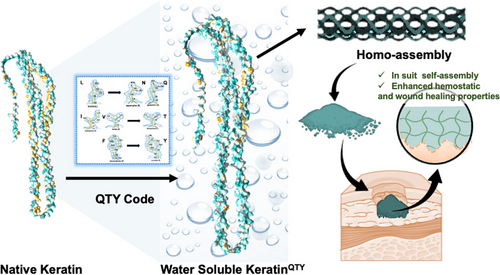
Rational design of water-soluble, homotypic keratins self-assembly with enhanced bioactivities
Abstract
Recombinant keratins possess strong hemostatic and wound healing properties but suffer from poor water solubility that restricts their bioactivities in biomedical applications. Herein, we report the rational design and synthesis of water-soluble keratins using a simple methodology named the QTY code. In vitro biophysical analyses and molecular dynamic simulation demonstrated a 200-fold increase in the water solubility of QTY variant keratins without apparent structural changes compared to native proteins. Homotypic self-assembly was observed for the first time in recombinant keratins in an aqueous environment, without urea and after QTY modification. Cell and animal experiments showed the in situ gel-forming capability of QTY variant keratins with superior hemostatic and wound healing activities at the wound sites compared to native recombinant keratins. Our work not only presented a simple and feasible pathway to produce large amounts of water-soluble keratins using QTY modification but also validated the enhanced self-assembly, hemostasis, and wound healing properties of these novel keratin species that may open up new venues for biomedical applications.
1 INTRODUCTION
Keratins are a family of ubiquitous biological materials that have been used in biomedical applications for more than 400 years.[1-4] Until now, keratin materials have been commonly applied in various fields, such as tissue engineering, hemostasis, cell culture, robot engineering, etc.[2, 5-8] Obtaining water-soluble keratins represents one of the notable landmarks in the progress of modern keratin studies. The first patent for keratin extraction was issued to John Hoffmeier in 1905, which described a process of horn keratin extraction using a lime solution.[9] In the following century, various methods were developed to extract keratins from different biomasses, including human hair, wool, feather, and so on.[10, 11] In some sense, soluble keratin extraction opens up a new avenue for keratin materials in biomedical applications. However, the extracts typically contain multiple kinds of keratins with difficulties in controlling amino acid compositions and batch-to-batch consistency.[12]
Recombinant expression is an alternative way to improve the purity and quality of keratin proteins. We previously expressed 17 kinds of recombinant human hair keratins to investigate the hemostatic mechanisms of different keratin materials.[8] Recombinant keratins exhibited superior hemostatic and wound healing activities because of their higher purity compared to the extracted keratins.[12, 13] However, recombinant keratins are insoluble in water in their native states, which leads to additional difficulties in their synthesis and purification.[14] Thus, we asked if it is possible for recombinant keratins to become soluble to enhance their bioactivities. The recombinant keratins have been treated following the reductive extraction process to enhance their hydrophilicity and stronger wound healing capabilities.[9] However, the solubilization of recombinant keratins using these reductive agents also results in the uncertainty of amino acid composition.
There are many approaches of rational protein design to improve the water solubility of proteins.[15] The QTY code, as one methodology of this type, is based on the side chain structure and electron density map similarities in certain pairs of 20 amino acids, through which the water solubility of a given protein can be accurately controlled. Specifically, the hydrophobic amino acids of L, I/V, and F were correlated with the hydrophilic residues of Q, T, and Y.[16, 17] The water-soluble chemokine receptors of CCR9 and CXCR2, interleukin (IL) receptors of IL4R and IL10R, and two variants of interferon receptors, IFNγR1 and IFNλR1, have been successfully designed via the QTY code in our previous study as antibody-like decoys to neutralize the excessive cytokines associated with cytokine release syndrome.[18] Therefore, we hypothesized that more water-soluble keratins could also be rationally designed using the QTY code. To test this hypothesis, we redesigned two variants of type I and II human hair keratins, namely, K31, K33A, K81, and K83, through the QTY code. The solubilities of recombinant keratins were significantly enhanced after the design (Scheme 1). More interestingly, the self-assembling model of human hair keratins changed from hetero-self-assembly to homo-self-assembly, demonstrating that the water-soluble keratins self-assembled by themselves. We further demonstrated that QTY-designed variants had superior hemostasis and wound healing performances compared with native recombinant keratins, suggesting a promising means to produce functional water-soluble keratin materials for various biomedical applications.
