A chiral spirofluorene-embedded multiple-resonance thermally activated delayed fluorescence emitter for efficient pure-green circularly polarized electroluminescence
Abstract
The simultaneous achievement of chiral multiple-resonance thermally activated delayed fluorescence (MR-TADF) emitters with narrowband and circularly polarized electroluminescence (CPEL) poses a challenge. Herein, a MR-TADF emitter, Spiro-BNCz, embedding spirofluorene structure was developed and chiral separated, whose emission peaks at 528 nm in toluene with Commission Internationale de L'Eclairage coordinates of (0.26, 0.69). The conjugation extension caused by embedding sulfur substituted spirofluorene on B/N framework shortens the singlet-triplet energy gap and increases spin-orbital coupling matrix element. Therefore, a fast reverse intersystem crossing rate constant of 2.8 × 106 s−1 and a high photoluminescence efficiency of 92% in film were achieved. The organic light-emitting diode (OLED) exhibits a maximum external quantum efficiency of 32.3%. Particularly, the circularly polarized OLEDs based on Spiro-BNCz enantiomers show symmetric CPEL with dissymmetry factors (|gEL|) ≈ 10−3.
1 INTRODUCTION
Circularly polarized organic light-emitting diodes (CP-OLEDs) have gained significant attention and have been extensively studied due to their potential applications in 3D displays.[1] The development of high-performance CP-OLEDs relies on efficient chiral emitters with key parameters such as CP luminescence (CPL), dissymmetry factor (g), high efficiency, and color purity, etc.[2] Chiral emitters can be categorized into chiral fluorescent molecules,[3] chiral phosphorescent materials,[4] and chiral thermally activated delayed fluorescence (TADF) emitters[5] based on the relaxation path of excited state electrons. Among them, the research focus has shifted towards chiral TADF materials due to their low cost, flexible molecular design, and wide color gamut emission.
In the pursuit of efficient chiral emitters and CP-OLEDs, it is important to consider high color purity emission. Multiple-resonance TADF (MR-TADF) emitter has been proposed in recent years as a solution to the problems of wide emission spectrum and poor color purity in OLEDs.[6] The specific design strategy is to introduce complementary localization effects, such as electron-withdrawing group (B, carbonyl, phosphine oxide groups) and electron-donating atoms (N, O, S), to separate the frontier molecular orbitals (FMOs) of the emitters. This approach shortens the singlet-triplet energy gap (∆EST) and induces a short range charge transfer (SR-CT) effect.[7] To achieve more efficient TADF performance, accelerating kRISC by introducing S atoms has been proven to be an effective strategy.[8] Yasuda group demonstrated that the introduction of S atoms can enhance the spin orbit coupling (SOC) between singlet and triplet states for TADF or MR-TADF emitters.[8–8] Similar strategies have also been successfully replicated in MR-TADF materials by Yang and Zheng group.[8] Notably, the SR-CT effect is particularly advantageous as it results in a narrowband emission spectrum and a high radiation transition rate for MR-TADF emitters,[8] surpassing traditional MR-TADF materials. Therefore, integrating chiral structures with MR frameworks allows for the efficient development of CP-MR-TADF emitters for narrowband CP-OLED applications.
