Aggregation-induced emission artificial enzyme (AIEzyme) with DNase-like activity: Imaging and matrix cleavage for combating bacterial biofilm
Abstract
DNase-catalyzed hydrolysis of extracellular DNA (eDNA) has been widely employed to eradicate intractable biofilms. Although aggregation-induced emission (AIE) has become the ideal tool for killing planktonic bacteria, AIE luminogens (AIEgens) often lack DNase-mimetic activity, so as to suffer from poor anti-biofilm capacity. Here, an “AIEzyme”, a kind of AIE nanomaterial with enzyme-like activity, is designed and synthesized, where the AIEgens are used as the ligands of Zr-based coordination polymer nanoparticles. Not only does AIEzyme have enduring DNase-mimetic activity with low activation energy, but also structural rigidity-stabilized fluorescence. Due to the long-acting hydrolysis for eDNA in biofilm, AIEzyme can efficiently disorganize the established biofilms with good penetrability and realize the healing of superbug-infected wounds under only one dose of AIEzyme. This work provides a strategy to endow ordinary AIEgens with DNase-like and anti-biofilm activities. Moreover, AIEzymes can be observed by virtue of their own AIE character, facilitating the study on self-positioning and residual amount of AIEzymes in wounds. The concept “AIEzyme” would hopefully stimulate the tremendous expansion for the tool kits and the application of AIEgens and artificial enzymes.
1 INTRODUCTION
DNA, the carrier of genetic information, is a highly stable polymer, which is linked by phosphate ester bond with the inert phosphate backbone.[1-3] The cleavage of the phosphate ester bond is essential for biological phenomena, biomedicine, and biosensors.[1, 4] For example, the DNase-catalyzed hydrolysis of phosphate ester bond has been widely used for combating bacterial communities. Bacteria generally tend to attach to surfaces of organisms and devices by self-synthesized extracellular polymeric substances (EPS) to generate biofilm.[5] In EPS, extracellular DNA (eDNA) plays the role of “bridge” and “glue” in connecting bacteria with other EPS components and enhancing bacterial adhesion, which is pivotal for the formation of mature and sessile bacterial communities.[6-8] Under the aegis of EPS, biofilm-enclosed bacteria are prominently more resistant to antibiotics, reactive oxygen species (ROS), high temperature, and host immune defenses than planktonic ones.[9-13] Hence, biofilms involve in the majority of bacterial infections, causing chronic wounds, implant failure, persistent infections, and even death.[10-13] Although DNase and its composites have been recognized as anti-biofilm reagents to destroy eDNA and then eradicate intractable biofilms,[14-16] they have some drawbacks, including complicated preparation process, high cost, and poor stability.[17] Moreover, they generally fail to monitor their self-position during the antimicrobial process in real time, because of the lack of imaging function. Therefore, it is urgent to excavate a powerful agent to simultaneously realize the eDNA cleavage and imaging.
To expediently realize the real-time observation during the antimicrobial process, fluorescence imaging is a highly desirable tool.[18, 19] However, the conventional fluorophores usually suffer from aggregation-caused quenching, greatly limiting their applications related to biology.[20] As an opposite phenomenon, aggregation-induced emission (AIE) solved the above problem, where AIE luminogens (AIEgens) show no or weak fluorescence in dilute solution, but strong emissions in the aggregate or solid state,[21, 22] due to the restriction of intramolecular motion (RIM) mechanism.[23, 24] So, AIEgens become a kind of ideal tool for imaging microorganisms, peptides, cancer cells, biological processes, and so on.[25-28] On the other hand, many AIEgens can also kill planktonic bacteria by photoinduced ROS, photothermal effect, and their own toxicity.[29-34] However, they are experts at killing planktonic bacteria rather than anti-biofilm,[19, 29-32] because the biofilm is markedly resistant to environment stresses (such as antibiotics, ROS, and high temperature).[9-13] Therefore, it is interesting and meaningful to develop an AIEgen with imaging function and DNase-like activity for improving the biofilm resistance performance of the AIE family.
Inspired by natural enzymes, artificial enzymes, particularly burgeoning types of nanomaterials with enzyme-like characteristics (i.e. nanozymes),[35, 36] have attracted extensive interest due to their high catalytic activity, high stability, and convenient preparation.[37-40] So far, most of the nanozymes show oxidoreductase-like character and catalyze small molecules as substrates.[41-43] In contrast, a few nanozymes mimic the activities of non-oxidoreductase and catalyze macromolecules (such as DNA and protein) as substrate.[4, 44] Although the cleavage of eDNA by DNase-like nanozymes has been reported less, DNase-like nanozymes still demonstrate the amazing application potential on biofilm disintegration.[8, 17, 45] Numerous studies have certified that the distinctive properties of nanozymes can stimulate their unique applications accordingly.[46-50] If DNase-mimicking nanozymes also possess AIE properties, this will be very interesting and valuable scenario for the simultaneous disintegration and imaging of biofilm.