2 RESULTS
2.1 Water-soluble keratin design using the QTY code
Human hair keratins belong to intermediate filaments (IFs) with a total of 17 types, which can be divided into type I and type II. The molecular weight of type I keratin is relatively low, whereas that of type II keratin is relatively high.[19, 20] To verify the feasibility of QTY code in the design of water-soluble keratin, two type I keratins, namely, RK31 and RK33A, and two type II keratins, namely, RK81 and RK83, were selected for modification. There were three α-helical domains in each protein, denoted as 1Aα, 1Bα, and 2α (Figure 1A). Following the design principle in G protein-coupled receptors,[15-17] we targeted these helical domains in keratins for QTY code modifications in our study. The amino acids of L, I/V, and F in all helices were replaced by Q, T, and Y, respectively. The redesigned recombinant keratin variants were named RK31QTY, RK33AQTY, RK81QTY, and RK83QTY. The change ratios of RK31QTY, RK33AQTY, RK81QTY, and RK83QTY were 18.02%, 18.56%, 13.66%, and 13.60%, respectively (Figure 1B). An increase in the molecular weights of QTY variants was observed compared to the native proteins, but no significant changes in isoelectric point were found.

Subsequently, we assessed whether the hydrophilicity of keratins was enhanced after the QTY modification. The grand average of hydropathicity (GRAVY) was calculated by the ExPASy-ProtParam prediction web server (https://web.expasy.org/). As shown in Figure 1C, positive values indicated the hydrophobic residue and negative values were related to the hydrophilic residue.[21] The hydrophobic amino acid sites in proteins showed a wave-like variation over their sequence ranges, while more positive values were found in native keratins compared to QTY variants. In the rod regions ranging from approximately amino acids 50 to 360, a significant decrease in GRAVY value was observed, implying the overall improvements in protein hydrophobicity by QTY code modification. Furthermore, the surface molecular lipophilicity potential of native and redesigned keratins was calculated with UCSF ChimeraX (Figure 1D),[22] where the protein surface was colored from dark cyan (most hydrophilic) to white to golden rod (most lipophilic).[23] The amino acids colored in gold turned to cyan or white after QTY modifications, especially in the α-helical regions, which suggested that the hydrophilicity of QTY variants of keratins was significantly improved. We further assessed the structural similarity between native and QTY keratins by using AlphaFold2 (Figure 1E).[24] Similar predicted structures were observed between the two variants. The template modeling score (TM-score) measure was also calculated.[25] The resulting TM-score was <0.7, indicating strong similarities between native and QTY keratins (Table S1).[26] Meanwhile, the structures of native keratins and QTY variants were also predicted via the SWISS-MODEL algorithm, and highly similar predicted structures were observed (Figure S1).
2.2 QTY–keratin variants show good water solubility
The redesigned QTY variant proteins, namely, RK31QTY, RK33AQTY, RK81QTY, and RK83QTY, were synthesized through the Escherichia coli system. SDS-PAGE gel showed that the protein bands of RK31, RK33A, RK81, and RK83 were at about 48, 47, 56, and 55 kDa, respectively. In contrast, the native PAGE gel showed the bands corresponding to the molecular weights of RK31QTY, RK33AQTY, RK81QTY, and RK83QTY were at around 104, 101, 119, and 117 kDa, respectively (Figure 2A). The homotypic self-assembly of recombinant keratins after QTY modification was observed in the aqueous environment for the first time, and the keratin–QTY variant could self-assemble itself. The native PAGE gel was used to assess the molecular weight of water-soluble keratins. The tandem mass spectrometry/mass spectrometry (MS/MS) analysis was then performed to verify the target protein using RK33AQTY as an example. Ninety percent coverage was observed in the mass spectrometry. The redesigned keratin variants mostly existed in the form of dimers and higher order multimers. All purified proteins exhibited similar morphologies and were cream-white spongy after freeze drying (Figure 2B).
