Microalbuminuria sensitive near-infrared AIE probe for point-of-care evaluating kidney diseases
Abstract
Urinary microalbumin (mALB) serves as an exceptionally sensitive indicator for the early detection of kidney damage, playing a pivotal role in identifying chronic renal failure and kidney lesions in individuals. Nevertheless, the current fluorescent methodologies for point-of-care (POC) diagnosis of mALB in real urine still exhibit suboptimal performance. Herein, the development and synthesis of QM-N2, an albumin-activated near-infrared (NIR) aggregation-induced emission (AIE) fluorescent probe, are presented. The strategic incorporation and positioning of quaternary ammonium salts within the quinoline-malononitrile (QM) scaffold significantly influence solubility and luminescence characteristics. Specifically, the quaternary ammonium salt-free variant, QM-OH, and the quaternary ammonium salt integrated at the donor function group (DFG) site, QM-N1, display limited solubility in aqueous solutions while demonstrating a distinct fluorescence signal. Conversely, the incorporation of quaternary ammonium salt at the conformational functional group (CFG) site in QM-N2 imparts superior dispersibility in water and reduces the initial fluorescence. Furthermore, the integration of a well-defined D-π-A structure within QM-N2 enables itself with near-infrared emission, which is crucial for mitigating interference from autofluorescence present in urine samples. Upon interaction with albumin, QM-N2 forms a tight bond with the IIA site of the subdomain of human serum albumin (HSA), inducing alterations in protein configuration and constraining the intrinsic motion of fluorescent molecules. This interaction induces fluorescence, facilitating the sensitive detection of trace albumin. Ultimately, QM-N2 is applied for POC testing of mALB using portable equipment, particularly in the diagnosis of mALB-related diseases, notably chronic renal failure. This positioning underscores its potential as an ideal candidate for self-health measurement at home or in community hospitals.
1 INTRODUCTION
Urinary proteins consist of a variety of components, including albumin, microglobulin, macroglobulin, transferrin, and other proteins, with albumin constituting approximately 60% of the total urinary protein. Especially in the initial phases of glomerular diseases, such as early-stage diabetic nephropathy, early-stage hypertensive nephropathy, and early-stage chronic renal failure, the predominant protein found in urine is primarily albumin.[1, 2] As a consequence, the prompt assessment of the microalbumin (mALB) content in a patient's urine is imperative for the early detection of renal insufficiency.[3] Presently, the clinical detection of mALB predominantly relies on the enzyme-linked immunosorbent assay (ELISA).[4] Nonetheless, for routine testing, self-testing through point-of-care testing (POCT) is considered as the most pragmatic approach.[5] Therefore, there exists an urgent need to develop a highly sensitive probe to facilitate the transition from “clinical testing” to “home self-testing”.
In pursuit of this objective, numerous studies have harnessed fluorescence technology to advance the detection of urinary microalbumin.[6] By finely tuning the probe's dispersion and the nature of the recognition group, these aggregation-induced quenching (ACQ) molecules are designed to aggregate and extinguish fluorescence in the absence of albumin, and conversely, to disperse and rekindle fluorescence in the presence of albumin.[7] Nonetheless, the inherent propensity of these probes to aggregate poses challenges in the precise quantification of high concentrations of albumin. Furthermore, their relatively shorter emission wavelength complicates the analysis of real patient urine samples.[8] Consequently, the development of a high-performance probe capable of rectifying ACQ limitations and extending the emission wavelength is of paramount significance.[9]
Materials with aggregation-induced emission (AIE) characteristics are anticipated to address the aforementioned limitations effectively and are well-suited for the design of probes intended for the detection of urinary microalbumin content.[10-14] Consequently, building upon the AIE framework QM developed by our research group, this study synthesized QM-OH, QM-N1, and QM-N2, and systematically investigated the impact of introducing hydrophilic quaternary ammonium salt units and their substitution positions on fluorescence properties and microalbumin detection efficacy (Figure 1A). The findings reveal that QM-OH, lacking quaternary ammonium salt substitution, and QM-N1, with quaternary ammonium salt units substituted at the donor functional group (DFG) position, both exhibit varying degrees of aggregation in an aqueous environment, leading to pronounced fluorescence signals. By slightly adjusting the position of the quaternary ammonium salt unit to the conformational functional group (CFG) position within the molecule's main chain, QM-N2 attains favorable dispersion in the aqueous system, resulting in the absence of any fluorescence signal. This property is advantageous in minimizing initial fluorescence interference during detection.

