Smart multi-functional aggregates reoxygenate tumor microenvironment through a two-pronged strategy to revitalize cancer immunotherapy
Yan Zhang and Luoqi Liang contributed equally to this work.
Abstract
PD-1/PD-L1 inhibitors have emerged as standard treatments for advanced solid tumors; however, challenges such as a low overall response rate and systemic side effects impede their implementation. Hypoxia drives the remodeling of the tumor microenvironment, which is a leading reason for the failure of immunotherapies. Despite some reported strategies to alleviate hypoxia, their individual limitations constrain further improvements. Herein, a novel two-pronged strategy is presented to efficiently address hypoxia by simultaneously adopting atovaquone (ATO, inhibiting oxygen consumption) and oxyhemoglobin (HbO2, directly supplementing oxygen) within a multifunctional aggregate termed NPs-aPD-1/HbO2/ATO. In addition to eliminating hypoxia with these two components, this smart aggregate also includes albumin and an ROS-responsive cross-linker as a controlled release scaffold, along with PD-1 antibody (aPD-1) for immunotherapy. Intriguingly, NPs-aPD-1/HbO2/ATO demonstrates exceptional tumor targeting in vivo, exhibiting ≈4.2 fold higher accumulation in tumors than in the liver. Consequently, this aggregate not only effectively mitigates hypoxia and significantly assists aPD-1 immunotherapy but also simultaneously resolves the targeting and systemic toxicity issues associated with individual administration of each component. This study proposes substantial implications for drug-targeted delivery, addressing tumor hypoxia and advancing immunotherapy, providing valuable insights for advancing cancer treatment strategies.
1 INTRODUCTION
The landscape of anti-tumor treatment has undergone a profound transformation with the advent of immunotherapies based on immune checkpoint inhibitors (ICIs), particularly programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) inhibitors.[1, 2] However, the response rates to PD-1/PD-L1 inhibitors in solid tumors still remain relatively low, resulting in a limited beneficiary population. Subsequent studies have indicated that the lack of infiltration by CD8+ T cells is a critical factor in the resistance of solid tumors to the treatment of PD-1/PD-L1 inhibitors.[3] Consequently, researchers have termed tumors lacking CD8+ T cell infiltration immunologically “cold” tumors, implying a stronger resistance to ICIs therapy.[4, 5] Moreover, “cold” tumors also exhibit an enrichment of numerous immunosuppressive cells, such as myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs) and so on, further diminishing their responsiveness to immunotherapy.[6, 7]
Hypoxia is a crucial characteristic of solid tumors due to inadequate blood flow and oxygen supply and is widely recognized to be closely associated with a poor prognosis.[8] Recent studies suggest a close relationship between hypoxia and “cold” tumors, indicating the potential involvement of hypoxia as one of the early factors in the formation of “cold” tumors.[9] For instance, hypoxia has the potential to impede the maturation and function of antigen-presenting cells, leading to the suppression of dormant T cell activation. This, in turn, contributes to the formation of an immunosuppressive tumor microenvironment (TME).[10, 11] Furthermore, hypoxia enrolls more immunosuppressive cells such as MDSCs, Tregs and tumor-associated macrophages (TAMs) into the tumor stroma through hypoxia-inducible factor 1α (HIF1α)-driven secretion of various cytokines and growth factors.[12, 13] In addition, under hypoxia, CD47, PD-L1 and several immunosuppressive checkpoint molecules are upregulated by HIF1α, adding to the immune escaping propensity.[14, 15] Therefore, addressing hypoxia has become a key factor in transforming “cold” tumors into “hot” tumors and enhancing the effectiveness of immunotherapy.[16]
Currently, there are several strategies to alleviate hypoxia, including direct oxygen delivery, tumor vascular normalization, in situ oxygen generation and mitochondrial inhibition.[17-19] Based on these strategies, numerous materials and methods have been developed, achieving certain success.[20, 21] For instance, direct oxygen delivery is the most widely used strategy and is usually achieved by oxygen carriers such as perfluorocarbons (PFCs)[22] and hemoglobin (Hb)[23] to quickly supplement oxygen. Although these treatments provide partial alleviation for hypoxia, they each have intrinsic limitations such as toxicity and low efficiency. Therefore, it is imperative to develop more refined approaches to overcome the constraints of the existing strategies and elevate the capacity for alleviating tumor hypoxia to a more advanced stage.
In this study, we originally proposed a two-pronged strategy to achieve an extremely effective reoxygenation of the tumor microenvironment through a multifunctional aggregate, denoted as NPs-aPD-1/HbO2/ATO. The framework of this aggregate comprises albumin, oxyhemoglobin (HbO2) and aPD-1, covalently crosslinked with a reactive oxygen species (ROS)-responsive linker, constructing a stable nanostructure using a simple and convenient method. Subsequently, atovaquone (ATO), a clinically used drug for antimalarial treatment known for its ability to block mitochondrial respiration,[12] is introduced into the smart aggregate through non-covalent adsorption, ultimately forming the NPs-aPD-1/HbO2/ATO. Within this NP, we take full advantage of HbO2 for oxygen delivery while introducing ATO to inhibit tumor oxygen consumption, thereby achieving a highly effective reversal of tumor hypoxia. It is noteworthy that ATO also possesses the ability to increase tumor ROS, further enhancing the efficiency of ROS linker-responsive release and improving the specificity of the encapsulated drug accumulation. With the aid of this intelligent aggregate, the challenges posed by the short circulation time and low efficiency of delivering oxygen using Hb alone have been ingeniously addressed. Simultaneously, the targeting specificity and target concentration of ATO have been significantly improved. Moreover, this smart aggregate not only overcomes the low bioavailability and insufficient effective concentration issues associated with individual HBO2, ATO and PD-1 delivery but also addresses the systemic toxicity problems of each drug, fully demonstrating the principle that the whole is greater than the sum of its parts (Scheme 1).