In 2021, Li et al. successfully prepared the first CP-MR-TADF materials OBN-4CZ-BN (Figure 1 (1)) by attaching chiral octahydrobinaphthol derivatives to the periphery of DtBuCzB core for CP-OLEDs with a maximum external quantum efficiency (EQEmax) of 29.8% and an electroluminescence (EL) dissymmetry factor (|gEL|) of 10−3.[9] Subsequently, Zhu et al. further disrupted the symmetry of the peripheral region by incorporating phenothiazine and acridan substituents (BN4), increasing the racemic barrier and inducing CPL.[10] Recently, Yang et al. achieved a blue-green emitting CP-MR-TADF material (BN-MeIAc) by introducing the chiral donor units, and the corresponding CP-OLEDs showed a maximum EQE of 37.2% and |gEL| factors of 10−4.[11] Although the above CP-MR-TADF emitters exhibited high device performances and significant gEL values, there is a gap in color purity compared to the pure-blue and pure-green emissions defined by National Television System Committee (NTSC). Recently, our group achieved nearly pure-blue circularly polarized electroluminescence (CPEL) by utilizing a chelated N/B/O resonance system on chiral octahydrobinaphthol. The CP-OLEDs exhibited Commission Internationale de L'Eclairage (CIE) coordinates of (0.14, 0.10), accompanied by |gEL| ≈ 10−3.[12] Subsequently, a green CP-MR-TADF emitter (Czp-POAB) was developed by bonding chiral paracyclophane unit to the periphery of 2PXZBN, resulting in a CP-OLED with an EQE of 28.7% and CIE coordinates of (0.23, 0.65).[13] Even though the above chiral emitters exhibit CPEL signals, the possible configuration racemization may occur during the thermal evaporation process, which will greatly affect their practical application. Additionally, the reported CIEy values of aforementioned CP-MR-TADF emitters are not above 0.65, which is somewhat away from pure green emission. Therefore, efficient pure-green emitting CP-MR-TADF emitters with stable chiral configuration are worth further exploration.
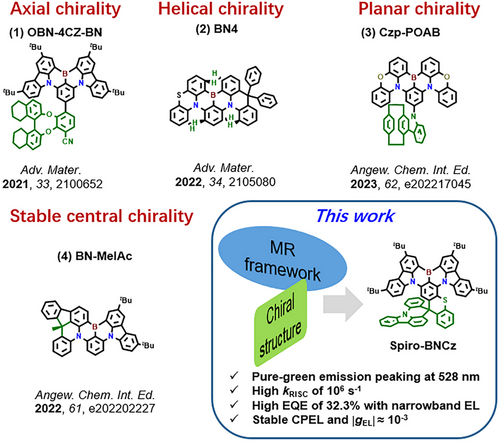
Given that the extension of the conjugated skeleton can effectively achieve the red-shift of narrowband characteristics, in this work, we introduced the sulfur-substituted spirofluorene structure featuring asymmetric chiral C-center into the MR framework. The resulting racemic MR-TADF material, named Spiro-BNCz, exhibited pure-green emission with a peak wavelength of 528 nm and a relatively narrow full width at half maximum (FWHM) of 42 nm, and CIE coordinates of (0.26, 0.69) in toluene. As expected, the introduction of S atom promotes the SOC between singlet (S1) and triplet (T1) states, endowing the Spiro-BNCz with a higher rate of reverse intersystem crossing (kRISC) of 106 s−1 in doped film. Through chiral resolution, a pair of chiral MR-TADF enantiomers, (R/S)-Spiro-BNCz, were obtained, which show symmetric CPPL in doped films, with a |gPL| factor of 10−3. Consequently, the OLEDs based on Spiro-BNCz exhibit a high EQE of 32.3% with pure-green narrowband emission. Notably, the stable chiral C-center structure avoids the racemization process of (R/S)-Spiro-BNCz during the evaporation plating process. The corresponding CP-OLEDs display symmetric CPEL, with a |gEL| factor of 10−3.
2 RESULTS AND DISCUSSION
As depicted in Scheme S1, the synthesis of racemic Spiro-BNCz involved a four-step reaction sequence utilizing readily available starting materials. The precursor unit, 2FTA, of spirofluorene structure was synthesized by catalytic intramolecular cyclization of 1-bromo-3-chloro-2,4-difluorobenzene with 2-mercaptobenzoic acid using CuI and H2SO4 as catalysts, respectively. Subsequently, a nucleophilic addition reaction between (2-(9H-carbazol-9-yl)phenyl) lithium and the carbonyl group of 2FTA was performed, followed by intramolecular dehydration to obtain Spiro-2FTA. Further introduction of the 3,6-di-tert-butylcarbazole group through nucleophilic substitutions resulted in the precursor compound Spiro-ClCz. Ultimately, the racemic Spiro-BNCz was synthesized through a one-pot lithiation-borylation-annulation reaction, providing a yield of 20% (Supporting Information).