Here, we successfully synthesized Zr-based coordination polymer nanoparticle (CPNP) by using 1,1,2,2-tetra(4-carboxylphenyl)ethylene (TCPE, a typical AIEgen) as a ligand (Figure 1A). On the one hand, Zr-CPNPs showed stable fluorescence in various solvents and temperatures due to the RIM effect in the rigid coordination structure (Figure 1B). On the other hand, CPNPs showed outstanding DNase-mimetic activity with high substrate affinity and low activation energy, which can hydrolyze various DNAs, including single-stranded DNA (ssDNA) and eDNA (Figure 1B). So, we call the AIE nanomaterial with enzyme-like characteristics as “AIEzyme”. Due to the high-efficiency DNase-mimetic activity, good durability, and strong penetrability, AIEzyme not only enduringly prevented biofilm formation (Figure 1C) but also dispersed mature biofilms (Figure 1D). On the account of the stable AIE character, AIEzyme also showed the capacity of imaging bacterial cells and self-positioning in biofilm. Particularly, CPNPs can maintain prolonged and stable anti-biofilm efficiency on the superbug-infected wound with acceptable safety and simultaneously realize the real-time monitoring of AIEzyme residue (Figure 1E). We expect that the proposed AIEzyme would not only open an interesting avenue for the biofilm combating and the wound healing with process monitoring but also provide a new opportunity for expanding the applications of AIEgens and nanozymes.
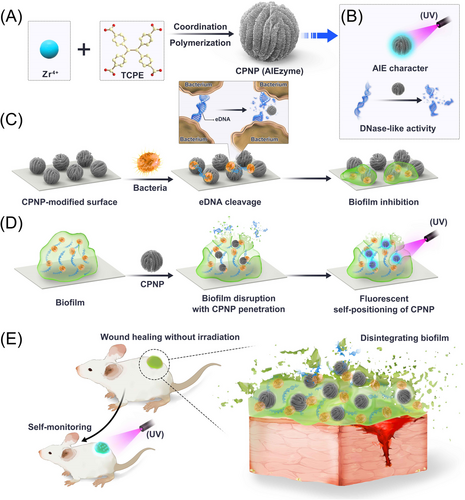
2 RESULTS AND DISCUSSION
2.1 Synthesis and characterization of Zr-CPNPs
Zr-CPNPs were synthesized via the coordination polymerization approach at 90°C with ZrCl4 and AIE ligand (TCPE) as precursors (Figure 1A) and emitted sky-blue fluorescence in solid state (Figure 2A). Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images demonstrate that CPNPs show well monodisperse and uniform walnut-like morphology with the average diameter of about 200 nm (Figure 2B). Chemical composition and distribution of CPNPs are confirmed by energy-dispersive X-ray spectroscopy (Figure 2C and Figure S1). Powder X-ray diffraction pattern of CPNPs (Figure 2D) gives well-resolved peaks, suggesting high crystallinity. Fourier transform infrared spectra after the formation of CPNPs shows an obvious redshift of CO stretching frequency from 1682 to 1608 cm−1 (Figure 2E), confirming the coordination of Zr4+ ions with the carboxylate groups of TCPE ligands.[51] TCPE and CPNP have the typical OH hydrogen stretching bonds, as a broad absorption band at 3410 and 3430 cm−1, respectively.
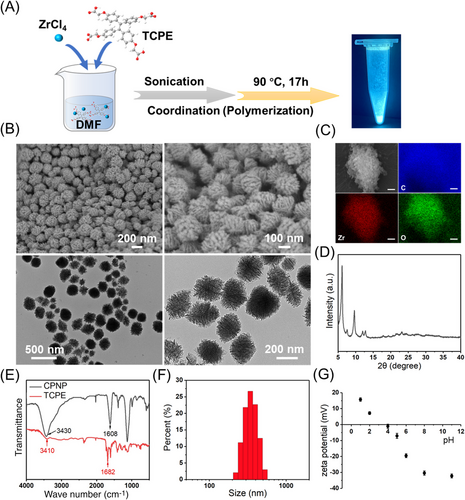
Dynamic light scattering test shows that the hydration diameter of Zr-CPNPs was about 300 nm, suggesting the existence of the hydration layer (Figure 2F). In addition, the hydrous size of CPNPs was stable within wide pH range (5–11), whereas the size increased sharply at pH 4 (Figure S2). To explain this phenomenon, the influence of pH on zeta potentials of CPNPs was investigated (Figure 2G). The surface charge of CPNPs tends to zero at pH 4, suggesting the isoelectric point is approximately at pH 4. The disappearance of electrostatic repulsion at pH 4 leads to the aggregation of CPNPs, but otherwise CPNPs have stable size due to the strong electrostatic repulsion when the pH is not near the isoelectric point.