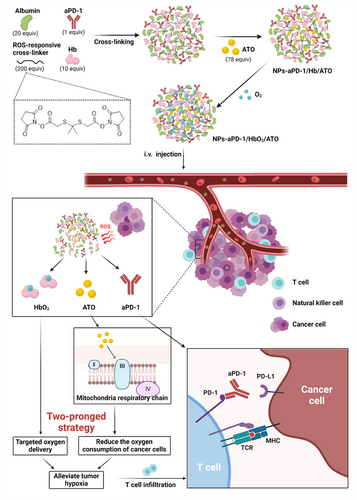
2 RESULTS AND DISCUSSION
2.1 Design and characterization of NPs-aPD-1/HbO2/ATO
The antimalarial drug atovaquone (ATO) enters complex III and blocks mitochondrial respiration.[24] Numerous studies have demonstrated that ATO partially relieves tumor hypoxia in patients with non-small cell lung cancer and reduces the oxygen consumption of tumor cells in vitro and in vivo.[25-28] Despite the long record of clinical use of ATO, it frequently causes adverse effects, which are all related to its high lipophilicity and lack of specific targeting ability.[19-21] Although Hb has been identified as an efficient oxygen donor, its short circulation time, susceptibility to oxidation-induced inactivation and damage to the heart and kidneys severely limit its further application.[23, 29] Considering the higher ROS level in tumors than in normal tissues, a ROS-responsive aggregate was designed to address the aforementioned challenges by virtue of its ROS-controlled release property and distinct nano-accumulation effect.[30, 31] The ROS-responsive cross-linker used in this study, N-hydroxysuccinimide disubstituted 2,2′-[propane-2,2-diylbis(thio)]diacetic acid, was synthesized according to a reported procedure.[32] The linker, as a representative thioketal compound, has been demonstrated to be susceptible to ROS, resulting in the removal of the ester groups and leaving only the thiol group on the cargoes after cleavage, thus minimizing the impact on the activity of therapeutic molecules.[32, 33] Then, through amide formation reactions, the amino groups on the side chains of albumin, aPD-1 and Hb can be linked to the ROS-responsive cross-linker, allowing the construction of a nanocomplex. Subsequently, ATO was compatibly incorporated into the hydrophobic structure of the NPs by non-covalent interactions to finally form the smart aggregate, NPs-aPD-1/Hb/ATO (Scheme 1). Of note, before use, NPs-aPD-1/Hb/ATO needs to be oxygen-saturated.
After building this aggregate, the fundamental morphology and size were characterized by performing transmission electron microscopy (TEM) imaging and dynamic light scattering (DLS) analysis. As shown in Figure 1A,B, NPs-aPD-1/HbO2/ATO were uniformly spherical with a diameter of 175.23 ± 22.89 nm. The polydispersity index (PDI: 0.22 ± 0.04) from DLS also indicates good uniformity. Next, the encapsulation rates of aPD-1 and ATO were measured by enzyme-linked immunosorbent assay (ELISA) analysis (data not shown) and high-performance liquid chromatography (HPLC) analysis (Figure S1), respectively. The encapsulation rates of aPD-1 and ATO were calculated as 73.73 ± 3.70% and 67.20 ± 1.51%, respectively. Additionally, the result of the encapsulation rate of aPD-1 was also supported by the result of western blotting (78.83 ± 6.79%, Figure S2). Moreover, the absorbance detection of Hb at 540 nm and standard curve (Figure S3) were performed, and the Hb encapsulation rate was up to 79.09 ± 4.00%.
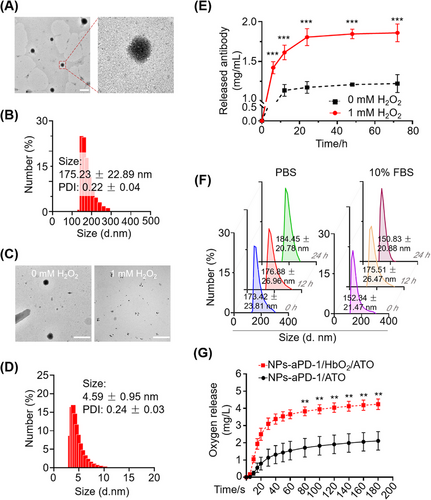
To investigate the response of NPs-aPD-1/Hb/ATO to ROS, we performed TEM imaging and diameter detection of NPs-aPD-1/HbO2/ATO before and after H2O2 treatments. According to the TEM images and particle size in Figure 1C,D, the NPs-aPD-1/HbO2/ATO lost their structural integrity following exposure to 1 mM H2O2 for 30 min. Meanwhile, the supernatants of the NPs-aPD-1/HbO2/ATO solution added with H2O2 at different times were subjected to SDS-PAGE gel electrophoresis and silver staining. It was found that the protein components, including albumin, aPD-1 and Hb, were significantly present in the supernatant (Figure S4), suggesting the achievement of highly efficient ROS-responsive controlled release. Moreover, the supernatants were detected by ELISA against IgG, which further confirmed the excellent release capability of NPs-aPD-1/HbO2/ATO when exposed to ROS, e.g., H2O2 (Figure 1E). However, according to the DLS analysis, the NPs-aPD-1/HbO2/ATO remained structurally stable for 24 h in PBS and 10% Fetal Bovine Serum (FBS), respectively (Figure 1F). Via silver staining of SDS-PAGE and HPLC experiments, it was found that the major components in the NPs, including aPD-1, Hb and ATO, were not released during 24 h observation (Figure S5).
