Light-controlled molecular tweezers capture specific amyloid oligomers
Abstract
Amyloid-β peptide (Aβ) oligomers, characteristic symptom of Alzheimer's disease (AD), have been identified as the most neurotoxic species and significant contributors to neurodegeneration in AD. However, due to their transient and heterogeneous nature, the high-resolution structures and exact pathogenic processes of Aβ oligomers are currently unknown. Using light-controlled molecular tweezers (LMTs), we describe a method for precisely capturing specific Aβ oligomers produced from synthetic Aβ and AD animal models. Light irradiation can activate LMTs, which are composed of two Aβ-targeting pentapeptides (KLVFF) motifs and a rigid azobenzene (azo) derivative, to form a tweezer-like cis configuration that preferentially binds to specific oligomers matching the space of the tweezers via multivalent interactions of KLVFF motifs with the oligomers. Surprisingly, cis-LMTs can immobilize the captured oligomers in transgenic Caenorhabditis elegans under light irradiation. The LMTs may serve as spatiotemporally controllable molecular tools to extract specific native oligomers for the structure and function studies via reversible photoisomerization, which would improve the understanding of the toxic mechanisms of Aβ oligomers and development of oligomer-targeted diagnosis and therapy.
1 INTRODUCTION
Protein misfolding and aggregation in the brain is a typical sign of a variety of neurodegenerative disorders.[1] The oligomeric aggregates of amyloid-β peptides (Aβ) are the principal neurotoxic species to the onset of Alzheimer's disease (AD),[2, 3] which is the most prevalent neurodegenerative illness associated with dementia.[4] Aβ oligomers not only cause various neuronal dysfunctions, like loss of learning and memorial abilities,[5] but also activate other pathological processes of AD, such as tau hyperphosphorylation, oxidative stress, and mitochondrial dysfunction.[6] As a result, Aβ oligomers are recognized as legitimate targets for the diagnosis and therapy of AD.[7] Nevertheless, the term “Aβ oligomer” is rather ambiguous because it refers to numerous soluble Aβ species or a combination of diverse types of metastable oligomeric species with varying sizes, shapes, and conformations as a result of Aβ’s dynamic assembly.[8] The structural changes may cause variances in the molecular toxicity of Aβ oligomers.[9] Therefore, accurate capture of the oligomers at the single aggregation level from Aβ mixtures is a critical step in elucidating the structural elements behind oligomer toxicity in AD pathological circumstances.
Due of the tremendous metastability and heterogeneity of Aβ oligomers, it is unfortunately very difficult to grasp a specific oligomer. Although significant effort has been made to identify Aβ oligomers using a variety of biosensors, including oligomer-specific antibodies,[10, 11] small-molecule-based probes,[12-14] and electrochemical reporters[15, 16] in combination with large-scale instruments, no one is able to distinguish a particular oligomer at a degree of aggregation from the others in the mixture. Through covalent interactions, certain techniques coupled with electrophoresis have even been used to isolate specific oligomers from Aβ mixtures.[17-19] However, they are unable to accurately reflect the toxicity of naturally occurring oligomers since the captured oligomers may exhibit different cytotoxicity from that of the oligomers formed in self-aggregation due to differing structures.[20, 21] Thus, new methods are urgently needed to capture and stabilize specific Aβ oligomers for the development of oligomer-targeted diagnosis and therapeutic strategies in anti-AD.
To this aim, we present here the invention of light-controlled molecular tweezers (LMTs) to capture and stabilize the specific Aβ oligomers by making use of the conformation features of Aβ oligomers (Figure 1). In general, Aβ oligomers adopt solvent-exposed hydrophobic patches and antiparallel β-sheets. The KLVFF region (Aβ16−20) as the central hydrophobic core of Aβ is responsible for Aβ aggregation via self-interactions between neighboring strands (Figure 1).[22] Accordingly, the pentapeptide KLVFF has been successfully employed to target Aβ through interactions with the exposed KLVFF motif of Aβ aggregates.[23] Small compounds containing photoresponsive groups, on the other hand, have demonstrated great promise for precise spatiotemporal manipulation of protein behavior and function, relying on their photoswitchable structures.[24-26] Notably, azobenzene (azo) is the most commonly utilized photochromic chemical in biomedical applications;[27, 28] it undergoes trans-cis photoisomerization with significant geometrical alterations between planar trans- and non-planar cis-configurations. In this context, we created the LMTs by fusing the functions of KLVFF and azo groups. Through multivalent interactions of KLVFF motifs between the LMTs and Aβ oligomers, the LMTs are able to precisely “freeze” the chosen oligomers produced from both synthetic and natural Aβ upon light irradiation. For the first time, these LMTs provide a molecular technique to precisely capture individual Aβ oligomers at a single aggregation level, which may remarkably advance studies on the structural and functional aspects of amyloid oligomers.