2.2 Photophysical properties of Zr-CPNPs
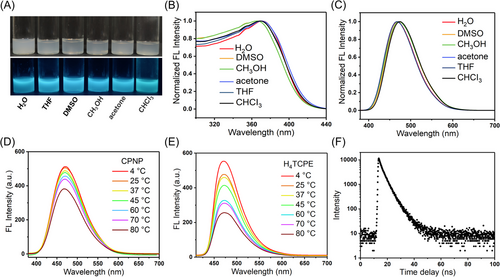
As expected, CPNPs emit bright sky-blue fluorescence UV light in solution state (Figure 3A). Interestingly, the fluorescence of CPNPs shows negligible solvent effects (Figure 3B,C). The excitation and emission spectra are not affected by the polarity of solvents (Figure S3), suggesting insolubility in various solvents and the negligible twisted intramolecular charge transfer effect in polar media. Considering temperature has a strong effect on the degree of rotation of these dynamic phenyl rings, the fluorescence of CPNPs was researched at various temperatures (Figure 3D). Upon increasing temperature from 4 to 80°C, the emission intensity of CPNPs at 470 nm gradually decreases. For many AIEgens, rotations of the phenyl rings speed up upon increasing temperature, and then, the increased molecular motions can reduce and even quench the fluorescence.[52] The temperature-dependent fluorescence of CPNPs is consistent with many other AIEgens.[52-54] It is worth noting that the change in fluorescence intensity of CPNPs is much smaller than free ligand TCPE (Figure 3E and Figure S4). These above results strongly indicate that the phenyl ring rotors of CPNPs are partially restricted in the stable rigid structure of CPNPs and then RIM effect in the rigid construction induces the stable emission. In addition, the fluorescence lifetime of CPNPs was determined to be 3.66 ns (Figure 3F). The absolute quantum yield of CPNPs is 18.6% in water.
2.3 DNase-like activity of CPNPs
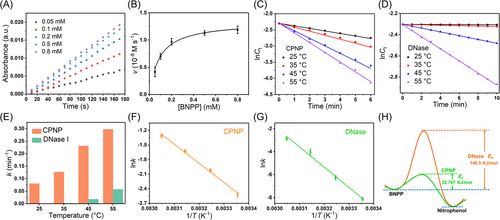
To comprehensively verify the DNase-like activity of CPNPs, various DNA model substrates, including bis(4-nitrophenyl)phosphate (BNPP), ssDNA, and bacterial genome DNA, were employed (Figure 5). As shown in Figure 5A–C, CPNPs can catalyze the hydrolysis of BNPP (DNA dinucleotide analog) into nitrophenol, which has absorbance peak at 410 nm. When the mixture of Zr4+ and TCPE was used to replace CPNPs, there was no observable absorption peak. These results indicate that DNase-mimetic activity occurs only after the formation of coordination polymer. In addition, the DNase-like activity of CPNPs shows pH dependence and achieves highest activity at pH 8.0 (Figure 4D and Figure S5), which is similar to natural DNase.[55]
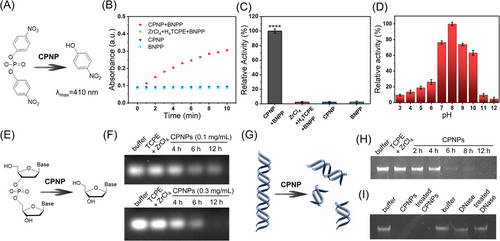
To further verify the DNase-like activity of CPNPs, the agarose gel (1%) electrophoresis was used on recording the hydrolysis of ssDNA (Figure 4E). Under the catalysis of CPNPs (0.1 mg mL−1), the DNA bands gradually became weak over time, indicating that ssDNA was cut into smaller fragments (Figure 4F). As for the higher dose of CPNPs (0.3 mg mL−1), the hydrolysis of ssDNA was accelerated, and the DNA bands disappeared completely after 12 h, demonstrating the complete degradation of ssDNA. In contrast, the control with the mixture of Zr4+ and TCPE showed that ssDNA remained stable under the same conditions, suggesting that the mixture of the precursors of CPNPs is ineffective and their coordination is important for the DNase-like activity.
Subsequently, the genomic DNA of Escherichia coli was extracted and used as the substrate (Figure 4G). As shown in the agarose gel electrophoresis (Figure 4H), the CPNPs can effectively degrade genomic DNA into fragments with increasing reaction time, and all the genomic DNA can be degraded within 12 h. Like the cases of BNPP and ssDNA, the precursors of CPNPs (the mixture of Zr4+ and TCPE) were still unable to catalyze the hydrolysis of genomic DNA, confirming that DNase-like activity appears only after the coordination of Zr4+. Further, when Zr4+ was replaced by Fe3+ and Mn2+, the obtained coordination polymers had no DNase-mimetic activity (data not shown), suggesting Zr4+ center in the coordination structure could act as the active site of DNase-like activity. According to the above results, we believe that the DNase-like activity indeed originates from the coordination structure of Zr4+ and TCPE, where Zr4+ acts as the active center, and the coordination structure stabilizes the substrate. Furthermore, numerous Zr4+ centers on each CPNP provided dense active sites, facilitating the DNA hydrolysis reactions. More detailed investigation on the catalytic mechanism will be conducted in the future work.
Particularly, CPNPs incubated at 37°C for 24 h still exhibited high catalytic activity, suggesting the stable rigid structure and activity of CPNPs (Figure 4I). In sharp contrast, DNase weakly cleaves the genomic DNA with 12 h, and treated DNase almost lost its activity, because of its unstable structure. In addition, CPNPs did not show obvious degrading effect toward the other two main EPS components, proteins (Figure S6) and polysaccharides (Figure S7). Considering that eDNA in biofilm originates from chromosomal DNA and is similar to the bacterial genome DNA,[56, 57] CPNPs are expected to degrade eDNA when combating biofilms.