Due to challenges encountered in chiral resolution, including incomplete peak separation and trailing phenomenon of Spiro-BNCz, a modified route was adopted to obtain the racemic precursors (R/S)-Spiro-ClCz, which served as the foundation for subsequent boration. Chiral separation of Spiro-ClCz was achieved in the mobile phase consisting of n-hexane and isopropanol, resulting in a separation ratio of 99:1. Two enantiomers, Spiro-ClCz-P1 and Spiro-ClCz-P2, were obtained in nearly equal proportions, with an enantiomeric excess (ee) value of 99% each (Figure S9 and S10). Additionally, thermogravimetric analysis (TGA) revealed that the Spiro-BNCz has a high decomposition temperature of 477°C at 5% weight-loss (Figure S11), demonstrating its excellent thermal stability.
Figure 2A illustrates the ultraviolet-visible (UV-vis) absorption and photoluminescence (PL) spectra of Spiro-BNCz in dilute toluene solution, and the key data are summarized in Table 1. In comparison to commonly reported MR-TADF materials,[14] Spiro-BNCz exhibits a distinct and intense peak indicative of SR-CT characteristic at 494 nm. Under excitation at 350 nm, Spiro-BNCz emits green light with a narrow bandwidth, characterized by a peak emission at 528 nm and CIE coordinates of (0.26, 0.69), which is consistent with pure-green display standard defined by NTSC. Meanwhile, a FWHM of 42 nm and a Stokes shifts of 34 nm are also recorded. To confirm the ICT nature of Spiro-BNCz, solvatochromism measuements of the absorption and emission spectra in solvents with different polarities were performed. As the polarity of the solvent increases, UV-visible absorption spectra of Spiro-BNCz exhibit almost identical behavior in different solvents. But the maximum emission peaks of Spiro-BNCz are 515 nm in n-hexane, 528 nm in toluene, 526 nm in tetrahydrofuran, 533 nm in dichloromethane and 527 nm in ethyl acetate, demonstrating its ICT nature (Figure S12).

| Emitter |
λabsa (nm) |
λPLb (nm) |
FWHMc (nm) |
S1d (eV) |
T1e (eV) |
∆ESTf (eV) |
ΦPLg (%) |
τph (ns) |
τdh (μs) |
kfi (108 s−1) |
kISCi (108 s−1) |
kRISCi (105 s−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spiro-BNCz | 494 | 528 | 42 | 2.56 | 2.46 | 0.10 | 92 | 1.6 | 3.6 | 4.9 | 1.3 | 2.2 |
- Abbreviation: FWHM, full width at half maximum.
- a Absorption peak corresponding to ICT nature.
- b Emission peak at room temperature measured in toluene solution (5.0 × 10−5 M).
- c Full width at half maximum of PL spectrum.
- d Calculated by the fluorescence spectrum at room temperature.
- e Calculated by the phosphorescence spectrum at 77 K.
- f Estimated by S1 and T1.
- g Absolute photoluminescence quantum yield measured in doped film.
- h prompt decay and delayed fluorescence lifetime in doped film.
- i Rate constant of fluorescence radiative decay (S1→S0): kf = ΦP/τp; rate constant of ISC (S1→T1); kISC = (1 - ΦP)/τp; rate constant of RISC (T1→S1); kRISC = Φd/(kISC·τp·τd·ΦP).