Molecular docking results illustrate that QM-N2 can bind to the IIA site of the human serum albumin (HSA) subdomain, effectively constraining the intramolecular mobility of QM-N2 and resulting in fluorescence emission, thereby enabling the precise albumin detection. Furthermore, a strong positive correlation between the fluorescence intensity of QM-N2 and albumin concentration within the range of 0–8000 µg mL−1 is observed. QM-N2 was successfully employed for the detection of microalbumin (mALB) in urine samples, exhibiting a high level of consistency with the data obtained through conventional clinical testing methods. In addition, integration of the QM-N2 probe with a portable fluorescent device facilitates immediate mALB detection, thereby making a valuable contribution to the assessment of early kidney injury-related conditions, particularly in the early warning of chronic renal failure (Figure 1B).
2 RESULTS AND DISCUSSION
2.1 Design of hydrophilic AIE probe QM-N2 with NIR emissions
Hydrophilic aggregation-induced emission (AIE) probes display subdued fluorescence in aqueous environments and manifest strong fluorescence upon activation. This feature provides a substantial signal-to-noise ratio and high accuracy, rendering it highly suitable for point-of-care testing (POCT) of biological samples.[15-17] Typically, the integration of ionic or polar hydrophilic groups into AIE building blocks serves as a common strategy to create activatable hydrophilic AIE probes.[18-21] Moreover, probes with a positive charge are more favorable for binding with negatively charged albumin (isoelectric point 4.8) in physiological environments. Therefore, in this study, a systematic investigation was conducted to evaluate the influence of the presence and positioning of a quaternary ammonium salt unit with positive charges in QM derivatives on their fluorescence properties. As a result, three compounds, namely QM-OH, QM-N1, and QM-N2, were successfully synthesized (Figure 1A). QM-OH, lacking hydrophilic groups, displayed classical AIE properties with strong emission in aqueous environments. Subsequently, QM-N1 was synthesized by introducing a hydrophilic albumin recognition group in the form of a quaternary ammonium salt unit at the donor function group (DFG) site of QM. While this alteration marginally improved solubility, the photophysical AIE properties remained largely unchanged, and the compound maintained its ability to fluoresce in water. To address this limitation, we made a modification by relocating the hydrophilic albumin recognition group to the N-ethyl (CFG) site and introduced thiophene units to broaden the emission wavelength range. The resulting compound, QM-N2 (Figure 1), featured a D-π-A structure that extended the emission wavelength into the near-infrared (NIR), was conducted to evaluate the influence of the presence and positioning of a quaternary ammonium salt unit in QM derivatives on their fluorescence properties. As a result, three compounds, namely QM-OH, QM-N1, and QM-N2, were successfully synthesized (Figure 1A). QM-OH, lacking hydrophilic groups, displayed classical AIE properties with strong emission in aqueous environments. Subsequently, QM-N1 was synthesized by introducing a hydrophilic albumin recognition group in the form of a quaternary ammonium salt unit at the donor function group (DFG) site of QM. While this alteration marginally improved solubility, the photophysical AIE properties remained largely unchanged, and the compound maintained its ability to fluoresce in water. To address this limitation, we made a modification by relocating the hydrophilic albumin recognition group to the N-ethyl (CFG) site and introduced thiophene units to broaden the emission wavelength range. The resulting compound, QM-N2 (Figure 1), not only featured a D-π-A structure that extended the emission wavelength into the near-infrared (NIR) region but also demonstrated enhanced dispersibility in water. This unique behavior sets it apart from conventional aggregation-induced emission (AIE) dyes, as it displays a non-emissive state in water, which holds great promise for its applications in bio-detection. We systematically investigated the hydrophilicity, emission wavelength, and AIE properties of the three compounds, and comprehensive characterization data can be found in the supporting information (Figures S1 and S2).