2 RESULTS AND DISCUSSION
2.1 Design and photoisomerization of LMTs
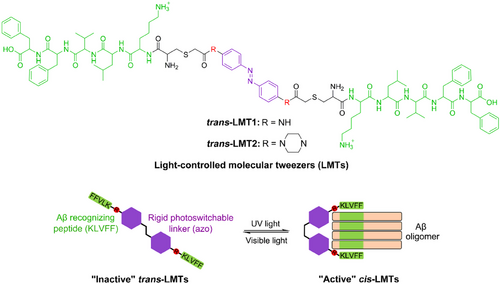
The two KLVFF motifs bridged by a flexible linker have been used to recognize low molecular weight (LMW) oligomers through binding to the KLVFF “ends” of Aβ side chains.[29] However, the flexibility or length of the linker severely restricts the probe's selectivity. For exact identification of a given Aβ oligomer, rigid linkers that can adjust the distance between the two KLVFF motifs would be ideal. As a result, we chose azobenzene (azo) derivatives as stiff linker backbones for the LMTs. The distance between the phenyl termini ranges from 9.0 to 6.0 Å,[30] and this is likely to change the distance between the two KLVFF motifs when exposed to light. We believe that a convertible structure with suitable rigid substituents (R) para to the azo group will identify certain Aβ oligomers (Figure 1). Accordingly, because the compounds adopt tweezer-like configurations with relatively short distances between the two KLVFF motifs, cis-LMTs may be “active” to a specific Aβ oligomer, As a result, a strong dual binding of KLVFF units of cis-LMTs to their “end” counterparts in the Aβ oligomer with a matching size would occur. On the contrary, trans-LMTs may be “inactive” to specific Aβ oligomers since they would adopt a single binding mode to Aβ oligomers with low affinity due to the considerable distance between the two KLVFF motifs. Thus, LMTs would offer light-controllable tools for capturing or isolating specific Aβ oligomers with great spatiotemporal accuracy via a reversible transition between the cis and trans forms.
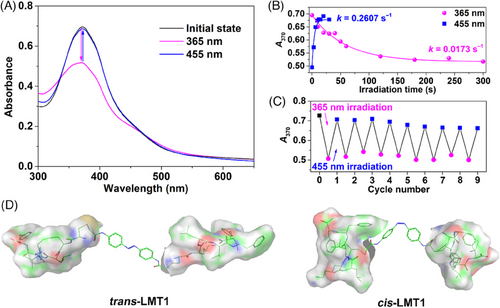
As a proof of concept, two models of LMTs, LMT1 and LMT2 (Figure 1), were synthesized by reacting the iodoacetamide moiety of azo derivatives with the thiol of Cys residue in NH2-CKLVFF-COOH peptide. The synthetic route and characterization of the LMTs are described in the Supplementary Information. The photoisomerization of the LMTs was investigated by Ultraviolet (UV)-Vis spectroscopy. As shown in Figure 2A, the original trans-LMT1 displayed two characteristic bands at ca. 370 and 475 nm in Tris-HCl buffer, they are π‒π* and n‒π* absorptions of azo, respectively. Light irradiation at 365 nm leaded to a significant decrease of the strong π‒π* transition and a slight increase of the n‒π* band, which is typical of trans→cis isomerization of azo.[31] The photostationary state (PSS) of LMT1 was attained after continuous irradiation for 300 s (Figure 2B), and it reached equilibrium with 66.7% of the cis form (Figure S1). Within 30 s of further irradiation at 455 nm, the cis configuration was quickly changed back to the trans one (Figure 2B). After nine cycles of alternating between 365 and 455 nm, there was barely any loss of absorption (Figure 2C). A complete recovery of cis-LMT1 to its trans form was achieved spontaneously by storing the solution at room temperature in the dark, with a half-life τ1/2 of 1.26 h (Figure S2). This time span is long enough for the binding of cis-LMT1 to an Aβ oligomer. The optimized geometries of the LMTs isomers were calculated using the semiempirical quantum chemical method PM7. As anticipated, the conversion of the LMTs from the stretched planar trans configuration to the tweezer-like cis form dramatically reduces the distance between the KLVFF motifs (Figure 2D and S3). Moreover, cis-LMT2 showed a greater separation between the KLVFF residues than cis-LMT1 due to the extension of azo group by piperazine at para positions. These findings suggest that the rigid “R” group and light irradiation can control the spacing between the KLVFF motifs.