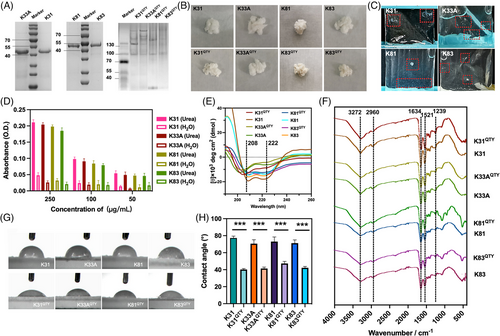
We next evaluated the water solubility of native recombinant human hair keratins and their QTY variants (Figure 2C). The native recombinant keratins were first dissolved in urea solution at different concentrations (50, 100, and 250 μg/mL) and then dialyzed using ultrapure water. The decrease in ultraviolet absorption values during dialysis suggested the supersaturation of keratins in solutions under the concentration. As shown in Figure 2D, the absorbance of the native proteins in water was significantly lower than that in the urea solution at concentrations of 50, 100, and 250 μg/mL, which suggested that the water solubility of all recombinant keratins was less than 50 μg/mL. However, the solubilities of RK31QTY, RK33AQTY, RK81QTY, and RK83QTY in water were up to 10.796, 10.072, 9.856, and 10.044 mg/mL, respectively. The water solubility of recombinant human hair keratins through the QTY code designs increased by more than 200 times. Furthermore, we performed circular dichroism (CD) and Fourier-transform infrared spectroscopy (FT-IR) analyses on synthesized proteins. The CD shows characteristic α-helix spectra with two negative peaks at 208 and 222 nm, indicating similar α-helical structures in both native and QTY keratins (Figure 2E). Figure 2F shows the FT-IR spectrum with characteristic peaks. The peaks at 3272 cm−1 resulted from the stretching vibration of O–H and N–H, which caused the band at 2960 cm−1 corresponding to the C–H stretching vibration and the band at 1634 cm−1 corresponding to the carbonyl C=O stretching vibration of the amide I bond. The band at 1521 cm−1 was caused by the N–H plane bending and C–H stretching vibration of the amide II bond, while the band at 1239 cm−1 was caused by the C–N stretching vibration, C–C stretching vibration, N–H bending vibration, and C=C bending of the amide III bond.[8] The characteristic peaks of QTY variants were consistent with native recombinant keratins, demonstrating similarity in their chemical structures.
Subsequently, we conducted contact angle measurements of native keratins and QTY variants to compare their hydrophobicity (Figure 2G).[9] According to the average of three measurements, the contact angles of RK31, RK33A, RK81, and RK83 were 73.73°, 70.60°, 73.03°, and 71.24°, respectively. Conversely, the contact angles of RK31QTY, RK33AQTY, RK81QTY, and RK83QTY decreased to 39.56°, 41.35°, 47.31°, and 42.23°, respectively (Figure 2H), which were closer to those from keratin extracts (approximately 30°).[9] Our results showed that the water solubility of native keratins was significantly enhanced by QTY modification, with no changes in their secondary and chemical structures.
2.3 Homotypic self-assembling water-soluble keratins
Self-assembling is one of the most remarkable features of keratins that enables many of their biomedical applications.[1, 7] The process of self-assembly is often affected by the placement of specific amino acids and varying degrees of hydrophilicity through the formation of hydrogen bonds. Herein, we tested the impact of QTY design on the self-assembling properties of keratins. The heterotypic self-assembly was commonly achieved by mixing type I keratin and type II keratins.[27] The processes for both RK31/RK83 and RK31QTY/RK83QTY were inspected using transmission electron microscopy (TEM) under different pH conditions (Figure 3A). Based on our previous study, the concentration of keratins was selected as 0.32 mg/mL, and the assembly time was 12 h.[7] RK31 and RK83 self-assembled with conventional gradient dialysis in urea at pH 7.4 and 3.4 but did not self-assembly at pH 5.4 due to the isoelectric point of protein. Similarly, the self-assembling of RK31QTY/RK83QTY was also observed at pH 7.4 and 3.4 without gradient dialysis in urea. The fiber diameters were then analyzed by ImageJ (Figure S2), and a larger size distribution was found at pH 3.4 than at pH 7.4. TEM images for other heterotypic self-assemblies with combinations of RK31, RK33A, RK81, RK83, as well as their corresponding QTY variants at pH 3.4 are shown in Figure 3B. Higher values in fiber diameters, including RK31QTY/RK81QTY (68 nm), RK31QTY/RK83QTY (62 nm), RK33AQTY/RK81QTY (62 nm), and RK33AQTY/RK83QTY (67 nm), were observed in QTY keratin groups (Figure S3), whereas the native keratin groups showed RK31/RK81 (59 nm), RK31/RK83 (53 nm), RK33A/RK81 (46 nm), and RK33A/RK83 groups (53 nm). This indicated that larger diameters of fiber represented the stronger self-assembling properties of QTY variants.