2.2 Photophysical properties of QM-derivatives
First, the photophysical properties QM-OH and QM-N1 in a DMSO/water mixture, and QM-N2 in an EtOH/water solution, were systematically evaluated. Specifically, QM-OH exhibited absorption in the range of 300–500 nm (Figure 2A) and emitted light within the 500–700 nm range (Figure 2B), with a maximum emission wavelength at 560 nm. Additionally, QM-OH demonstrated traditional AIE behavior, as it dissolved well in DMSO without emitting fluorescence, but gradually formed aggregates with increasing water fraction, accompanied by enhanced fluorescence signals (Figure 2C). Upon the introduction of the quaternary ammonium salt unit at the donor functional group (DFG) site, a discernible improvement in the solubility of QM-N1 was observed, as evidenced by the higher absorbance values (Figure 2D). However, the photophysical properties of QM-N1 remained akin to those of QM-OH, exhibiting short emission wavelengths and enhanced fluorescence intensity with the increasing water content (Figure 2E,F). In the case of QM-N2, the incorporation of a thiophene unit resulted in the expansion of the π-system, consequently broadening the absorption range to 300–600 nm (Figure 2G), surpassing that of QM-N1. QM-N2 demonstrated a NIR emission spectrum ranging from 600 to 840 nm (Figure 2H), effectively minimizing potential interference caused by biological spontaneous fluorescence.[22-24] Significantly, the incorporation of the quaternary ammonium salt unit at the CFG site exerts a profound influence on the dispersibility of QM-N2 in water, enabling QM-N2 with initial fluorescence off state and continuously intensify the near-infrared fluorescence at 720 nm as the ethanol fraction (fe) progressively increases (Figure 2I). The aggregation behavior of QM-N1 and QM-N2 in the aqueous system was further investigated through dynamic light scattering (DLS) analysis. Figure S3 illustrates that QM-N2 has superior dispersibility in water compared to QM-N1, as evidenced by its smaller size distribution when water was the predominant solvent (Water:DMSO = 99:1, v/v). Based on these findings, we believe that the strategic placement of quaternary ammonium salt units at CFG site profoundly influenced the dispersion state of QM-based fluorescent molecules, rendering them highly suitable for a broad range of biological applications.
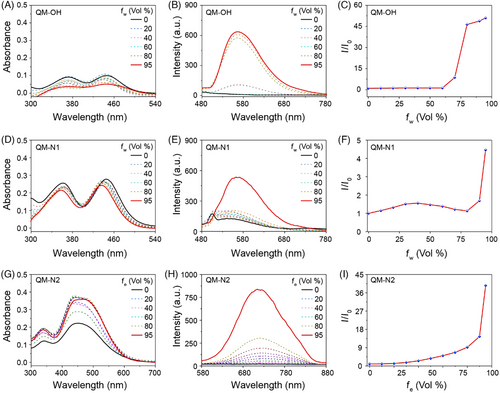
2.3 Detection performance and morphological analysis of QM-N2 in albumin sensing
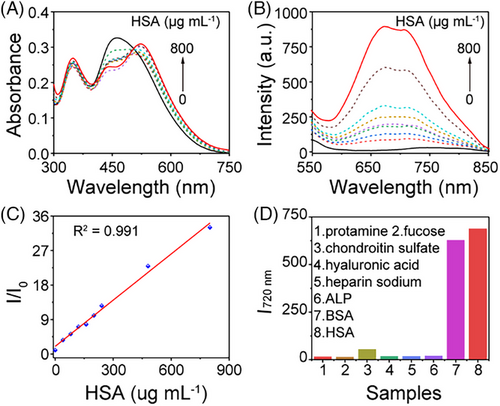
Microalbumin (mALB) has emerged as a pivotal biomarker for assessing renal parenchymal damage, exhibiting significant relevance to conditions such as chronic renal failure, diabetic nephropathy, hypertensive nephropathy, and systemic lupus erythematosus nephropathy.