2.2 cis-LMTs bind to specific oligomers of gel-separated Aβ
To test the light-driven binding of LMTs to synthetic Aβ oligomers, native polyacrylamide gel electrophoresis (PAGE) was conducted, followed by a modified Western blotting. In the assay, biotin-labeled LMTs (LMTs-biotin) containing biotinylated KLVFF were used to investigate the binding properties of LMTs to Aβ oligomers. KLVFF was biotinylated at C-terminal Lys (Figure 3A). Briefly, the synthetic Aβ mixture was initially separated by Tris-Tricine-PAGE.[32] LMTs-biotin was added to the membrane containing gel-transferred Aβ species and co-incubated. Because the unbound LMTs-biotin can be easily washed away, only the adducts of LMTs-biotin with Aβ were photographed on the membrane using streptavidin (SA)-conjugated horseradish peroxidase (HRP) solution with enhanced chemiluminescence (ECL). As a result of the selective and stable interaction of SA with biotin, the bands of LMTs-biotin-preferred Aβ oligomers can be observed on the membrane (Figure 3B). In addition, standard Western blotting with anti-Aβ antibody 6E10 confirmed the whole distribution of the Aβ species on the membrane as a control.
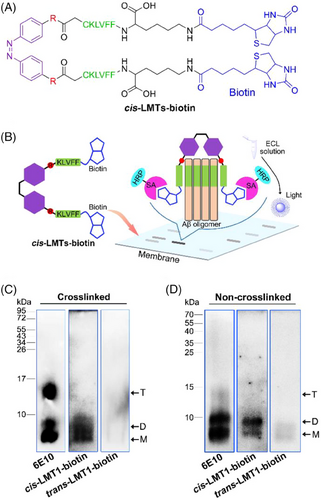
Initially, the photoisomeric behaviors of LMTs-biotin were explored. LMT1-biotin exhibited almost the same spectral profile as LMT1 after irradiation at 365 and 455 nm (Figure S4), demonstrating that the biotin groups had no impact on LMT1 isomerization. Because of the addition of piperazine groups, LMT2-biotin showed a spectacular red-shifted π‒π* band at ca. 425 nm (Figure S5).[33] It exhibited sensitive and reversible photoisomerization between trans and cis configurations without degradation after 6 cycles of alternating low-power irradiation at 455 and 620 nm, respectively (Figure S5D), although its thermal relaxation is faster than that of LMT1. Thus, LMTs-biotin can reveal the binding preference of LMTs to Aβ oligomers.