Subsequently, the low-temperature fluorescence and phosphorescence spectra of Spiro-BNCz in toluene were also measured at 77 K to evaluate the ΔEST value. As depicted in Figure 2B, the energy levels of S1 and T1 were determined to be 2.56 and 2.46 eV, respectively, yielding a ΔEST value of 0.10 eV, which is favorable for achieving TADF behavior through efficient up-conversion from T1 to S1. Owing to the rigidly multiple resonance framework, Spiro-BNCz exhibits a high photoluminescence efficiency (ΦPL) of 92% in a doped film using 2,6-Dczppy (2,6-bis(3-(9H-carbazol-9-yl)phenyl)pyridine) as the host material (Figure S13). Additionally, short transient fluorescence lifetime (τp) of 1.6 ns (Figure S14b) and long-lived delay lifetime (τd) of 3.6 μs were recorded at 300 K. Based on the aforementioned ΦPL and τp/τd values in the doped film,[15] the rate constants for fluorescence radiative decay (kf), intersystem crossing (kISC) and reverse intersystem crossing (kRISC) were calculated, and listed in Table 1. To the best of our knowledge, the kRISC value of 2.2 × 105 s−1 of Spiro-BNCz is relatively high compared to previously reported MR-TADF emitters, mainly due to the introduction of S atom, which enhances the SOC value, and the fusion strategies employed, which reduce the ∆EST value, leading to efficient reverse intersystem crossing.
The chiroptical properties of (R/S)-Spiro-BNCz were investigated in both the ground and excited states using circular dichroism (CD) and CPL spectra, respectively. As shown in Figure 2E, the chiral enantiomers display distinct mirror-image CD spectra in both toluene solutions and neat films. Based on the measured and simulated CD data in toluene solution, P1 compound was assigned as (R)-Spiro-BNCz, while compound P2 was assigned as (S)-Spiro-BNCz (Figure S15). The observed Cotton effect signals in the range of 300–400 nm can be attributed to the characteristic absorption of chiral spirofluorene moiety. The enantiomers display mirrored Cotton effect signals due to the SR-CT absorption at around 500 nm, which were relatively weaker compared to the absorption signals of the chiral spirofluorene moiety, indicating that the chiral moiety contributes less to the distribution of FMO.[16] The CPL spectra of doped films based on (R/S)-Spiro-BNCz exhibit almost mirror-image relationships with a gPL factor of 1.3 × 10−3 for (R)-Spiro-BNCz and −1.3 × 10−3 for (S)-Spiro-BNCz at 528 nm. This observation confirms that the fusion strategy of chiral units induces the formation of chiral configurations and leads to the emergence of CPL signals in the MR-TADF enantiomers.
To better understand the photophysical properties of spiro-BNCz, the time-dependent density functional theory (TD-DFT) using B3LYP/6-31 g(d,p) and SCS-CC2/cc-pVDZ methods[17] were further performed. Thehighest occupied molecular orbital/ the lowest unoccupied molecular orbital (HOMO/LUMO) electron distributions, oscillator strengths (f), S0-S1 transition energies (∆E, λ) and SOC matrix elements between the T1 and S1 states are depicted in Figure 3.

The observed classical MR effect in Spiro-BNCz is attributed to the relative localization effect of the B and N atoms. Specifically, the LUMOs are localized on the B atom and its ortho- and para- positions, while the HOMOs are localized on the N atoms and the meta-position relative to the B atom. Due to the effective extension of the HOMO by the introduction of S atom, Spiro-BNCz exhibits a more delocalized electron distribution of FMO. Meanwhile, the extension of π-conjugation length of the molecule and the reduction in energy gap (Eg) of the FMO result in green emission. Notably, Spiro-BNCz displays a large f value of 0.2426, ensuring the strong PL intensity. Similar to our previous work,[7, 18] the fusion strategy employed in Spiro-BNCz increases the delocalization of FMOs. The natural transition orbital (NTO) analyses of Spiro-BNCz (Figure S17), demonstrated charge transfer nature of S1 state. According to theoretical calculations using SCS-CC2/cc-pVDZ method, the ∆EST value was 0.10 eV, which is in good agreement with the experimental value. Furthermore, a large SOC matrix element, < S1|ĤSOC|T1 > , of 0.36 cm−1 was obtained for Spiro-BNCz in the T1→S1 channel, which is beneficial for spin-flipping exciton-transformation to achieve efficient TADF process. The T2 energy level is 3.23 eV, which is higher than S1, and the SOC value of S1 and T2 is 1.08 cm−1. Obviously, SOC value of S1 and T2 is three times higher than SOC value of S1 and T1. And, from the energy level diagram, it can be found the ΔEST value of S1-T2 is 0.43 eV, which is larger than ΔEST value of S1-T1. Therefore, the RISC process from T1 to S1 is dominated.