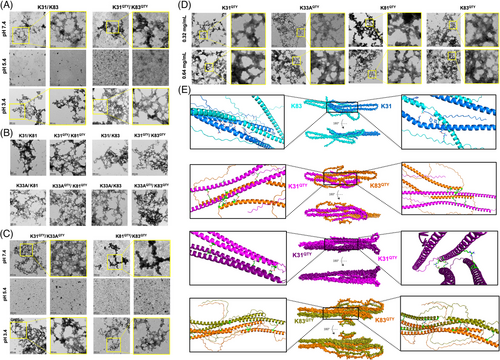
We next investigated whether the water-soluble keratins could perform homotypical self-assembly, which was not achieved for the native recombinant keratins according to previous publications.[7, 28] Unexpectedly, homotypical self-assembly was observed for all water-soluble QTY–keratin variants at both pH 7.4 and 3.4 (Figures 3C and S4). TEM images of the RK31QTY/RK33AQTY group display a more interconnected self-assembled fiber network at pH 3.4, whereas the self-assembly of RK81QTY/RK83QTY was weaker at both pH 7.4 and 3.4 compared to RK31QTY/RK33AQTY, which was attributed to the formation of only short rods and low-order aggregates at pH 7.4. To further explore the homotypic self-assembling property of water-soluble keratins, we evaluated their behaviors at different concentrations of 0.32 and 0.64 mg/mL (Figures 3D and S5). RK31QTY, RK33AQTY, RK81QTY, and RK83QTY assembled to form large areas of tightly interwoven fiber networks at pH 3.4, with average diameters of 40, 41, 35, and 39 nm at 0.32 mg/mL, respectively. Increasing keratin concentration resulted in fiber filaments with feathers of thicker diameter, more tightly woven and larger network structures. The average diameters of RK31QTY, RK33AQTY, RK81QTY, and RK83QTY increased to 50, 47, 44, and 46 nm at 0.64 mg/mL, respectively. Our results showed that the design of water-soluble keratins not only enhanced their heterotypic self-assembling property but also endowed the QTY variants with homotypic self-assembling ability. To better understand the self-assembling mechanisms of native and QTY keratins, interactions between RK31/RK83, RK31QTY/RK83QTY, RK31QTY/RK31QTY, and RK83QTY/RK83QTY were assessed by molecular docking using HADDOCK.[29, 30] The cluster with the least HADDOCK score was selected to further analyze the fold reliability using the ProSA server. As shown in Figure 3E, RK31/RK83 complex contained interacting residues that were involved in H-bonding and salt bridges, which was accounted for the assembly. Similar interactions were also observed in the complexes RK31QTY/RK83QTY, RK31QTY/RK31QTY, and RK83QTY/RK83QTY, and more extensive networks were observed in the assembly of QTY keratins. The increasing van der Waals and electrostatic interactions both contributed to the homotypic self-assembly of water-soluble keratins (Table S2). The simulation data further supported our results about enhancing self-assembling properties of keratins with QTY redesigns.