Additionally, we investigated the oxygen delivery ability of NPs-aPD-1/HbO2/ATO by measuring the oxygen release profile. As shown in Figure 1G, compared with NPs-aPD-1/ATO without Hb, NPs-aPD-1/HbO2/ATO significantly increased the concentration of dissolved oxygen in the deoxygenated PBS, demonstrating the excellent biological activity of Hb within NPs-aPD-1/HbO2/ATO in terms of oxygen transport and release.
2.2 In vivo distribution of NPs-aPD-1/HbO2/ATO
In order to investigate the in vivo distribution of NPs-aPD-1/HbO2/ATO, we established a mouse subcutaneous xenograft model. Albumin labeled with Cy5 (Cy5-albumin) was used to replace the normal albumin to build a new NPs-aPD-1/HbO2/ATO, named Cy5-NPs-aPD-1/HbO2/ATO. The tumor-bearing mice were then randomly divided into two groups and treated with Cy5-NPs-aPD-1/HbO2/ATO and Cy5-albumin through tail vein injections (2.4 mmol Cy5 for each mouse), respectively. Then all treated mice were imaged at the predetermined time points to collect the fluorescence signals. As shown in Figure 2A,B, the fluorescent signals in Cy5-NPs-aPD-1/HbO2/ATO-treated mice quickly appeared in the tumor region and reached a peak at 8 h, followed by a slow decline. Notably, at 48 h after Cy5-NPs-aPD-1/HbO2/ATO treatment, the fluorescence intensity of tumors only decreased by 40% compared to its peak intensity at 8 h. Even at the time point of 72 h, a remarkably strong signal was still detectable at the tumor sites of the mice treated with Cy5-NPs-aPD-1/HbO2/ATO. In contrast, free Cy5-albumin was mainly distributed in the liver instead of the tumor area. At the time point of 48 h, the fluorescence signal of the tumor region in Cy5-NPs-aPD-1/HbO2/ATO-treated mice was ≈13 times stronger than that in Cy5-albumin-treated mice. These results strongly emphasize the pronounced advantage of efficient tumor enrichment achieved by this smart aggregate. Besides, from the organ distribution of the fluorescence signal (24 h post injection) shown in Figure 2C,D, the fluorescence signals were mainly localized within the tumors of the mice treated with Cy5-NPs-aPD-1/HbO2/ATO and were significantly higher than those of other organs. Particularly, the fluorescence signal in the tumor surpassed that in the liver by more than 3.4 times, which is relatively challenging to achieve in terms of nanodelivery. These results indicate that this smart aggregate exhibits a high degree of tumor targeting and retention capacity, highly facilitating the bioavailability and effectiveness of the encapsulated drugs.
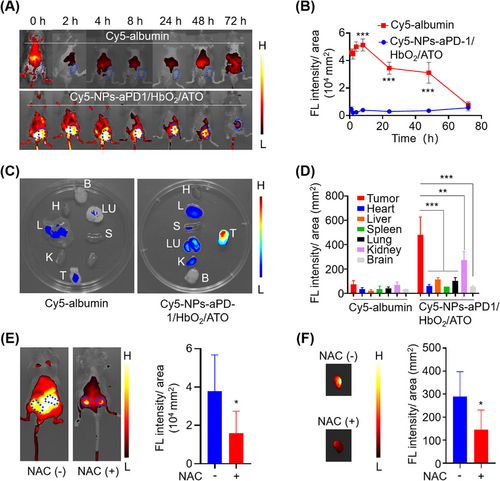
Numerous studies have extensively reported that ATO significantly amplifies mitochondria-derived ROS by several hundredfold.[27, 34] This is likely to allow the TME to work as a catalyst for the breakdown of ROS-responsive cross-linkers. To investigate whether the preferential selectivity of NPs-aPD-1/HbO2/ATO to tumor tissues was up to the ROS environment induced by ATO, we neutralized the ROS with N-acetylcysteine (NAC) in vivo and then examined the distribution of Cy5-NPs-aPD-1/HbO2/ATO again. As shown in Figure 2E, the fluorescence intensity of Cy5 in the subcutaneous tumors was much lower in mice given NAC by nearly 2.4-fold than in mice not given NAC. In vitro imaging of the separated tumors also supported this finding (Figure 2F). Therefore, ATO released from ROS-depolymerized NPs-aPD-1/HbO2/ATO induces a heightened production of ROS by cancer cells, which improves the responsive efficiency of the ROS-responsive cross-linker and leads to further nanoparticle breakdown and drug release, including Hb, ATO and aPD-1. Thus, a positive feedback loop for consistent depolymerization and redistribution of the NPs-aPD-1/HbO2/ATO within tumors has been established successfully. This partially elucidates the reasons for the efficient tumor enrichment and accumulation mentioned above. Consequently, we underscore the pivotal role of ATO in augmenting targeted accumulation in tumors when employing a ROS-responsive nanodelivery system.