[25, 26] Therefore, our initial examination focused on the probe's responsiveness to albumin, with all experiments conducted in a PBS buffer at pH ≈ 7.4. In the case of probe QM-OH, the addition of BSA in the range of 0–8000 µg mL−1 resulted in a gradual increase in fluorescent intensity (500–660 nm) accompanied by a blue shift (Figure S4A). However, the initial fluorescence signal and its irregular response characteristics make this probe unsuitable for albumin sensing (Figure S4B,C). The response of QM-N1 to BSA exhibited an “on-off” type behavior (Figure S4D–F), which is not conducive to the detection of biological samples. Conversely, when employing probe QM-N2, the addition of BSA resulted in a slight red-shift in the absorbance spectrum (Figure S4G). Furthermore, a discernible blue-shift in the resulting fluorescence spectrum was observed (Figure 2H; Figure S4H), affirming the transition of QM-N2 from a twisted molecular structure to a comparatively planar one. This transformation can be attributed to the mitigation of the twisted intramolecular charge transfer (TICT) effect.[27-29] Notably, there was a significant enhancement in fluorescence with increasing BSA concentration, and the I/I0 value exhibited a strong linear relationship with BSA in the range of 0–8000 µg mL−1 (R2 = 0.998, Figure S4I). Furthermore, even in the low concentration range of 0–800 µg mL−1, a favorable linear relationship was maintained between BSA concentration and fluorescence intensity (Figure S5A–C) with detection limit as low as 3.1501 µg mL−1, underscoring the probe QM-N2's high sensitivity for BSA detection. Additionally, the performance of QM-N2 remains unaffected by changes in pH, as evidenced by its consistent strong linear relationship at pH levels of 5.0 and 8.0 (Figure S6). The results obtained using QM-N2 for the detection of human serum albumin (HSA) closely resembled those obtained for the detection of BSA, underscoring evident sensitivity and a concentration-dependent relationship (Figure 3A–C). Subsequently, a selectivity experiment was conducted using QM-N2 by monitoring its fluorescence response following exposure to a variety of potential interfering substances, including protamine, fucose, chondroitin sulfate, hyaluronic acid, heparin sodium, and alkaline phosphatase (ALP).[30-33] As depicted in Figure 3D, QM-N2 exhibited specific fluorescence enhancement upon the addition of 800 µg mL−1 serum albumin (BSA/HSA), while the presence of other components resulted in negligible changes in fluorescence intensity. This observation underscores QM-N2's capacity to selectively differentiate albumin (BSA/HSA) from interfering substances.
2.4 Sensing mechanism and binding analysis of QM-N2 with albumin
Molecular docking calculations were performed to elucidate the binding mechanism between QM-N2 and HSA (PDB 4K2C), providing insights into the intrinsic binding sites.[34, 35] Results showed that QM-N2 could interact with the Site 1 (subdomain IIA) of HSA (Figure 4A, left). Specifically, the residues ASP451, LYS195, GLU153, LYS199 HIS242, TRP214 and GLU450 could form as a tailored pocket to accommodate probe QM-N2 (Figure 4A, right). Conventional van der Waals, carbon hydrogen bond Pi-lone pair, Pi–Pi stacked and Pi-alkyl are the predominant interaction force to assist the docking position (Figure S6), whereas the attractive charges between the quaternary ammonium salt of QM-N2 and HSA facilitated the interaction.