Given the metastability of Aβ oligomers, we investigated the capacity of LMT1-biotin to bind stable synthetic Aβ oligomers created by cross-linking unmodified Aβ under irradiation.[17] The control group showed that Aβ40 included a significant number of monomers and trimers, but only a small amount of dimers (Figure 3C). After imaging with SA-conjugated HRP, the bands of Aβ40 monomer and dimer were seen on the membrane that had been pretreated with cis-LMT1-biotin (365 nm), but no trimer band was observed. trans-LMT1-biotin, on the other hand, did not show any bands. These results suggest that the dimer is the favored binding target for cis-LMT1 in the Aβ40 oligomers. Encouraged by the findings, we further studied the binding of LMT1-biotin to the dynamic oligomers of Aβ40 without cross-linking pretreatment. The noncrosslinked Aβ40 samples showed monomers, dimers, and a few of trimers on the membrane (Figure 3D). The presence of dimers was confirmed by Western blotting using Aβ oligomer-specific antibody A11 (Figure S6). cis-LMT1-biotin (365 nm) still exhibited preferential binding to the dimers, as shown in Figure 3D. trans-LMT1-biotin, on the other hand, scarcely bound to dimers, which is consistent with the binding to the crosslinked counterparts. Because Aβ42 is more aggregation-prone than Aβ40,[12] the distribution of Aβ42 oligomers differed from that of Aβ40, with a high proportion of high molecular weight (HMW) oligomers in control groups. cis-LMT1-biotin (365 nm) still showed preferential binding to the dimers in both crosslinked and non-crosslinked Aβ42 samples (Figure S7), confirming the selectivity of cis-LMT1 for Aβ dimers. The bands of monomers were also found in the crosslinked samples of both Aβ40 and Aβ42, when treated with cis-LMT1. The distinct preparation between non-crosslinked and crosslinked samples may result in the difference on the membranes. For the crosslinked samples, a few Ru(II) catalyst [Ru(bpy)3]2+ may remain due to its binding affinity to Aβ monomers,[34] which would facilitate the adsorption of cis-LMT1 at the bands of monomers. The presence of [Ru(bpy)3]2+ in monomers was verified by high resolution mass spectrometry (Figure S8). Considering almost no monomer bands were observed in the non-crosslinked Aβ, it can be concluded that cis-LMT1 can specifically capture Aβ dimers in a light-driven manner, which is attributed to the appropriate distance between the two KLVFF units of cis-LMT1 for the two “end” KLVFF motifs of dimers, thereby forming stable adducts via multivalent KLVFF interactions. In contrast, trans-LMT1 was washed away from the membrane due to its low affinity for each Aβ species through a single binding mode between KLVFF, resulting in the subtle bands.
The binding affinities of LMT1 to different amyloid protein species, for example, LMW Aβ oligomers, Aβ protofibrils, Aβ fibrils, and tau oligomers were measured by biolayer interferometry assay. The equilibrium dissociation constants (KD) were obtained by Octet Data Analysis to represent the affinity of the binary interactions. As shown in Figure S9, cis-LMT1 demonstrated highest binding affinity (KD = 8.52 × 10‒7 M) to LMW Aβ oligomers. In contrast, trans-LMT1 showed lower binding affinity (KD = 1.35 × 10‒6 M) than that of the cis form to LMW Aβ oligomers, but similar KD values for different Aβ species. Interestingly, both the isomers showed an incomplete profile with low response values (∼0.01 nm) upon interaction with tau oligomers, revealing their extremely poor binding to the tau oligomers. The results strongly support the binding selectivity of cis-LMT1 for LMW oligomers over other Aβ aggregates and amyloid oligomers, attributed to its preferential binding to dimers.
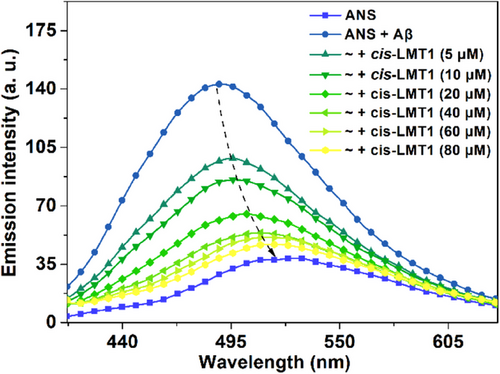
To validate the KLVFF-mediated binding mode of LMT1 to Aβ oligomers, a fluorescence competition assay was performed using 8-anilinonaphthalene-1-sulfonate (ANS), which is a “turn-on” fluorescence probe for Aβ oligomers through binding to the solvent-exposed hydrophobic patches of Aβ oligomers, particularly the region of residues 16–22 (KLVFFAE),[35-37] As shown in Figure 4, when 40 μM (2 eq. of ANS) of cis-LMT1 was added, the fluorescence of Aβ-bound ANS was decreased and red-shifted to that of ANS per se, suggesting that ANS was totally replaced by cis-LMT1. In contrast, the cis form of 4,4′-bis(iodoacetamide)azobenzene (2), the precursor of LMT1, cannot reduce the fluorescence of Aβ-bound ANS without KLVFF moieties (Figure S10), consolidating that the binding of cis-LMT1 to Aβ relies on the multivalent interactions of KLVFF motifs. Reasonably, such interactions at the region of KLVFF would weaken Aβ’s connection with ANS, leading to the detachment of the fluorescent probes from Aβ.