2.3 NPs-aPD-1/HbO2/ATO inhibit tumor growth in vitro and in vivo
ATO has shown the capacity to delay tumor growth by inhibiting oxygen consumption by interfering with the function of the mitochondrial respiratory chain and increasing ROS generation. Therefore, we subsequently investigated if ATO encapsulated in aggregates still preserved its pharmacological activity. It is noted that cancer cells in vitro typically exhibit a significant amount of ROS compared to non-cancer cells (Figure S6),[35] which could facilitate the initiated release of ATO from ROS-responsive smart aggregates (NPs-aPD-1/HbO2/ATO). Briefly, MC38 (a mouse colorectal carcinoma cell line) cells were subjected to cell proliferation assays under the treatment of NPs-aPD-1/HbO2 (ATO: 0 µg/mL) and different doses of NPs-aPD-1/HbO2/ATO (ATO: 0.19–3.00 µg/mL). Based on the findings from Figure S7, compared with NPs-aPD-1/HbO2, NPs-aPD-1/HbO2/ATO significantly inhibited the colony formation of MC38 cells in a dose-dependent manner but had marginal effects on 293T and NIH3T3 cells (commonly used non-cancer cell lines). To provide further evidence that ATO impedes tumor growth by reducing oxygen consumption, enzymatic activity assays were performed on mitochondrial complex III using MC38 cells after corresponding treatments. As displayed in Figure S8, NPs-aPD-1/HbO2/ATO remarkably inhibited the activity of mitochondrial complex III compared to the groups treated with PBS and NPs-aPD-1/HbO2. The findings demonstrated that NPs-aPD-1/HbO2/ATO can release ATO in the endogenous ROS environment of cancer cells and effectively initiate the inhibitory function of ATO on cellular oxygen consumption.
Next, to explore the in vivo effect of NPs-aPD-1/HbO2/ATO on the TME and cancer immunity, tumor-bearing mice were randomly assigned to 7 groups, namely “PBS”, “ATO”, “NPs-IgG/HbO2/ATO”, “aPD-1”, “aPD-1/HbO2/ATO”, “NPs-aPD-1/HbO2” and “NPs-aPD-1/HbO2/ATO” groups (Figure 3A). The grouping nomenclature directly reflects the drugs used in each group. In the “NPs-IgG/HbO2/ATO” group, IgG was used instead of aPD-1 in the nanocomplex. All tumor-bearing mice received intravenous injections of corresponding drugs three times with a two-day interval between each injection and carefully monitored the tumor growth until day 15. Then all mice were sacrificed, and tumor tissues were collected for further analysis. As depicted in Figure 3B-D, there was no significant difference observed in relative tumor volume or weight between the “ATO” and “PBS” groups. However, in comparison to both of these groups, tumors treated with NPs-IgG/HbO2/ATO exhibited slower growth and a lower weight. These results suggest that the efficient delivery and release of ATO were facilitated by the ROS-responsive aggregate formulation (NPs-IgG/HbO2/ATO). In comparison with “PBS”, “ATO” and “NPs-IgG/HbO2/ATO” groups, the growth of the tumors in the “aPD-1” and “aPD-1/HbO2/ATO” groups (without forming nanoaggregates) was partially inhibited. Nevertheless, their therapeutic effects were still markedly inferior compared to the groups treated with nano-sized aPD-1 formulations, including NPs-aPD-1/HbO2 and NPs-aPD-1/HbO2/ATO. In particular, the mice treated with NPs-aPD-1/HbO2/ATO obtained the largest tumor-inhibitory effect, revealing the importance of simultaneous use of ATO and Hb in maximizing the efficacy of combined aPD-1 immunotherapy.
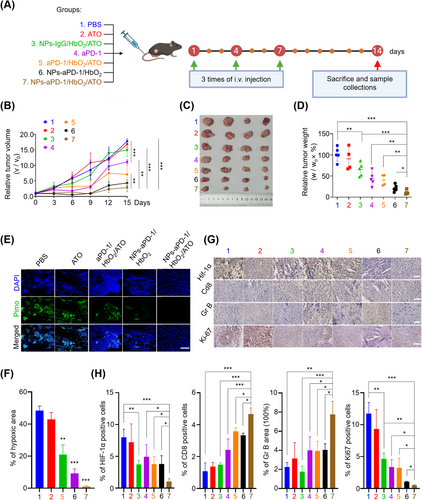
To further illustrate the mechanism by which ATO and HbO2 enhance the efficacy of aPD-1, we performed pimonidazole staining to visualize the hypoxic region of tumor tissues. As depicted in Figure 3E,F, in comparison to PBS, free ATO and the combined treatment of aPD-1/HbO2/ATO, the administration of NPs-aPD-1/HbO2 and NPs-aPD-1/HbO2/ATO substantially mitigated tissue hypoxia. Notably, the largest reversal of tumor hypoxia was achieved by NPs-aPD-1/HbO2/ATO, which almost completely eliminated the positive signal of pimonidazole in the tissues. The immunohistochemistry (IHC) analysis of hypoxia-inducible factor 1-alpha (HIF-1α) that positively correlated with hypoxia was also performed. As illustrated in Figure 3G (1st line) and Figure 3H (left 1st), the tumors within the “NPs-aPD-1/HbO2/ATO” group manifested minimal positive signals, aligning well with the observations in Figure 3E. Moreover, CD8 and GrB staining that revealed the infiltration and activity of cytotoxic T lymphocytes (CTLs) were conducted to assess the tumor conversion from immunogenic “cold” to “hot”. Based on the findings, the staining intensities of the tumors that underwent aPD-1-involved treatments, including aPD-1, aPD-1/HbO2/ATO, NPs-aPD-1/HbO2 and NPs-aPD-1/HbO2/ATO, were significantly stronger than those of non-aPD-1 treatments, in other words, PBS, ATO and NPs-IgG/HbO2/ATO. As expected, tumors treated with NPs-aPD-1/HbO2/ATO displayed significantly more prominent staining of CD8 and GrB (Figure 3G, second and third lines and Figure 3H, left second and third). This indicates the possibility of optimizing tumor immunity from “cold” to “hot”, which is a crucial determinant for enhancing the efficiency of aPD-1 immunotherapy. Additionally, the proliferation marker Ki-67 was highly expressed in tumors treated with PBS and ATO, whereas it was dramatically reduced in the other groups. Notably, tumors treated with NPs-aPD-1/HbO2/ATO showed an almost absence of Ki-67 signals (Figure 3G, bottom line and Figure 3H, right 1st). Collectively, these findings underscore the crucial role of co-delivery of HbO2 and ATO by this intelligent aggregate to effectively alleviate tumor hypoxia, optimize tumor immunity and significantly revitalize aPD-1 immunotherapy.