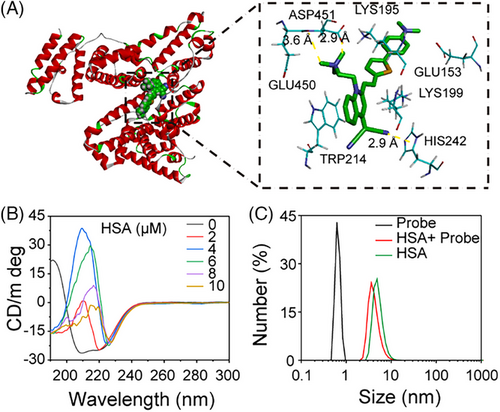
Furthermore, circular dichroism (CD) spectroscopy was employed to examine the structural alterations of the protein. As depicted in Figure 4B, isolated HSA (1 µm in PBS, pH = 7.4) displayed two negative shoulder bands at 222 and 208 nm, attributable to the negative Cotton effect, along with a positive band around 192 nm, affirming its α-helical structural features. Additionally, HSA exhibited a negative band at 216 nm and a spectral band spanning the positive and negative regions within the 195–200 nm range, indicating the presence of a certain β-sheet structure. Upon introducing QM-N2 to the HSA solution, the negative peaks at 222 and 208 nm gradually weakened to positive, suggesting a reduction in α-helix content. Simultaneously, the negative peak at 216 nm diminished, and the bands within the 195–200 nm range shifted into negative territory, signifying a decrease in β-sheet content. The interaction outcomes between BSA and QM-N2 mirrored those observed with HSA, both revealing a reduction in α-helix and β-sheet content (Figure S7). These findings affirm that QM-N2 has the capacity to effectively induce spatial structural changes in albumin, consequently constraining the intramolecular mobility of QM-N2 and generating NIR AIE signals.
To gain a deeper understanding of the morphology of the QM-N2 and albumin complex, a comprehensive study utilizing dynamic light scattering (DLS) and transmission electron microscopy (TEM) was conducted. As illustrated in Figure S8, the interaction of QM-N2 with HSA resulted in the disappearance of the initial DLS signal of QM-N2 and the emergence of a distinct DLS signal corresponding to the QM-N2@HSA complex. This new DLS signal partially overlapped with the signal of HSA, accompanied by a decrease in the Zeta potential. During the incubation process of QM-N2 with BSA, we observed similar changes in particle size and Zeta potential (Figure S8A,C), comparable to those observed with HSA. Additionally, TEM analysis of QM-N2, BSA, and the QM-N2+BSA mixture provided visual evidence supporting the formation of QM-N2@BSA complexes (Figure S8D). These findings provide unequivocal evidence that QM-N2 has the ability to bind with HSA, leading to emit its intrinsic AIE fluorescence signal.
2.5 Point-of-care diagnosis of mALB-related diseases with portable equipment
Early kidney injury will cause a significant increase in albumin content in urine, so urinary microalbumin (mALB) has become a pivotal clinic indicator for chronic renal failure, diabetic nephropathy, hypertensive nephropathy, and systemic lupus erythematosus nephropathy. Given the selective affinity of QM-N2 towards albumin, we conducted an investigation using a set of authentic urine samples (78 samples, Excel 1 in supporting information) to explore the relationship among fluorescence changes, mALB and other clinical indexes.
Initially, we recorded the fluorescence spectra of QM-N2, each urine sample, and their respective complexes. Subsequently, we calculated the difference in fluorescence intensity between the complex and the urine sample at 725 nm, referred to as ΔF (ΔF = FL1−FL2, Excel 1 in supporting information). Following this, heat maps were utilized to scrutinize the correlation between urine test parameters and ΔF for all urine samples. The findings indicated that ΔF exhibited limited correlation with blood urea nitrogen (BUN), serum creatinine (Scr), blood uric acid (Ua), glomerular filtration rate (GFR), and other indicators reflective of kidney function, while showing a robust positive correlation with mALB (Figure 5A). Furthermore, we normalized the mALB of all urine samples using ΔF values and represented them on a 3D scatter plot. The results demonstrated that larger ΔF values corresponded to higher levels of mALB in urine (Figure 5B). Subsequent curve fitting analysis underscored a significant positive correlation between mALB content and ΔF, with an impressive Pearson correlation coefficient (Pearson r) of 0.92. This result signifies that the QM-N2 fluorescent probe can accurately reflect urine mALB concentration in the sample (Figure 5C).