Furthermore, the binding properties of LMT2-biotin to the crosslinked Aβ42 oligomers were evaluated to confirm the critical function of the distance between two KLVFF units of the tweezer-like structure in conformation-specific recognition of oligomers. Unlike cis-LMT1, which preferentially bound to Aβ dimers, the increased distance between the two KLVFF motifs in cis-LMT2 allows for selective binding to oligomers bigger than dimers. As expected, cis-LMT2-biotin (455 nm) exhibited much higher binding selectivity to tetramers than to monomers and dimers (Figure S11). There was no binding to the other Aβ42 species discovered. The results strongly support that cis-LMTs would provide a universal method for precise recognition of distinct Aβ oligomers at the single aggregation level by altering the spacing between the two KLVFF motifs via modifying the rigid “R” group para to the azo.
2.3 cis-LMT1 captures dimers in synthetic Aβ mixture
Encouraged by the binding preference of cis-LMT1 to Aβ dimers, we next examined whether cis-LMT1 could capture the dimers in the Aβ mixture using Western blotting with antibody 6E10. The aggregation profile of Aβ monomers was measured by thioflavin (ThT) fluorescence in Tris buffer at 25°C (Figure S12).[38] Accordingly, Aβ monomers were co-incubated with LMT1 for 6 h beyond the plateau phase, and 30 min irradiation was set at the lag phase from the beginning of incubation, the process for oligomers formation. After incubating Aβ40 per se for 6 h, only a minor number of LMW oligomers (∼10 kDa) were seen on the membrane (Figure 5A), indicating that the bulk of Aβ would convert to fibrils at this phase, consistent with the result of aggregation profile (Figure S12). However, when Aβ40 was co-incubated with cis-LMT1, an increase of oligomers (∼10 kDa) assignable to the adducts of cis-LMT1 with Aβ40 dimers was detected (Figure 5A). Moreover, 365-nm irradiation yielded a better impact than darkness. Contrarily, trans-LMT1 hardly affected the Aβ aggregation. The results suggest that cis-LMT1 can capture the dimers and prevent them from further aggregation in the Aβ mixture, which is probably attributed to the preferential binding of cis-LMT1 clamps to the dimers’ two “end” KLVFF regions. Owing to the partial conversion to the “inactive” trans form via thermal relaxation during the co-incubation, treatment of Aβ with cis-LMT1 thus yielded relatively fewer captured dimers in the dark for 6 h. Morphological examination using transmission electron microscopy (TEM) was also carried out to assess the dimer-capturing effect of cis-LMT1 on Aβ aggregation (Figure 5B). Aβ self-aggregation generated abundant fibrils, and trans-LMT1 had little effect on them. In the presence of cis-LMT1, tiny granules rather than fibrils were observed. Irradiation at 365 nm exhibited a greater inhibitory effect than darkness (Figure 5B). In agreement with the results of Western blotting, Aβ was “frozen” at a low aggregation degree by cis-LMT1 under irradiation owing to its capacity to capture and stabilize dimers.
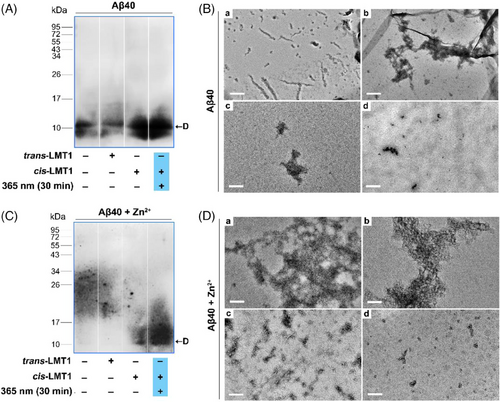
Considering that Zn2+ can promote Aβ aggregation, leading to greater heterogeneity of Aβ mixture,[36] we also studied the effect of cis-LMT1 on the Aβ dimers during Zn2+-induced Aβ aggregation. Because Zn2+ favored the formation of bulky aggregates,[39] no Aβ oligomers were found in the presence of Zn2+ after just 30 min (Figure 5C). Interestingly, cis-LMT1 can create considerable quantities of LMW oligomers (∼10 kDa) when exposed to 365-nm light for 30 min (Figure 5C). On the contrary, trans-LMT1 was almost ineffective to stabilize Aβ dimers. TEM assay manifested that only the cis-LMT1-treated group could clearly suppress Zn2+-induced Aβ aggregation by producing tiny aggregates under irradiation (Figure 5D). These findings suggest that cis-LMT1 is able to freeze the Aβ dimers in the mixture of Zn2+-induced Aβ aggregates. Although the PSS of LMT1 has a limited number of cis-isomers, continual irradiation created new cis-LMT1 as the original cis-LMT1 was consumed, ensuring effective dimer binding. Therefore, by sequestering the dimers in its clamps, cis-LMT1 might serve as an effective light-driven capture for synthetic Aβ dimers.