2.4 Catalytic reaction kinetics of CPNPs
To reasonably evaluate the DNase-mimetic activity of CPNPs, the steady-state kinetics were investigated by changing the concentration of BNPP at a fixed concentration of CPNPs. The initial reaction rate increases with the increase of BNPP (Figure 5A), further manifesting the DNase-mimetic activity of CPNPs. These data are conformed to the Michaelis–Menten equation (Figure 5B), and the Km of CPNPs for BNPP is 0.09 mM. In addition, the concentrations of BNPP (Ct) under the catalytic hydrolysis of CPNPs and DNase were monitored at various temperatures (Figures S8 and S9). The hydrolysis rate increases with the temperature, indicating the higher temperature would accelerate the interactions between reactants and catalysts. A good linear correlation of lnCt (Ct indicates the concentration of BNPP) versus reaction time (t) is observed (Figure 5C), indicating the good pseudo-first-order kinetics of CPNPs, which is similar to DNase (Figure 5D). Further, the kinetic rate constant k is obtained to be 8.902 × 10−2 min−1 at 298 K, 1.270 × 10−1 min−1 at 308 K, 2.309 × 10−1 min−1 at 318 K, and 2.974 × 10−1 min−1 at 328 K, respectively, which are integrally higher than those of DNase (2.968 × 10−4 min−1 at 298 K, 1.92 × 10−3 min−1 at 308 K, 1.779 × 10−2 min−1 at 318 K, and 5.742 × 10−2 min−1 at 328 K) (Figure 5E). According to the Arrhenius equation, the activation energy (Ea) of the BNPP hydrolyzation catalyzed by CPNPs or DNase was calculated by plotting lnk versus 1/T (Figure 5F,G). As shown in Figure 5H, the Ea value of CPNPs was 32.77 kJ mol−1, which was dramatically smaller than the one of DNase (146.5 kJ mol−1). The above results indicate that under the CPNPs, the minimum energy required for the occurrence of hydrolysis reaction is significantly reduced, which makes the reaction much easier.
2.5 Bacterial imaging based on AIEzyme
Considering CPNP simultaneously has the both AIE property and DNase-like activity, we call CPNP as “AIEzyme”, which represents a type of AIEgens with enzyme-like characteristics (Figure 1B). On account of the AIE property of CPNPs, the bacteria-staining performance of CPNPs on methicillin-resistant Staphylococcus aureus (MRSA) was evaluated. When incubating bacteria with CPNPs, the fluorescent signal can be clearly observed on bacterial cells with extremely low background fluorescence, showing the excellent bacterial imaging ability (Figure S10). To investigate the location of CPNPs on bacterial cells, untreated bacteria and CPNPs-strained bacteria were observed by TEM. CPNPs adsorb on the surfaces of cells, and all bacteria maintain their regular shape with well-defined cell walls and borders (Figure S11). The good staining capacity is presumably caused by the electrostatic interactions between the positively charged metal ions of the coordination compound,[58, 59] and the negative charges of bacteria, and the hydrophobic interaction between extracellular polysaccharides of bacteria and aromatic groups of AIEgens.[60] To further confirm the toxicity of CPNP to bacteria, a traditional plate counting method was conducted. For all groups, bacteria grew and reproduced smoothly on the agar plates (Figure S12), implying CPNP, TCPE, and Zr4+ cannot kill the planktonic bacteria. The results verify not only the staining capacity of AIEzyme on the surface of bacterial cells but also the inherent hypotoxicity of AIEzyme to bacteria.
2.6 Prohibiting biofilm generation by AIEzyme
These above results inspired us to evaluate the anti-biofilm performance of AIEzyme. Subsequently, its ability of prohibiting bacterial adhesion and biofilm generation was investigated (Figure 1C). MRSA suspensions were statically incubated with CPNP- and DNase I-coated glass slides for different lengths of time. As for the crystal violet staining assay (Figure 6A), MRSAs are abundantly adhered to the bare surfaces, whereas the adhesions of bacteria to CPNP- and DNase-coated surfaces are obviously reduced. Interestingly, biomass attached on the DNase-coated surface significantly increases after 24 h, whereas dramatically reduced adhesion biofilm is observed on the CPNP-coated surfaces even after 120 h (Figure 6B). Then, 3D confocal laser scanning microscopy (CLSM) demonstrates that the biofilm formation is intensively inhibited on CPNP-coated surfaces, obviously superior to DNase-coated surfaces (Figure 6C). With the aid of the excellent DNase-like activity of CPNPs, the average thickness of biofilms was less than 5 μm even after 24 h (Figure 6D), which was much thinner than the case on DNase-coated surfaces. To further confirm the anti-biofilm performance, E. coli were employed, and the results are similar to MRSA (Figure S13).