2.4 NPs-aPD-1/HbO2/ΑTO inhibit tumor metastasis in vivo
As previously established, hypoxia facilitates cancer cells to escape from immunity.[36] In addition, recent studies have demonstrated that metastatic cancer cells block the recruitment of CTLs by myeloid cells through PD-L1/PD-1 signaling, thereby escaping immunity.[1] Given this information, we intend to further explore the effects of NPs-aPD-1/HbO2/ATO on the immune escape of metastatic cancer cells. We established a lung metastasis model via tail vein injection of the melanoma cell B16, which was then subjected to different treatments (Figure 4A). At the end of the experiment, the lungs of mice treated with PBS or ATO were almost occupied by melanoma cells, and the tumor burden score (TBS) was the highest; the TBS of lungs in the group treated with NPs-IgG/HbO2/ATO, aPD-1 and aPD-1/HbO2/ATO was intermediate; whereas the lungs treated with NPs-aPD-1/HbO2 and NPs-aPD-1/HbO2/ATO attended rare melanoma foci, and TBS was the lowest (Figure 4B and Figure S9). Moreover, according to the results of pimonidazole and HIF-1α staining, treatments of HbO2 or/and ATO in nano-sized regimens, such as NPs-IgG/HbO2, NPs-αPD-1/HbO2 and NPs-αPD-1/HbO2/ATO, significantly alleviated the hypoxia of metastatic cancers when compared to other treatments (Figure 4C–E and Figure S10). In comparison to the treatments with free aPD-1, the lungs of the mice treated with NPs-aPD-1/HbO2/ATO demonstrated the most significant CD8 and granzyme B (Gr B) staining, indicating that the infiltration and activity of CTLs were the most prevalent (Figure 4D,E). Correspondingly, Ki-67 staining was drastically suppressed in NPs-aPD-1/HbO2/ATO treated metastatic tissues compared to the others, indicating that the growth of metastatic cells was maximally inhibited (Figure 4D,E). These findings confirmed the inhibitory effects of NPs-aPD-1/HbO2/ATO on cancer metastasis and indicated that the responsiveness was reliant on the usage of aPD-1 or the targeted delivery of ATO, while the alleviation of hypoxia via co-delivery of HbO2 and ATO strengthened the efficacy of the aPD-1 treatment.
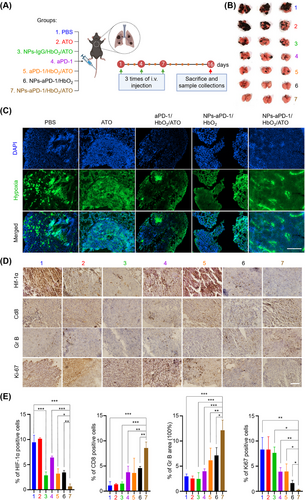
In both the subcutaneous xenograft tumor and cancer metastasis models, the cancer inhibitory effects of single ATO treatment were less pronounced compared to nano-sized regimens of ATO. This provided solid evidence that targeted delivery and TME-specific release of ATO significantly enhanced its bioavailability. The advantages of hypoxia-targeted cancer therapy have been extensively investigated.[37-39] According to our findings, the tumor-targeted delivery of ATO and HbO2 significantly alleviated the hypoxia of tumors, on the basis of which aPD-1 successfully activated tumor immunity, further highlighting its practical role in immunotherapy. In addition, NPs-IgG/HbO2/ΑTO inhibited tumor growth independently of aPD-1, presumably associating with the oxidative damage induced by ROS generation via ATO-mediated inhibition of mitochondrial complex III. The enhanced immune response observed in NPs-aPD-1/HbO2/ATO-treated cancer tissues compared to NPs-aPD-1/HbO2-treated tissues underscores the pivotal role of ATO. This multifaceted role involves synergistically alleviating hypoxia by reducing oxygen consumption, facilitating the release of components from the ROS-responsive aggregate through elevated tumor ROS levels, and potentially contributing to antigen release via oxidative damage.[40] Therefore, the exploration of these hypotheses is poised to offer valuable insights for subsequent research endeavors.