2.4 In situ gel-forming property of water-soluble keratins
Considering the self-assembly of QTY variant keratins in physiological environments, we supposed that they could also be able to directly form gels at wound sites for their most common usage as hemostats.[31, 32] To test our hypothesis, an open full-thickness wound of 1.5 cm × 1.5 cm was created on the back of SD rats after anesthesia, followed by the application of 50 mg keratin extract, RK31QTY, RK83QTY, RK31QTY/RK83QTY, and RK31/RK83 at the wound sites (Figure 4A). Interestingly, keratin extract group completely dissolved within 3 min, which finally became flowing liquid instead of a cross-linked gel. The RK31/RK83 group was infiltrated by tissue exudate and did not form gel due to their poor water solubility. In contrast, RK31QTY, RK83QTY, and RK31QTY/RK83QTY groups all absorbed tissue exudate to form a translucent and gel-like protective layer on the wound site within 3 min. This structure achieved good gas exchange and maintained the moisture environment at the wound site. The morphology of the keratin hydrogels was observed using SEM (Figure 4B). Gel-like substances were formed by RK31QTY and RK83QTY alone or in the RK31QTY/RK83QTY mixture group with highly crosslinked network and micropores as their internal architectures. However, the SEM image of wet RK31/RK83 also showed a porous structure, but the mixture was fragile and did not form hydrogels. In order to further investigate the properties of keratin gels, their rheological properties were examined. In the test frequency range, the energy storage modulus (G′) of the QTY variant keratins was much higher than the loss modulus (Gʺ), indicating that the protein had obvious gelation behavior and good viscoelasticity. The combination of RK31QTY and RK83QTY exhibited better rheological properties than RK83QTY and RK31QTY alone. These results were consistent with the self-assembling behaviors we observed in experiments.

2.5 Enhanced wound healing property of keratin through a water-soluble design
Hemostasis is one of the primary biomedical applications for keratins.[8] We thus compared the hemostatic performance of different types of keratins including keratin extract, RK31/RK83, RK31QTY, RK83QTY, and RK31QTY/RK83QTY using a liver hemorrhage model (Figure 5A,B). The quantification of blood loss per unit body weight of animals was determined to be 1.61, 1.83, and 1.59 mg/g for the RK31QTY, RK83QTY, and RK31QTY/RK83QTY treatment groups, respectively (Figure 5C). The values were notably lower than those of the groups treated with keratin extract (1.86 mg/g) and RK31/RK83 (2.08 mg/g). In addition, the hemostatic time was significantly shortened after treatments with RK31QTY, RK83QTY, and RK31QTY/RK31QTY, suggesting superior hemostatic performance of QTY variant keratins (Figure 5D). Next, we evaluated the wound healing property of QTY variant keratins by establishing a full-layer back skin resection model in SD rats. After administration of keratins in each group, wound sites were photographed and tissues were collected at 0, 3, 7, 14, and 21 days (Figure 5E). Hematoxylin–eosin (H&E) staining (Figure 5F) and Masson staining (Figure 5G) were performed for the histopathological and collagen deposition investigation. A difference in wound healing rate was observed among different treatment groups at 21 days. On day 7, the healing rates of the RK31QTY, RK83QTY, and RK31QTY/RK83QTY groups were approximately 58.68%, 56.90%, and 62.26%, respectively, which were significantly higher than the ∼42.83% of the RK31/RK81 group and 47.99% of the keratin extract group (Figure 5H). On day 14, the wound healing rates of the RK31QTY, RK83QTY, and RK31QTY/RK83QTY groups were approximately 95.01%, 94.55%, and 96.51%, respectively, and the wounds were completely covered by new tissues. In comparison, the healing rates of RK31/RK81 group and keratin extract group were approximately 84.49% and 82.37%, respectively, and no complete epidermis formed. All wounds treated with QTY variant keratins were almost completely healed on day 21, showing a good therapeutic effect compared with other treatment groups and the control group.