As shown in Figure 3, the gPL factor of chiral enantiomers was calculated using the Equation g = 4 × |μm|·|μe|·cosθe,m/(|μm|2 + |μe|2) (μm and μe are the magnetic transition and electric transition dipole moment vectors, respectively, and θ is the angle between the μm and μe).[19] For Spiro-BNCz, μm, μe, and θ values were estimated as 4.91 × 10−18 esu cm, 7.42 × 10−21 erg G−1, and 79.0°, respectively. Consequently, gPL was calculated as 1.2 × 10−3, which is in good agreement with the experimental value.
Motivated by pure-green emission and excellent MR-TADF property of Spiro-BNCz, an OLED (D-Spiro-BNCz) was fabricated and investigated with the optimized device structure of ITO/ HAT-CN (hexaazatriphenylene-hexacarbonitrile, 5 nm)/ TAPC (di-(4-(N,N-ditolylamino)phenyl)cyclohexane, 40 nm)/mCP (1,3-Di-9-carbazolylbenzene, 5 nm)/ 2,6-Dczppy : 5 wt% Spiro-BNCz (20 nm)/ TmPyPB (1,3,5-tri((3-pyridyl)-phen-3-yl)benzene, 45 nm)/ LiF (1 nm)/ Al (100 nm). The energy levels of each functional material are summarized in Figure 4A.
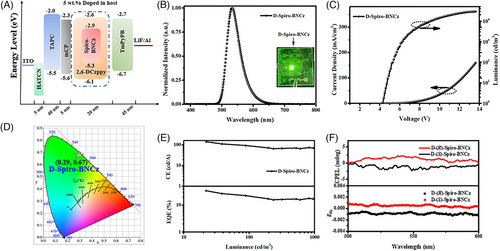
The EL characteristics of D-Spiro-BNCz are illustrated in Figure 4, and the key EL data are summarized in Table 2. D-Spiro-BNCz displays EL emission peaking at 533 nm with a FWHM of 48 nm and CIE coordinates of (0.29, 0.67), indicating good color purity of electrogenerated green emission. Notably, the CIEy value (0.67) of D-Spiro-BNCz surpasses that of previously reported green CP-MR-TADF materials-based OLEDs, to the best of our knowledge, demonstrating the highest purity among them. Furthermore, the variations in the EL spectra are negligible as the driving voltage increases from 5 to 9 V, demonstrating excellent spectral stability (Figure S18).
| Device |
λELa (nm) |
FWHMb (nm) |
Vonc (V) |
Lmaxd (cd/m2) |
CEe (cd/A) |
PEf (lm/W) |
EQEg (%) |
CIEh (x, y) |
|---|---|---|---|---|---|---|---|---|
| D-Spiro-BNCz | 533 | 48 | 4.2 | 29266 | 140.5/92.3/70.2 | 90.1/55.7/35.5 | 34.2/22.5/17.2 | 0.29, 0.67 |
- Abbreviations: CIE, Commission Internationale de L'Eclairage; EQE, external quantum efficiency; FWHM, full width at half maximum;
- a The electroluminescence peak.
- b Full width at half maximum of electroluminescence spectrum.
- c Turn-on voltage at 1 cd/m2.
- d Maximum luminance.