2.5 Biological safety of NPs-aPD-1/HbO2/ΑTO
To assess the biological safety of the different treatments involved in this study, we periodically recorded the body weight of the mice during the experiment. According to the body weight curve, there was no significant difference in mice's body weight across the various treatments (Figure S11), suggesting that none of the treatments in this study had any serious toxic or adverse consequences. Additionally, the tissue sections of the heart, liver, spleen, lung, kidney and other organs were subjected to H&E staining and microscope analysis, and there was no evidence of inflammation or injury in these organs in the mice given different treatments (Figure 5A). However, we found that aPD-1 and its combination with HbO2 and ATO resulted in an abnormal rise in blood alanine transaminase (ALT) and creatinine (sCr), indicating that these two treatments may have potential effects on liver and kidney function (Figure 5B). Of particular significance is the fact that no abnormal alterations in liver and kidney tests were found in the animals given the same dose of treatment in nano-sized regimens (Figure 5B).
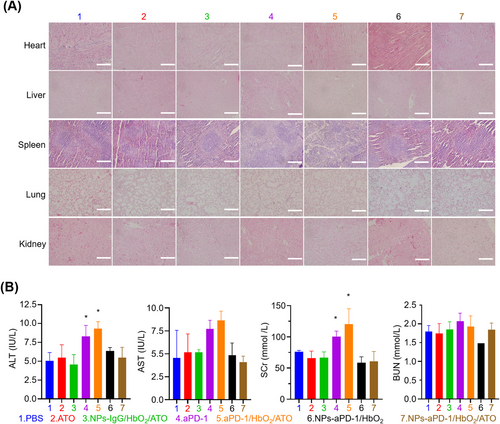
Organ failures brought on by aPD-1 therapy-induced cytokine storm syndrome can occasionally be fatal,[41] while such extreme situations were completely avoided in our investigation. Moreover, although the use of aPD-1 and the combination of aPD-1 and ATO did provoke some degree of liver and renal injury, the mice treated with nano-sized regimens of aPD-1 and ATO did not exhibit this sort of condition. This fact further confirmed the significance of targeted delivery for aPD-1 and ATO-related treatments in terms of minimizing systemic side effects.
3 CONCLUSIONS
Herein, for the first time, we propose a two-pronged strategy to ameliorate hypoxia: ATO is employed to block tumor oxygen consumption, while Hb is utilized to directly transport oxygen to the tumor. Notably, we have developed an intelligently responsive multifunctional aggregate through a one-step approach to deliver ATO, Hb and aPD-1 with ROS-responsive cross-linkers. The aggregate addresses critical challenges associated with the independent administration of ATO, Hb and aPD-1, including low bioavailability and high systemic toxicity. Moreover, this aggregate achieves exceptionally high tumor-targeted delivery efficiency and prolonged retention time, representing one of the most promising outcomes reported to date based on ROS nanodelivery strategies. On the basis of these advantages, each component in the aggregate successfully fulfills its obligation, significantly reducing the hypoxia of tumor tissues and thus maximizing the performance of immunotherapy in a safe mode.
4 EXPERIMENTAL SECTION
4.1 Synthesis of ROS-responsive cross-linkers and NPs-aPD-1/HbO2/ATO
ROS-responsive cross-linker was synthesized according to the previous study.[15] Briefly, N-hydroxysuccinimide (NHS) (5.1 mg), 1-ethyl-(3-dimethylaminopropyl) carbodiimide (EDC) (15.6 µL) and 2,2′-[propane-2,2-diylbis(thio)]diacetic acid (5.0 mg) (Tianjin Kailiqi Biopharma Technology) were mixed in dimethyl sulfoxide (DMSO) and stirred for 6 h at room temperature. To prepare the NPs-aPD-1/HbO2/ATO, Hb (7.5 mg) (Solarbio, China) was pretreated with vitamin C (230 µL of 100 mm) for 4 h. The processed Hb was added to PBS together with BSA (15 mg) (Solarbio, China) and aPD-1 (2.3 mg) (cat. no. BE0237, Clone: 29F.1A12, Bioxcell, USA) and mixed well. Then ATO (6.67 g/L, 50 µL) (Aladdin, China) and ROS-responsive cross-linker (20 µL) dissolved in DMSO were slowly added to the mixture and reacted overnight at 4°C. After the reaction, the free reagents, such as the linkers and ATO, were removed by dialysis (MWCO: 10 kDa) for 12 h. The products were lyophilized and reconstituted to the required volume and concentration. In order to oxygenate Hb, oxygen was introduced into the NPs before use. NPs-IgG/HbO2/ATO, NPs-aPD-1/ATO and NPs-aPD-1/HbO2 used as control complexes were prepared using mouse IgG to replace aPD-1 or synthesized without Hb or ATO following the same procedure.