We next examined whether LMT1 could dynamically regulate Aβ aggregation with light in situ. The aggregation of Aβ40 in the presence of LMT1 with or without light irradiation was monitored by ThT fluorescence. As expected, cis-LMT1 exhibited greater inhibition of Aβ aggregation than trans-LMT1 (Figure S13A), consistent with the TEM data. However, during aggregation, illumination of the trans-LMT1-treated Aβ sample with 365-nm light leaded to a significant reduction in ThT fluorescence in the interval (Figure S13B). This effect can be repeated for many times until the aggregation reaches a plateau, finally causing a higher inhibition of Aβ aggregation than trans-LMT1 without light irradiation due to photoinduced isomerization of the trans form to the “active” cis configuration. Thus, Aβ aggregation could be spatiotemporally controlled by employing the LMT1 through dimer capturing in a light-driven manner.
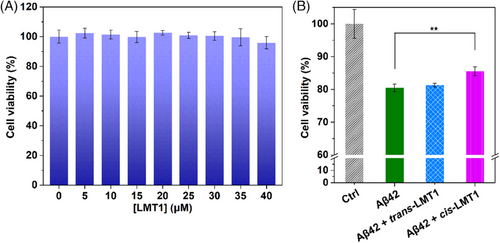
Intervening Aβ aggregation may alter the Aβ-induced neurotoxicity. Using the 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) assay, we thus tested the ability of cis-LMT1 to capture dimers and its effect on Aβ-induced neurotoxicity in PC12 cells. LMT1 per se was nontoxic even at 40 μM (Figure 6A). After a 24-h co-incubation in the dark, Aβ42 self-aggregation decreased the cell viability to ∼80% due to the neurotoxicity of aggregates. While the cell viability was recovered to ∼86% in the presence of cis-LMT1 (Figure 6B), probably resulting from its inhibitory effect on Aβ aggregation. In contrast, trans-LMT1 did not influence the Aβ-induced cytotoxicity due to its inability to interfere with Aβ aggregation. Given the toxicity of oligomers, the limited inhibitory effect of cis-LMT1 on Aβ-induced neurotoxicity is very likely attributed to the dimer stabilization that would make the Aβ staying at LMW oligomeric states, although the binding of cis-LMT1 to dimers may reduce their toxicity to some extent. These results imply that cis-LMT1 can also freeze Aβ dimers and prevent Aβ aggregation in living cells.
2.4 cis-LMT1 captures natural Aβ dimers in Caenorhabditis elegans
Inspired by the light-driven action of cis-LMT1 on synthetic Aβ dimers, we further investigated its effect on natural Aβ42 in the AD model—transgenic Caenorhabditis elegans (C. elegans). C. elegans strain CL4176 expresses Aβ42 in the body wall muscle cells; higher temperature (25°C) can stimulate Aβ42 aggregation, resulting in toxic aggregates in the worms and a progressive paralysis.[40, 41] To determine whether cis-LMT1 could capture natural Aβ dimers in C. elegans, Western blotting assay was carried out to examine the distribution of Aβ in the worms following the treatment of LMT1 with or without irradiation at 365 nm for 8 h. As shown in Figure 7A, both the vehicle and trans-LMT1 groups displayed weak bands of oligomers, indicating HMW aggregates would be the dominant Aβ species in the worms. Contrarily, cis-LMT1-treated group clearly showed the adduct of cis-LMT1 with Aβ42 dimer at a band (∼10 kDa) under irradiation, signifying that cis-LMT1 is effective for capturing natural Aβ dimers in vivo. Besides dimers, some LMW oligomers at MW 15–40 kDa were also observed in the cis-LMT1 group, implying that cis-LMT1 can also prevent Aβ aggregation into HMW aggregates to some extent through capturing dimers.