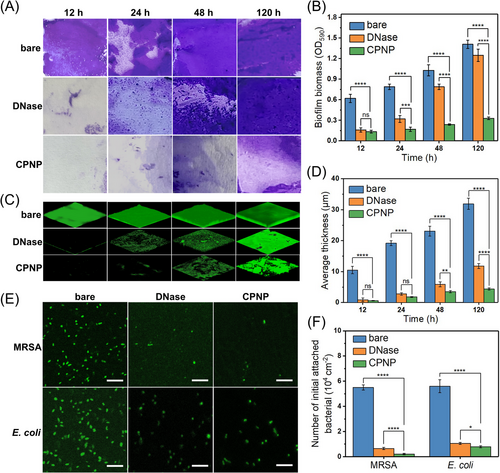
In addition, at the initial stage of the bacterial adhesion (after 60 min incubation), a lot of bacterial cells (MRSA and E. coli) are attached onto the bare surfaces (Figure 6E), whereas the adhesion amounts of bacteria to the CPNPs- and DNase-coated surfaces are dramatically reduced by more than 86.07% and 81.25%, respectively, compared to the bare surface (Figure 6F). Although both DNase and CPNP can reduce the bacteria adhesion amount, there is the obvious difference on the long-term biofilm inhibition performance of DNase and CPNP, which could be attributed to the vulnerable structure of natural DNase and the good stability of CPNPs. Hence, AIEzyme has an excellent biofilm inhibition performance superior to DNase.
2.7 Disorganizing formed biofilm by AIEzymes
To investigate whether AIEzyme can disperse formed biofilms, 24 h-old E. coli and MRSA biofilms were treated with different concentrations of CPNPs, followed by the crystal violet staining and 3D CLSM (Figure 7A). The remaining biomass and average thickness of the biofilms are significantly diminished by CPNPs in a dose-dependent manner (Figure 7B). Further, the abilities of CPNPs and DNase toward dispersing various ages of biofilms were evaluated (Figure 7C). DNase only causes the slight disruption for young biofilms (less than 12 h old), whereas CPNP strongly disintegrates biofilms at all time points (Figure 7D).

In general, the intact biofilms physically shield foreign materials from inward penetration in order to protect themselves. Considering the excellent anti-biofilm capacity of CPNPs, we speculated CPNPs could penetrate the inside of biofilms (Figure 1D). Subsequently, the location of CPNPs was observed by the virtue of AIE property. CPNPs could effectively penetrate the internal biofilms, and even a few of CPNPs have arrived at the bottom of the biofilm within 10 min (Figure 7E). This might be due to the synergetic effect of DNase-like activity and the sedimentation of CPNPs. The unchanged bacterial viability of MRSA and E. coli after different times of incubation with various concentrations of CPNPs (Figure 7F and Figure S14) also proved the poor biotoxicity of CPNP to bacterial cells, which was consistent with the other results (Figure S12). The low toxicity of CPNPs to planktonic bacteria excludes the possibility that the anti-biofilm ability of CPNPs is caused by the effect of CPNPs on cell viability. Taken together, it can be deduced that CPNPs can rapidly penetrate biofilms and deeply destroy eDNA due to the synergetic effect of DNase-like activity and sedimentation of CPNPs. Then, the biofilms will be finally disorganized, because of the collapse of the “bridge” of all biofilm components. More interestingly, the deep penetration of CPNPs can be self-certified by fluorescence, which will facilitate the study on the anti-biofilm process (Figure 1D).
2.8 In vivo antibacterial therapy and wound healing
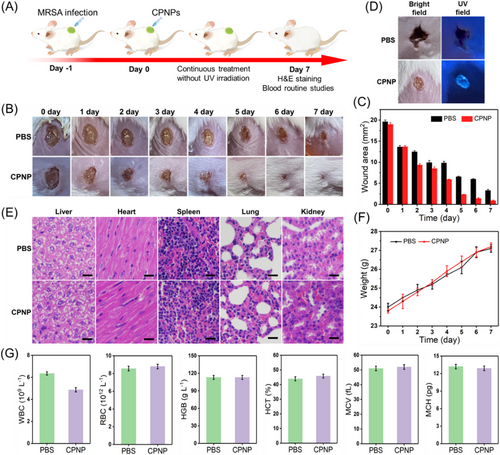
To assess the in vivo anti-biofilm and wound healing performance of AIEzyme, a circular full-thickness skin wound model (0.5 cm diameter) on the backs of mice was infected with MRSA (Figure 8A). The mice were separated into two groups treated with phosphate buffer saline (PBS) and CPNPs, respectively. To monitor visually the therapeutic effects, the wound healing processes were photographed over time, and the infected mice in CPNPs group achieved almost full recovery on seventh day (Figure 8B,C). In contrast, the infected wounds without CPNPs exhibit delayed recovery, and partial recovery is observed on seventh day. It is worth noting that only one dose of CPNPs was administered on the first day (Figure 8A). To investigate the reason for the long-acting wound healing performance, the durability of CPNPs was visually monitored by the AIE property of CPNPs under the transitory UV irradiation during the healing process of the larger wound (1 cm diameter) (Figure S15). The fluorescence of CPNPs at wound suggests that CPNPs persisted at the wound even on the seventh day (Figure 8D). As the further evidence, the TEM result indicates the morphology and structure of CPNPs were stable in physiological saline (Figure S16). Moreover, the DNase-like activity of CPNPs for BNPP remained stable and reusable over at least five catalytic cycles (Figure S17). After six cycles, CPNPs still showed good DNase-like activity for genomic DNA (Figure S18). Taken together, the good stability of AIEzymes realizes the persistent anti-infection after only one dose of CPNPs.