4.2 Morphology, ROS responsiveness and stability of NPs-aPD-1/HbO2/ATO
In order to determine the structure and size of NPs-aPD-1/HbO2/ATO, we performed transmission electron microscopy (TEM) and dynamic light scattering analysis. Typically, NPs were filtered with a 0.45 µm filter, mounted on the carbon film-coated grid and allowed to naturally dry before being analyzed using an TEM (JEOL JEM-1200EX, Tokyo, Japan). Zetasizer Nano (Malvern Instrument, Worcestershire, UK) was used to further determine the average size and polydispersity index (PDI) of NPs. To evaluate the effects of ROS on the nanostructures, NPs-aPD-1/HbO2/ATO with and without 1 mM H2O2 treatment were compared morphologically and in size using TEM and Zetasizer Nano. In order to assess the stability of NPs-aPD-1/HbO2/ATO, PBS and 10% FBS were used as comparative solvent environments. After fully dispersion, NPs-aPD-1/HbO2/ATO were kept at 4°C for 24 h and DLS was used to assess the changes in particle size. All measurements were performed in triplicate at 25°C.
4.3 Encapsulation efficiency
To evaluate the encapsulation efficiency of ATO, NPs-aPD-1/HbO2/ATO (1.70 mg/mL aPD and 262.8 µg/mL ATO) were subjected to treatment with 1 mm H2O2 for 12 h, and the supernatants after the reaction were determined by a HPLC system. Briefly, HPLC analyses were carried out on a C18 column (Waters XBridge, 4.6 × 150 mm, 3.5 µm) running for 22 min at 40°C, using 10% acetonitrile in water containing 0.1% TFA for the first 10 min, followed by 90% acetonitrile for the next 5 min and 10% acetonitrile for the last 7 min at a flow rate of 1 mL/min. The amount of aPD-1 within the supernatant of NPs-aPD-1/HbO2/ATO was measured by both the ELISA Mouse IgG ELISA Kit (NeoBioscience Technology Co., Ltd., Shenzhen, China) and western blotting (Supporting Information, Materials and Methods). To examine the amount of Hb in NPs, we detected the absorbance of Hb standards of different concentrations at 540 nm. The amount of Hb in NPs-aPD-1/HbO2/ATO was calculated according to the standard curve. The ATO, Hb and aPD-1 encapsulation efficiency were then calculated using the formula below: Encapsulation efficiency (EE%) = encapsulated content of a reagent / total amount of the reagent added during synthesis ×100%.
4.4 Oxygen release profile of NPs-aPD-1/HbO2/ATO
To characterize the oxygen release behavior of NPs-aPD-1/Hb/ATO, a comparative analysis was conducted using NPs-aPD-1/ATO as a control. Both NPs were subjected to oxygenation by aeration. The PBS buffer was deoxygenated under 1% oxygen for 15 h in a hypoxic workstation (Ruskinn Technology Ltd., UK). The oxygen-charged NPs-aPD-1/Hb/ATO and NPs-aPD-1/ATO (1.70 mg aPD-1, 262.8 µg ATO and 5.90 mg Hb) were transferred to 15 mL of deoxygenated PBS, respectively. An optical luminescent dissolved oxygen sensor (Chongqing Xuetong Instrument, China) was used to detect and record the dissolved oxygen in PBS at different time points.
4.5 Cell culture
The murine colon cancer cell line MC38, human embryonic kidney 293 cell line HEK293T, mouse embryonic fibroblasts NIH3T3 and melanoma cell line B16F10 were provided by the American Type Culture Collection (ATCC; Manassas, VA, USA). Cell lines were cultured at 37°C and 5% CO2 in Dulbecco's Modified Eagle Medium (DMEM) (Gibco, Grand Island, NY) medium containing 100 U/mL penicillin and 10% fetal bovine serum (FBA). Cell passage and mycoplasma removal were done regularly.
4.6 In vivo distribution of NPs-aPD-1/HbO2/ATO
In order to investigate the in vivo distribution of NPs-aPD-1/HbO2/ATO, we first modified BSA with sulfo-cyanine-5 maleimide (Lumiprobe, Hong Kong, China), which was named Cy5-BSA. To be specific, BSA and sulfo-cyanine-5 maleimide (1:10 in molecules) were mixed in PBS buffer and stirred at 4°C overnight, followed by dialysis in PBS for 6 h (MWCO: 10 kDa). The fluorescence intensity of the dialyzed product and the source reagent, sulfo-Cyanine-5 maleimide, were detected using a BioTek Cytation 5 Multimode Reader (Agilent, USA). The fluorescent percent that Cy5-BSA took in the source reagent was over 95%, which was considered successful, and the products were used for the further experiments. Cy5-NPs-aPD-1/HbO2/ATO were synthesized with Cy5-BSA following the same processes depicted above.
C57BL/6 mice were injected with 1 × 106 MC38 cells subcutaneously. After the neoplasm formation, the mice were randomly grouped and administered tail vein injections of Cy5-BSA and Cy5-NPs-aPD-1/HbO2/ATO, respectively (both contain 2.4 mmol Cy5). Before the treatment, the mice's hair was shaved for better penetration depth. The IVIS Spectrum instrument (Perkin Elmer, USA) was used to detect and record the fluorescence intensity of the subcutaneous tumor region at different times, developing the temporal variation curves. 24 h after tail vein injections, some of the mice were euthanized and dissected for the major internal organs and tumor tissues, and the fluorescence intensity of the separated tissues was detected in vitro. To investigate the effect of ROS on the in vivo distribution of NPs-aPD-1/HbO2/ATO, 100 mg/kg of NAC was injected intraperitoneally 24 h before Cy5-NPs-aPD-1/HbO2/ATO administration, and fluorescence distribution analysis of the tumors was performed in vivo and ex vivo 8 h after Cy5-NPs-aPD-1/HbO2/ATO administration.