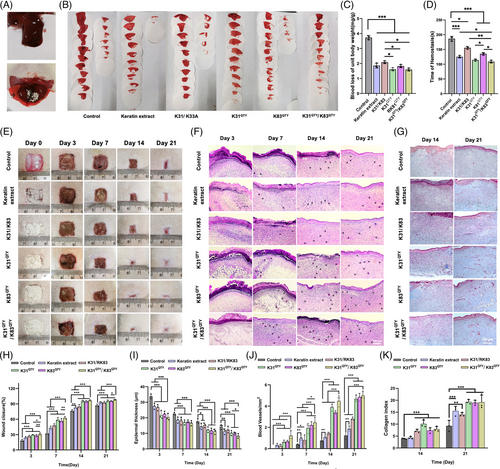
The epidermal thickness and angiogenesis were also quantified. On day 7, the epidermal thicknesses of the RK31QTY, RK83QTY, and RK31QTY/RK83QTY groups were approximately 17.07, 17.50, and 16.60 μm, respectively, which were thinner than 18.13 μm in RK31/RK83 group and 18.61 μm in keratin extract group (Figure 5I). On day 14, inflammatory cells decreased and fibroblasts increased significantly. The RK31QTY, RK83QTY, and RK31QTY/RK83QTY groups showed better healing effects than the other groups, with epidermal thicknesses of 12.35, 11.67, and 11.19 μm, respectively, which were much lower than the 15.23 μm in the RK31/RK83 treatment group and the 14.43 μm in the keratin extract treatment group. After 21 days of treatment, all testing groups were completely epithelialized and thickened, whereas the groups treated with QTY variant keratins maintained better performance throughout the process compared to other treatment groups. The water-soluble recombinant human hair keratin treatment group also showed stronger tissue angiogenesis at 14 and 21 days than the other groups (Figure 5J). Furthermore, Figure 5K shows collagen deposition in tissues at 14 and 21 days after wound repair. The collagen content of QTY variant keratin treatment groups was higher than that of the keratin extract group and the native recombinant keratin group. All results supported our claim that better hemostatic performance and wound healing properties of recombinant keratins were achieved by water-soluble design through the QTY code.
2.6 Biocompatibility evaluation of water-soluble recombinant keratins
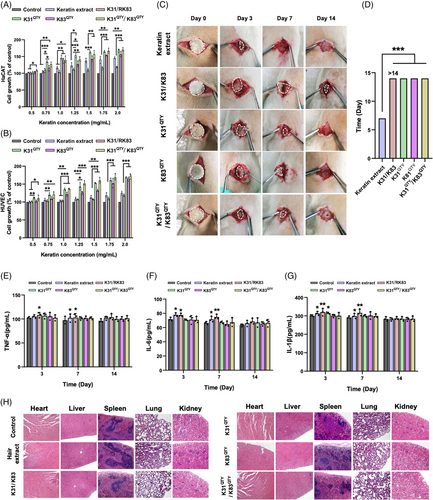
Finally, we tested whether water-soluble recombinant keratins would pose harmful effects on cell viability and animal health. The effects of water-soluble keratins on the proliferation of Human Immortalized Epidermal Cells (HaCaT) and Human Umbilical Vein Endothelial Cells (HUVEC) cells were evaluated by the Cell Counting Kit-8 (CCK-8) method. As shown in Figure 6A,B, the QTY variant keratins, keratin extract and native recombinant keratins all enhanced the proliferation of HaCaT and HUVEC cells, especially for QTY redesigned proteins with better performance. The growths of HaCaT and HUVEC cells were observed with keratins in a concentration-dependent manner. The effects of RK31QTY, RK83QTY, and RK31QTY/RK83QTY on cell proliferation were consistent with their bioactivities in tissue repair. Furthermore, in vivo biocompatibility of QTY variant keratins, keratin extract, and native recombinant keratins was assessed in Sprague-Dawley (SD) rats through subcutaneous implantation (Figure 6C). No animals died at the end of the experiment, and no abnormal manifestations were observed, including wound infections and behaviors of moving difficulty. As shown in Figure 6D, the size of keratins in subcutaneously implanted groups decreased with increasing time. The QTY variant keratins were completely degraded after 14 days post-surgery, whereas RK31/RK83 remained at the same time point. The QTY variant keratins showed faster degradation than the native recombinant keratins due to their higher water solubility. Additionally, the pro-inflammatory response after keratin implantation was evaluated by measuring the levels of inflammatory cytokines Interleukin (IL)-1β, IL-6, Tumor neccrosis factor alpha (TNF-α) in rats (Figure 6E–G). The QTY variant keratin group showed weaker responses in cytokine levels without serious inflammatory reactions compared with other keratin groups, implying that the water-soluble recombinant keratins were less likely to cause inflammation and had better biocompatibility. Besides, the H&E staining of major organs from SD rats, including the heart, liver, spleen, lung, and kidney, suggested no obvious organ damage or significant pathological differences in all groups (Figure 6H). Our findings identified the safety and biocompatibility of redesigned water-soluble recombinant keratins, which were used for hemostasis and wound healing applications without negative effects.