Taken together, the efficient, durable, and irradiation-free wound healing capacity of CPNP AIEzymes could stem from the persistent existence of AIEzymes on wounds, the long-acting DNase-like activity of AIEzymes, and the synergistic effect of host immune defenses. So far, many common AIEgens kill bacteria by photo-generated ROS, photothermal effect, and their own toxicity.[19, 29-32] For general antibacterial methods based on AIEgens, the regular irradiation and dressing change are usually required for wound healing. In contrast, the proposed anti-biofilm method based on AIEzymes does not require the irradiation and the dressing change, avoiding the frequent operations and the exogenous infection (Figure 1E). Because the DNase-like activity of AIEzymes can continuously catalyze the hydrolyzation of eDNA in EPS without irradiation, AIEzymes can eliminate the formation and recurrence of biofilms without irradiation at any time. Although AIEzymes cannot kill bacteria, the planktonic bacteria released from biofilm will be easily killed by the host immune system.[11, 61] The dissociated biofilm residues will be metabolized under the normal physiological mechanism. It is widely recognized that the resistance of microorganisms to drugs, host defenses, and environment stresses is mainly derived from their inherent ability to produce resilient biofilms, which does not involve the acquisition of genetic mutations.[9-12, 61] In this work, AIEzyme can disorganize the biofilm by the hydrolyzation of eDNA but not directly act on bacteria themselves. Therefore, the target bacteria would be hard to develop the resistance to AIEzyme. More interestingly, the AIEzymes added to the wound can be monitored in real time by their own AIE property, where only transitory UV irradiation is used with sound security (Figure 1E). Therefore, AIEzymes would show great application potential for anti-biofilm and wound healing.
To investigate the biosafety of CPNP, the hematoxylin and eosin (H&E) staining of organs (liver, heart, spleen, lung, and kidney) was performed, which showed no observable organ damage and inflammatory lesion for CPNP treatment (Figure 8E). Further, the body weights of the infected mice treated by CPNP and PBS were monitored under the condition of sufficient food and water, respectively (Figure 8F), which demonstrated a normal physiological trend. Moreover, blood routine results suggest that there is no difference between these mice with and without CPNP treatment (Figure 8G). To further evaluate the biocompatibility of CPNP, the cytotoxicity of CPNP to normal cells (NIH-3T3) was quantitatively researched by the standard methylthiazolyldiphenyl–tetrazolium bromide assay method. As shown in Figure S19, the increasements of CPNPs concentration have no effect on cell viability. The above results demonstrate that CPNP AIEzyme has reliable biocompatibility and negligible biotoxicity.
3 CONCLUSION
In summary, we demonstrated the rational design of an “AIEzyme”, a kind of AIE nanomaterial with enzyme-like characteristics for biofilm combating with process monitoring. Due to the high substrate affinity, low activation energy, and stable structure, AIEzyme shows high and enduring DNase-mimetic activity to hydrolyze eDNA in EPS of biofilm, which can prevent biofilm formation and disorganize established biofilms with good penetrability for prolonged period. AIEzyme can disrupt various biofilms of Gram-negative bacteria (E. coli) and Gram-positive bacteria (MRSA), because the DNase-like activity is not selective for eDNA from different bacteria. Moreover, AIEzyme has poor cytotoxicity and good biocompatibility. Due to the AIE character and RIM effect in the rigid structure, AIEzyme shows stable fluorescence for bacterial cell imaging and self-positioning in biofilm. Combining with above merits, CPNPs can maintain a long-acting anti-biofilm capacity on the superbug-infected wound with acceptable safety and simultaneously realize the real-time monitoring of AIEzyme residue. Although biofilm-enclosed bacteria can withstand the host immune system, the planktonic bacteria dissociated from biofilm can be killed and metabolized by the host immune defense. It should be noted that the anti-biofilm method based on AIEzyme does not require the UV irradiation, and only the fluorescence-related applications require the transitory UV-irradiation. Given the limitation of UV irradiation, the fluorescence character of AIEzyme is mainly used for the in vitro confocal imaging and the observation of AIEzyme residue on wound surfaces under the instantaneous UV irradiation. Considering AIEzyme-based anti-biofilm method does not require the continuous dressing change and light irradiation every day, AIEzyme would hopefully be used as the active constituent of wound dressing and antimicrobial preparation for clinical and veterinary applications. The as-prepared AIEzyme with DNase-mimetic activity would not only provide an effective anti-biofilm tool for the wound healing with process monitoring but also open a viable avenue for the application development of AIEgens and nanozymes.