4.7 Animal studies
C57BL/6 female mice of 6–10 weeks of age were provided by the Laboratory Animal Center, Xi'an Jiaotong University. All animal studies were conducted with the approval of the Animal Ethics Committee at Xi'an Jiaotong University (No. XJTUAE2023-2093). In accordance with Swiss guidelines, all mice were kept in a certified animal facility with a 12-h light/dark cycle in a temperature-controlled room (25 ± 1°C) with unrestricted access to water and food.
For the xenograft models, mice were subcutaneously injected with 1 × 106 MC38 cells to allow the growth of tumors. When the tumor volume reached 50–100 mm3, mice were randomly divided into seven groups and administered three tail vein injections containing: (1) PBS, (2) ATO (4.2 mg/kg), (3) NPs-IgG/HbO2/ATO (ATO: 0.35 µmol/kg, IgG: 5 mg/kg), (4) aPD-1 (5 mg/kg), (5) aPD-1/HbO2/ATO (ATO: 0.35 µmol/kg, aPD-1: 5 mg/kg), (6) NPs-aPD-1/HbO2 (ATO: 0.35 µmol/kg, aPD-1: 5 mg/kg) and (7) NPs-aPD-1/HbO2/ATO (ATO: 0.35 µmol/kg, aPD-1: 5 mg/kg) for each injection at three days-interval. During the process, we measured the diameters of the xenograft tumors every two days to estimate tumor volume and generate tumor growth curves. The volume of tumors was calculated by the equation: volume = length × width2/2. The relative tumor volume = v/vo, where v was the tumor volume at a different day, vo was the tumor volume at the first day. We also plotted the body weights of the mice. At the end of the experiment, the peripheral blood of the mice was collected for serum isolation, then the mice were euthanized, and the tumors were removed, pictured and weighed. The relative tumor weight was w/wo, where wo was the mean value of the PBS-treated tumor weights and w was the weight of the other tumors on the 15th day.
For the tumor metastasis models, mice were tail vein injected with 1 × 106 B16 cells to facilitate the establishment and growth of lung metastases. On the 14th day after tumor cell injection, mice were randomly assigned to one of the seven groups and exposed to the aforementioned treatments. At the end of the experiment, the lung tissues were separated and photographed. The TBS of the lungs was calculated as follows: TBS2 = (maximum tumor diameter)2 + (number of tumors)2. Major organs (heart, liver, spleen, lung and kidney) were also separated. All samples harvested were properly processed and kept for future use.
4.8 Assessment of tissue hypoxia
Pimonidazole hydrochloride (HypoxyprobeTM RedAPC Kit, Hypoxyprobe, USA) was administered intravenously for 90 min before tumor isolation at a dose of 60 mg/kg. Tumor tissues were paraffin-embedded and sectioned according to standard procedures. After deparaffination, hydration and blocking, tissue sections were stained with anti-pimonidazole antibody (PAb27, Hypoxyprobe, USA) and goat anti-rabbit polyclonal immunoglobulin G Alexa Fluor® 488-coupled secondary antibody (1:1000, Abcam, USA). DAPI (ThermoFisher, Beijing, China) was used to stain the nuclei. The slices were imaged using a confocal microscope (Leica, Leica Microsystems). Quantitative analysis of fluorescence pictures was done by ImageJ.
4.9 Histological analyses
The isolated tumor and visceral tissues were fixed at room temperature in a formaldehyde solution, followed by dehydration, paraffin embedding and sectioning. Hematoxylin and eosin (H&E) staining was performed to evaluate the morphological changes, and IHC was carried out to assess the expression of the antigens concerned. Briefly, after deparaffination, hydration and blocking, tumor tissue sections were stained with anti-Ki67 (ab15580, Abcam, Cambridge, UK), anti-CD8 (sc-7970, Santa Cruz Biotech, Texas, U.S.A.), anti-Gr B (44153, Cell Signaling, Massachusetts, U.S.A.) and anti-HIF-1α (NB100-134, Novus Biologicals, Minnesota, USA), respectively, and subjected to corresponding secondary antibodies and color development with the DAB kit (Zhongshan Golden Bridge, Beijing, China). After hematoxylin counterstaining, dehydration and sealing of the sections, Olympus DP71 microscopes were used to take pictures. Quantitative analysis of IHC pictures was done by ImageJ.
4.10 Liver and kidney testes
Serum ALT and aspartate transaminase (AST) activities, sCr and urea nitrogen (BUN) concentrations were determined using commercially available kits (Cusabio, China) following the manufacturer's instructions.
4.11 Statistical analysis
Statistical analysis was performed using SPSS19.0 software, and all results are shown as mean ± standard deviation. An unpaired two-tailed t-test was used for two group comparisons. ***p < 0.001; **p < 0.01; *p < 0.05.
ACKNOWLEDGEMENTS
The authors thank Ms. Mengjun Sui and Mr. Simeng Wang (The First Affiliated Hospital of Xi'an Jiaotong University) for helping with nanotechnology. This work was supported by the National Natural Science Foundation of China (Nos. 82073058, 32371449), Basic Research Projects of the Natural Science Foundation of Shaanxi Province (key program) (2021JZ-37), Youth Cultivation Project of the First Affiliated Hospital of Xi'an Jiaotong University (No. 2019QN-02), Nanjing Tianqing Research Fund Project (No. HX202324), and the Clinical Research Award of the First Affiliated Hospital of Xi'an Jiaotong University, China (No. XJTU1AF-CRF-2022-009).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest