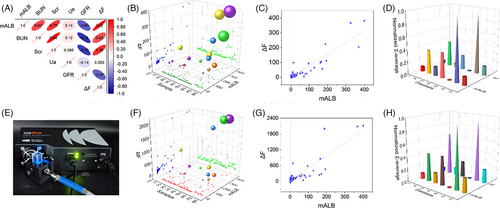
In addition, QM-N2 demonstrated robust detection performance and substantial Pearson r coefficients, whether in cases of chronic renal failure or other disease-related nephropathies, such as type 2 diabetic nephropathy (Pearson r 0.83) and hypertensive nephropathy (Pearson r 0.93) (Table 1; Figure S9). To assess detection accuracy, we segmented the 2D scatter diagrams of ΔF and mALB into four quadrants artificially and calculated them using the formula shown in Figure S9H. To establish a reference line, the dashed line parallel to the X-axis was determined from the intersection of fitted line (red) and the dashed line parallel to the Y-axis (mALB = 3 mg dL−1, mALB lower than 3 mg dL−1 is considered within normal range in clinic settings). Utilizing this classification, QM-N2 achieved an impressive high accuracy for chronic nephritis, type 2 diabetes and nephropathy (Table 1; Figure S9). Remarkably, by using 3 mg dL−1 as the threshold, it could completely discriminate between healthy individuals and those with renal impairment.
| Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Diseases | chronic nephritis | type 2 diabetes | high blood pressure | nephropathy | health people | chronic renal failure | other |
| Pearson's r | 0.98 | 0.83 | 0.83 | 0.93 | -0.68 | 0.93 | 0.90 |
| Accuracy (%) | 83.33 | 83.33 | 46.15 | 85.71 | NULL | 68.75 | 100.00 |
POCT not only hold immense significance in early disease detection and prevention, but also offer enhanced convenience for home and community hospitals.[36, 37] Herein, we employed a compact and user-friendly fluorescence detection equipment (Figure 5E; Figure S10) to evaluate the performance of QM-N2 in diagnosing mALB-related diseases. Remarkably, the results demonstrated the high sensitivity of QM-N2 and an approximately linear correlation between ΔF and mALB (Figure 5F,G; Figure S11), akin to the relationship depicted in Figure 5B,C, confirming the exceptional selectivity of QM-N2 in diagnosing mALB related diseases (Figure 5H) with high accuracy (Table S1). Conclusively, it is of high reproducibility for QM-N2 to diagnosis chronic renal failure with portable equipment, which would satisfy the demand of POCT at home or in community hospitals.
3 CONCLUSIONS
This study focuses on the developing of NIR AIE-active probe for diagnosing of mALB-related kidney diseases, and meeting the demand for portable medical equipment utilized in POCT at home or in community hospitals. By optimizing the substituent position of quaternary ammonium salt, the elaborately tailored probe QM-N2 with desirable hydrophilicity and activable NIR emitting was obtained on the basis of quinoline-malononitrile (QM). The extended emission wavelength of QM-N2 facilitates a decrease of interference from biological spontaneous fluorescence, making it a valuable tool for clinical pathological urine analysis. Significantly, the quenched initial fluorescence of QM-N2 substantially amplifies detection sensitivity, and meanwhile its high specificity for albumin binding coupled with constrained intramolecular motion remarkably enhances detection accuracy. Notably, in the analysis of clinical urine samples, QM-N2 distinguished a strong correlation observed for the concentration of urinary mALB. This pronounced correlation establishes QM-N2 as a promising tool for the diagnosis of mALB-related kidney diseases. Furthermore, when equipped with a portable equipment, QM-N2 retains its effectiveness in reflecting the concentration of mALB. The amalgamation of QM-N2 with portable devices provides an ideal paradigm to promote laboratory scientific achievements towards clinical applications.
AUTHOR CONTRIBUTIONS
Zhirong Zhu and Xiaoyan Chen contributed equally to this work.
ACKNOWLEDGEMENTS
This work was supported by the National Key Research and Development Program of China (2021YFA0910000), NSFC (22222803, 91959202 and 21974047), China Postdoctoral Science Foundation (2022M72142), and Shanghai Municipal Science and Technology Major Project (2018SHZDZX03).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
The study was approved by the ethics board of Ruijin Hospital (Ruijin Hospital Ethics Committee, Shanghai JiaoTong University School of Medicine) (reference number 2021-417).
Open Research
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are included within the article and the supporting information.