3 DISCUSSION
Here, we presented a simple methodology to design water-soluble variants of recombinant keratins that had enhanced bioactivities in terms of self-assembly, hemostasis and wound healing through QTY code redesign. Keratins have been extracted from various biomasses, including human hair, wool, feather, horn, and so on for biomedical applications.[33-36] Similar to many protein-based Chinese medicinal materials, keratins can benefit from enhanced water solubility to exert their full medicinal effects.[37] Although we have found some critical factors, such as protein purity, that significantly influence their hemostatic and wound healing properties,[12, 13] to a certain extent, the water solubility can be seen as a landmark for the development of keratin materials. The recombinant proteins have poor water solubility because keratin-rich biomasses, such as hair, wool, feather, and hoof, are insoluble in nature. We hypothesized that the bioactivities of recombinant keratins could be enhanced by increasing their water solubility. Thus, we randomly selected two type I keratins and two type II keratins as model proteins in this study. The in vitro and in vivo studies demonstrated that water-soluble keratins had superior activities of self-assembly, hemostasis, and wound healing than the native keratins. Surely, we will explore and assess the self-assembling, hemostatic, and wound healing properties of QTY variants of other keratins in the future experiments.
Water solubility evaluation of recombinant keratins was difficult since suspending keratins in water was impossible to remove, which would influence their absorbance detection. To resolve this problem, we first dissolved recombinant keratins into urea solution at different concentrations, which was then dialyzed using ultrapure water within a short time. Precipitates could be observed if the water solubility of recombinant keratin was lower than the pre-set concentration in urea solution. The solubility detection conducted at low keratin concentrations could avoid the interference of their self-assembly. In our study, we found that the water solubilities of recombinant native keratins were all below 50 μg/mL, but the solubilities of the corresponding keratins after QTY redesign were increased by at least 200 times. Yet human hair keratin extract still exhibited the highest water solubility among all samples, which could be explained by the fact that extracts with lower molecular weights were obtained during extraction process. Another reason could be that some other soluble proteins, such as keratin-associated proteins, were also included in human hair, which might contribute to improving the water solubility of keratin extract.[38] Furthermore, there are 17 kinds of keratins in human hair, but the content of each keratin is not equal.[9] Thereby, the improvement in the water solubility of recombinant keratins may be different after QTY code modification.
The QTY code is an efficient strategy for enhancing the water solubility of proteins but is also an applicable tool for solubilizing integral transmembrane proteins.[16-18] Our recent work also demonstrated that the reverse-QTY code was a feasible pathway to introduce specific hydrophobic interactions into functional hydrophilic proteins with clear-defined binding interfaces.[39] In all previous works, the proteins expressed in the E. coli system were typically obtained in the inclusion bodies and needed to be refolded before functional tests.[16-18] We here presented the first report where redesigned QTY variant proteins were obtained in the soluble fractions of E. coli expression. The soluble expression of keratin–QTY variants might mainly result from their highly abundant α-helices. All L, I, V, and F amino acids in α-helical domains were replaced in this study to enhance the water solubility of native keratins, and the change ratios ranged from 13.60% to 18.56%. Since the aim of QTY modification was to improve the solubility of hydrophobic proteins without structural changes, further research will be needed to investigate the relationships between structure and water solubility to decrease the transformation ratio of variants. In fact, many other approaches may also be potentially useful for the solubilization of keratins. For example, solubility-enhancing fusion tags, including Mal-bp, GST, DsbA, TRX, and T7PK, may inhibit protein aggregation and increase the solubility of fusion proteins.[40-42] Additionally, molecular chaperone fusion and soluble protein partner fusion are available approaches to enhance the expression of soluble proteins, and linking a soluble fragment into an insoluble protein is usually an efficient way to increase its solubility.[15]