4 EXPERIMENTAL SECTION
4.1 Synthesis of CPNPs
Ligand TCPE (9 mg) and anhydrous ZrCl4 (7.8 mg) and acetic acid (450 μL) were added into N,N-dimethylformamide (DMF, 3 mL). The mixture was dispersed through sonication for 5 min after being sealed. The mixed solution was heated at 90°C for 17 h. After cooling to room temperature, the products were collected by centrifugation (5000 rpm, 5 min) and washed twice with DMF and MeOH. After drying under vacuum, the final product is stored in the refrigerator (4°C).
4.2 DNase-like activity assay of CPNPs
Typically, the Tris–HCl buffer solution (50 mM, pH 8.0) containing BNPP (1 mM) and CPNPs (0.1 mg mL−1) was incubated at 25°C. And then, the characteristic absorbance of nitrophenolate (BNPP hydrolysate) at 410 nm was detected. The catalytic activity of CPNPs was calculated by the initial hydrolysis rate (product conversion < 5%). All reactions were repeated at least three times. The effect of pH on the activity of CPNPs was also researched by the above method, except for changing pH buffers (pH 3.0–12.0).
4.3 Steady-state kinetics assay
The steady-state kinetics analysis of the hydrolytic activity of CPNP nanozymes was conducted by changing the concentrations (0.05–0.8 mM) of BNPP at the constant CPNP concentration (0.1 mg mL−1) under the above assay method. The initial hydrolysis rates were employed for fitting to the Michaelis–Menten equation: ν = Vmax × [S]/(Km + [S]), where ν is the initial velocity, Vmax represents the maximal reaction velocity, [S] represents the concentration of substrate, and Km is the Michaelis constant.
4.4 Cleavage of various DNA by CPNPs
To examine the ssDNA cleavage ability of CPNPs, ssDNA was treated with CPNPs (0.1 mg mL−1) in Tris–HCl buffer (pH 8.0) at 37°C. After incubation for different times, CPNPs were taken away by centrifugation. Control experiments were carried out with DNase and buffer under the same reaction conditions. The hydrolysates of ssDNA were appraised by 1% agarose gel electrophoresis with ethidium bromide staining.
To evaluate the genomic DNA cleavage ability of CPNPs, the genomic DNA of MRSA was picked up by utilizing the DNA isolation kit, because the eDNA in biofilms was homologous to the genomic DNA of bacteria. Genomic DNA was treated with CPNPs (0.1 mg mL−1) in Tris–HCl buffer (pH 8.0) at 37°C. After incubation for different times, CPNPs were taken away by centrifugation. Control experiments were carried out with DNase and buffer under the same reaction conditions. The hydrolysates of genomic DNA were appraised by 1% agarose gel electrophoresis with ethidium bromide staining.
4.5 Bacterial cultivation and biofilm growth
A single colony of bacteria (E. coli or MRSA) was cultivated in Luria-Bertani (LB) medium (10 mL) at 37°C. The concentration of bacteria was determined by measuring optical density at 600 nm (OD600), and the stationary growth phase bacterial culture was diluted to the optical density of 1.0 (OD600 = 1.0) with about 1 × 109 CFU mL−1. Then, the diluted bacterial culture (10 μL, OD600 = 1.0) and Tryptone Soy Broth (TSB, 990 μL) were added in 24-well plates where there were glass coverslips in each well for biofilm growth at 37°C. After removing medium and unbound bacteria with sterile PBS, the established biofilms remained on glass coverslips.
4.6 Prohibiting biofilm generation by CPNPs
To evaluate the ability of prohibiting biofilm generation, CPNP- and DNase-coated glass coverslips were established for replacing bare glass coverslips during the above biofilm growth. First, glass coverslips (1 cm × 1 cm) were thoroughly washed by methanol/concentrated HCl solution (1:1 volume ratio) and water. Subsequently, CPNPs (0.1 mg mL−1) and DNase (20 mg mL−1) were dropped onto the surfaces of glass coverslips. After drying at room temperature, the obtained CPNP- and DNase-coated glass coverslips were obtained and applied to the above biofilm growth methods for different growth times. The obtained biofilms were analyzed by crystal violet staining and CLSM.
4.7 Initial bacterial attachment assays
To research the initial bacteria attachment, cell suspensions (OD600 = 1.0, 10 μL) were diluted to TSB (990 μL) and then incubated with different surfaces of glass coverslips for 60 min at 37°C, after which attached cells on different surfaces were stained by calcein-AM and observed by CLSM.
4.8 Eradicating established biofilms by CPNPs
To evaluate the ability of Eradicating established biofilm, the 120 h-old biofilms were treated with CPNPs in various concentrations (25, 50, and 100 μg mL−1) in the TSB medium for 12 h. The obtained biofilms were stained by calcein-AM and analyzed by CLSM.
Alternatively, the different ages (12, 24, 48, and 120 h) of biofilms were treated with CPNPs (0.1 mg mL−1) or DNase (20 mg mL−1) in TSB medium for 12 h. The obtained biofilms were stained by calcein-AM and analyzed by CLSM.
