of the major hurdles to the clinical translation for all the LnNP-containing nanoprobes. Very recently, a class of red-emitting biodegradable UCNP using K3ZrF7 as host matrix with a feature of dynamically “soft” crystal lattice has been reported.85] The K3ZrF7:Yb,Er nanoparticles injected intravenously were observed to biodegrade into nontoxic products and harmlessly excrete from the bodies of mice, in stark contrast to the nondegradable NaYF4:Yb,Er nanoparticles that primarily accumulated in the main organs of mice. The NIR dye/LnNP nanocomposites comprised of such biodegradable LnNP would be promising for future biomedical applications and clinical translations.
ACKNOWLEDGMENTS
This work was supported by the Science and Technology Cooperation Fund between Chinese and Australian Governments (no. 2017YFE0132300), the Strategic Priority Research Program of the CAS (no. XDB20000000), the NSFC (nos. 51672272, 21771185, 21771178, 21975257, 12074380), Youth Innovation Promotion Association of CAS (no. 2017347), and the CAS/SAFEA International Partnership Program for Creative Research Teams.
Biographies

Shan Lu earned her B.Sc. degree (2006) in Applied Chemistry and PhD degree (2011) in Chemical Engineering and Technology from Beijing University of Chemical Technology. She joined Prof. Xueyuan Chen's group in Fujian Institute of Research on the Structure of Matter as a research assistant professor in 2011 and was promoted to research associate professor in 2015. She was selected as a member of the Youth Innovation Promotion Association of the Chinese Academy of Sciences in 2017. Her research interest focuses on the synthesis and applications of lanthanide-based luminescent nanomaterials.

Jianxi Ke earned her B.Sc. from Fuzhou University (2016). She is currently a doctoral student in materials science and engineering at ShanghaiTech University. In September 2016, she joined Prof. Xueyuan Chen's group in the Fujian Institute of Research on the Structure of Matter. Her current research focuses on the controlled synthesis, optical spectroscopy, and biological applications of inorganic luminescent nanomaterials.

Xueyuan Chen is Editor-in-Chief of Journal of Luminescence. He earned his B.Sc. from the University of Science and Technology of China (1993) and his PhD from the Fujian Institute of Research on the Structure of Matter (FJIRSM), Chinese Academy of Sciences (1998). From 2001 to 2005, he was a postdoctoral research associate at the Chemistry Division of Argonne National Laboratory, US Department of Energy. In 2005, he joined the faculty at FJIRSM. His research focuses on the electronic structures, optical properties and applications of inorganic luminescent materials, such as lanthanide nanobioprobes and LED phosphors.