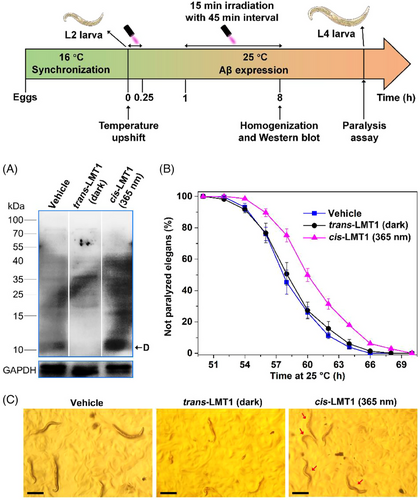
The worm paralysis experiment can reflect the influence of dimer-capturing of cis-LMT1 on Aβ neurotoxicity in vivo. The Aβ-induced paralysis was monitored after the temperature was upshifted to 25°C, which was hardly affected by light irradiation (Figure S14). For the same conditions as that in LMT1 groups, Dimethyl sulfoxide (DMSO) (1%, v/v) was used in the vehicle group, which would lead to the delayed onset of worm paralysis.[42] Compared to the vehicle group, treatment with cis-LMT1 and irradiation at 365 nm obviously postponed the paralysis (Figure 7B), suggesting that cis-LMT1 could alleviate the neurotoxicity of Aβ aggregation in C. elegans by blocking the aggregation via freezing Aβ42 dimers. In the dark, almost no delay in paralysis was observed in worms treated with trans-LMT1, confirming that trans-LMT1 cannot intervene Aβ aggregation in vivo. The effects of LMT1 on paralysis were certified by the motion mode of the CL4176 elegans at 60 h post-temperature shift to 25°C (Figure 7C and Movie S1–S3). The majority of the worm bodies stiffened in both the vehicle and trans-LMT1-treated groups, with just the heads moving slowly; however, when exposed to light (365 nm), the cis-LMT1-treated worms wriggled fast with curvy bodies. These results demonstrate that cis-LMT1 can also capture and stabilize the Aβ42 dimers in C. elegans, thereby inhibiting Aβ aggregation and attenuating toxicity. It should be pointed out that the amelioration of Aβ-induced neurotoxicity seems not good enough for therapeutic purpose, because cis-LMT1 do not capture and stabilize all the toxic LMW Aβ oligomers. Even so, we believe that LMTs could be promising candidates to construct the targeting scavengers for oligomers to reduce Aβ-induced neurotoxicity.
3 CONCLUSIONS
For the first time, we developed LMTs for the precise capture of specific Aβ oligomers. The LMTs were constructed by bridging two KLVFF motifs to the rigid photoswitchable azobenzene core. The distance between two KLVFF motifs could be varied by photoisomerization of azobenzene at different wavelengths, allowing tweezer-like cis-LMTs capable of seizing the two “end” KLVFF sequences in Aβ oligomers. The selectivity of cis-LMTs for specific Aβ oligomers was achieved by tuning the space between the clamp arms using substituents of azobenzene at para positions. Once the cis-LMTs possess a suitable room to accommodate the specific Aβ oligomers, they preferentially capture the oligomers via multivalent interactions between KLVFF motifs. As a proof of concept, cis-LMT1 successfully captures dimers of both synthetic and natural Aβ from mixture in vitro and in vivo under light irradiation. Moreover, this binding mechanism allows the cis-LMT1 to stabilize captured dimers in neuron cells and AD C. elegans. Given the structure's considerable tunability, the molecular tweezers presented here may offer a universal molecular platform to build a toolbox for specific amyloid oligomers in a variety of contexts. Based on the reversible photoisomerization, LMTs would be promising starting scaffolds to achieve extraction or elimination of specific natural Aβ oligomers for studying the pathogenesis and structures of oligomers and developing oligomer-targeted therapy of AD.
ACKNOWLEDGMENTS
The authors appreciate the financial support from the National Natural Science Foundation of China (grant number: 21771105 and 22377053), the Natural Science Foundation of Jiangsu Province (grant number: BK20170103), and the Six Talent Peaks Project in Jiangsu Province (grant number: SWYY-043).
CONFLICT OF INTEREST STATEMENT
The authors declare no competing financial interests.