4.9 Crystal violet assay for detection of biofilm biomass
Quantitative assay of biofilm biomass was carried out by the spectrophotometric method. The established biofilms were stained by solution (1.0 mL) containing 0.2% crystal violet, 1.9% ethanol, and 0.08% ammonium oxalate in PBS. Then, the biofilms were placed in air for 30 min of incubation. Then, the extra stain was carefully washed off by 1.0 mL sterile PBS buffer twice. After adding ethanol (1 mL), the remaining crystal violet-stained biofilms were detected by the optical density at 590 nm (OD590).
4.10 Morphology analysis of biofilms by CLSM
To visualize biofilms, biofilms treated with different agents were incubated with calcein-AM for 20 min. After rinsed with PBS buffer to remove excess dye and loose bacteria, the biofilms were observed by CLSM. Then images with the size of 386.87 μm × 386.87 μm and frame size of 1024 × 1024 (X × Y) were acquired using a top-of-the-line motorized upright CLSM (Leica, Germany). All z-sections were collected at 3-μm intervals. All samples were observed using the same parameters and analyzed utilizing LAS AF software. The average thickness of the biofilms was quantified with the COMSTAT 2 software by using at least three different images per plate from three independent experiments for each sample.
4.11 CLSM and TEM analyses for binding behavior of CPNPs toward pathogens
First, bacterial solution (OD600 = 1.0, 100 μL) was added to 400 μL of PBS solution containing CPNPS (0.1 mg mL−1). After incubation at 37°C for 20 min, bacteria were harvested by centrifugation (5000 rpm for 1 min) and resuspended in 10 μL of PBS. The suspension was employed on the TEM analysis. For bacterial imaging by CLSM, the suspension was added to clean glass slide followed by a slightly covering coverslip for immobilization. Subsequently, the specimen was observed by CLSM with a 488 nm laser.
4.12 Toxicity assay of CPNP to bacteria
The toxicity of CPNP to MRSA and E. coli was evaluated by plate colony counting method. PBS solutions of MRSA and E. coli (∼107 CFU mL−1) were treated with CPNP (25, 50, and 100 μg mL−1) at 37°C for 7 and 12 h, respectively. Next, the bacteria suspensions were serially diluted by 103-fold with PBS, and then diluted bacteria (50 μL) were spread on the solid LB agar plate. After the incubation for 12–18 h at 37°C, the bacterial viability was evaluated by colony forming units (CFU) counting. Bacteria treated with PBS, TCPE, and ZrCl4 were used as control groups. Each experiment was repeated three times.
4.13 Mouse wound model and in vivo anti-biofilm experiments
All animals were purchased from the Charles River (China). All animal experiments were approved by Qingdao Agricultural University Laboratory Animal Center and the Ethics Committee of Qingdao Agricultural University (Approval No. 20220069) in compliance with Chinese law for experimental animals and conducted in compliance with The ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines 2.0. All efforts were made to minimize animal suffering.
The bacteria-infected wound models were established on the back of KM mice (female, 6–8 weeks, 18–22 g). A wound was surgically cut on the back of each mouse (d = 5 mm) and covered with MRSA (1 × 107 CFU mL−1).
Then mice were randomly divided into two groups and three mice in each group. After infection for 24 h, PBS (50 μL) and PBS (50 μL) containing CPNPs (0.1 mg mL−1) were sprayed onto the bacteria-infected wound, respectively. Afterward, the healing processes of the infected areas were dynamically monitored by digital camera, and damaged areas and body weights of the infected mice were measured throughout the whole treatment period (7 days).
4.14 In vivo toxicity evaluation
The blood samples of mice with different treatments after 7 days were collected for blood routine examination. The organs (heart, liver, spleen, lung, and kidney) of mice with different treatments were also collected for the H&E staining. The collected tissues were fixed in 4% paraformaldehyde and then embedded in paraffin. The fixed tissues were cut into slices with a thickness of 4 mm, followed by the histological analysis.
4.15 Statistical analysis
Statistical analyses were performed using GraphPad Prism Version 9.5.1 (GraphPad Software, USA). All numeric data were presented as mean ± standard deviation from a minimum of three independent samples. Data were statistically analyzed by one-way analysis of variance (ANOVA) and Tukey's multiple comparison tests, and difference at p < 0.05 (two-tailed) was considered statistically significant. Statistical significance: *p ≤ 0.05, **p ≤ 0.01,***p ≤ 0.001, ****p ≤ 0.0001, and ns: not significant. In numeric figures, each bar represents the average of three independent experiments unless otherwise stated.
AUTHOR CONTRIBUTIONS
Lei Han conceived, designed, and supervised the work at all the stages. Yucui Zhang, Baojian Huang, and Xuhui Bian performed the experiments and visualized the data, and then Lei Han analyzed the results. Lei Han wrote and revised the manuscript with input from all the other coauthors.
ACKNOWLEDGMENTS
This work was financially supported by the Youth Innovation Team Project for Talent Introduction and Cultivation in Universities of Shandong Province (No. 096-1622002), and Research Foundation for Distinguished Scholars of Qingdao Agricultural University (No. 663-1117015). We thank staff from the Instrumental Analysis Center of Qingdao Agricultural University for technical support.
CONFLICT OF INTEREST STATEMENT
There is no conflict of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