- e Maximum current efficiency and current efficiency at 100 and 1000 cd/m2.
- f Maximum power efficiency (PE) and power efficiency at 100 and 1000 cd/m2.
- g Maximum external quantum efficiency and external quantum efficiency at 100 and 1000 cd/m2.
- h Commission Internationale de l’Éclairage color coordinates measured at 10 mA/cm2.
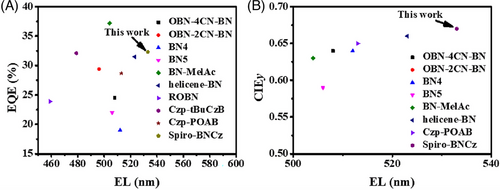
Significantly, D-Spiro-BNCz exhibits good OLED performances with a high maximum current efficiency (CEmax) of 140.5 cd/A and an EQEmax value of 34.2%, respectively, which are comparable to the current efficient green narrowband CP-OLEDs reported (Table S3). These results can be expalined by the calculation formula of EQE as: EQE = γ × ηeue × ΦPL × ηout,[20] where EQE depends mainly on ΦPL and ηout of TADF materials. Therefore, the distinguished EQE value of D-Spiro-BNCz can be attributed to the high ΦPL of emitter (92%) and horizontal emitting dipole ratio (75%) (Figure S19) of the emitting layers. Additionally, D-Spiro-BNCz displayed EQEs of 22.5% and 17.2% at 100 and 1000 cd/m2, respectively. Unbalanced carrier injection, transport, and combination within the devices are the main reasons for severe efficiency roll-off. Furthermore, the significantly lower EQE values in the high luminance region implies that kRISC is not high enough. Importantly, the EQEmax of D-Spiro-BNCz is only a little lower than that the highest efficient CP-OLED based on BN-MelAc (Figures 1 and 5), while its CIEy is the purest green emission among reported CP-OLEDs (Table S3 and Figure 5). Moreover, CP-OLEDs based on (R)-Spiro-BNCz and (S)-Spiro-BNCz as guests with the same device configuration were also fabricated to investigate the CPEL performances. The stable carbon center chirality of these compounds effectively prevents configuration racemization during the thermal evaporation plating process. Due to the obvious CPPL characteristics in doped films, the D-(R)-Spiro-BNCz and D-(S)-Spiro-BNCz display mirror symmetric CPEL spectra with gEL factors of 8.1 × 10−4/−1.0 × 10−3 (Figure 4F), respectively. As a result, efficient CP-OLEDs with pure-green CPEL were successfully achieved.
3 CONCLUSION
In conclusion, the fusion of sulfur-substituted spirofluorene and boron-nitrogen skeleton in Spiro-BNCz leads to the generation of pure green emission with CIE coordinates of (0.26, 0.69). Benefitting from the effective introduction of S atom, Spiro-BNCz shows a high SOC value of 0.36 cm−1, a high kRISC of 2.8 × 106 s−1, and a high ΦPL of 92% in the doped film. The separated (R/S)-Spiro-BNCz enantiomers show mirror imaged CPL spectra with a |gPL| factor of 1.3 × 10−3. Consequently, the OLED based on Spiro-BNCz achieves an EQEmax of 32.3% with CIE coordinates of (0.29, 0.67), representing one of the highest reported values for pure green MR-TADF materials-based OLEDs. Notably, the CP-OLEDs based on (R/S)-Spiro-BNCz enantiomers exhibit symmetric CPEL spectra with an approximate |gEL| factor ≈ 10−3. These promising results highlight the successful integration of the B/N skeleton and spirofluorene structure, which imparts the chiral CP-MR-TADF material with pure green CPL. These findings provide valuable insights for guiding the molecular design of efficient and full-color CP-MR-TADF emitters for CP-OLEDs.
ACKNOWLEDGMENTS
This work is supported by the National Natural Science Foundation of China (grant numbers: 92256304, 21975119, and 22171092).
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.