Self-assembly is considered as one of the most remarkable features of keratin-based biomaterials.[1, 7, 43] The hard keratin-based exoskeletons of animals exhibit many indispensable functions, including self-defense, communication, sensing, and temperature regulation. The mechanics of these hierarchical composites are controlled by the self-assembly of keratins.[1, 44] Besides, the formation of cytoskeletal IF in soft epithelia is also dependent on the assembly of cellular keratins.[45, 46] These self-assemblies of hard keratins or soft (cellular) keratins are typically hetero-assembly, which requires a type I keratin with its pairing type II keratin.[44] Our work demonstrated that QTY variant keratins had the characteristics of heterotypic self-assembly and homotypic self-assembly, which further improved their uses in more biomedical applications. The native sequences of keratins contain a large amount of cysteine, glycine, and proline, a low content of lysine and methionine, and almost no tryptophan. The sequence characteristics render keratins susceptible to disulfide bond formation, which in turn generates strong intermolecular and intramolecular interactions for causing higher stability and lower solubility.[47, 48] Such protein characteristics are further reinforced by hydrogen bonds, salt bonds, and disulfide bonds.[49, 50] Since cysteines are not mutated during QTY design, disulfide bonds are likely to contribute to the self-assembly of keratins. Our computer simulation results suggested that QTY keratin variant complexes had more van der Waals and electrostatic interactions compared to the native variant complexes. It was reasonable that an increase in molecular interactions between water-soluble keratin monomers could contribute to their homotypic self-assembly in the aqueous environment. Moreover, the in situ self-assembly of water-soluble keratins at the wound sites was demonstrated, which was impossible to achieve with recombinant native keratin and keratin extract hydrogels at the same concentration due to their low water solubility and weak self-assembly ability, respectively.
Several reports have shown that the water solubility and purity of keratins significantly influence their hemostatic and wound healing properties.[12, 13] We also previously solubilized recombinant keratins through the reductive reactions that resembled the extraction process of natural keratins, whereas redesigned QTY keratins exhibited superior wound healing performance.[9] Compared to the reductive route, the QTY redesign approach had better controls on the molecular weight and amino acid composition of the final proteins, which contributed to their superior performance. Finally, different self-assembling profiles were found among different combinations of keratins. The stronger the self-assembly of keratins, the shorter the bleeding time. Keratins with stronger self-assembly properties also displayed faster closure speeds. Besides, the QTY variant keratins exhibited stronger hemostatic and wound healing properties than the native recombinant keratins.
Beyond the scope of the current study, we will plan to conduct further structural analysis using cryo-EM to determine the structural differences between the native keratins and QTY variants and will develop keratin-based peptides based on the structure–bioactivity relationships extrapolated from molecular dynamic simulations. In addition, keratin biomaterials have been widely used in various biomedical, environmental, and engineering applications.[2, 51] The homotypic self-assembling of recombinant keratins in aqueous conditions is helpful for the tissue regeneration in vivo and cell culture applications. Moreover, the self-assembling of recombinant keratins forms porous structure, which will be used for metal ion adsorption in environmental applications. Meanwhile, recombinant keratins with excellent mechanical properties through homotypic self-assembling will have an opportunity to be used for robotic engineering.
4 CONCLUSION
In summary, we demonstrated in this work the application of the QTY code to efficiently design water-soluble variants of keratins. The water solubility of redesigned proteins increased by over 200 times compared to that of their native counterparts. Both heterotypic self-assembly and homotypic self-assembly were observed for the QTY variant keratins, while previous works only reported heterotypic assembly for the native proteins. More importantly, self-assembly was conducted in an aqueous environment without urea, which opens a new avenue for keratin materials in more biomedical applications. Moreover, the redesigned proteins showed superior hemostatic and wound healing activities at the wound sites based on the in situ gel-forming feature of QTY variant keratins, and had no negative effects compared to the native recombinant keratins. Our study proved the feasibility of water-soluble keratin design by QTY code and demonstrated their superior performance in various biomedical applications.
ACKNOWLEDGMENTS
We acknowledge support from the National Natural Science Foundation of China (11972099 and 82202340), the Venture & Innovation Support Program for Chongqing Overseas Returnees (cx2020079), and the Scientific and Fundamental Research Funds for the Central Universities (2023CDJXY-050 and 2023CDJXY-051).
CONFLICT OF INTEREST STATEMENT
The authors declare they have no conflicts of interest.
ETHICS STATEMENT
All animal experiments were approved by the Animal Ethics and Laboratory Committee of Chongqing University.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




