Photo-induced fusion of monodisperse polymer microspheres: A novel strategy for constructing topological microsphere clusters with improved stability
Abstract
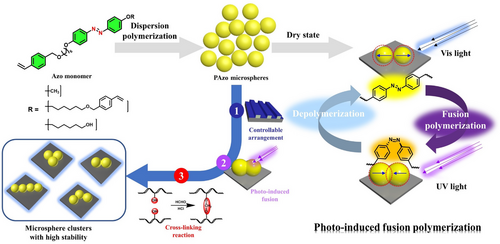
The preparation of monodisperse azobenzene (Azo) polymer microspheres by dispersion polymerization was reported. The photo-induced fusion of Azo polymer microspheres was successfully achieved during the process of reversible trans-cis-trans photoisomerization of Azo units, and induced various unique “microsphere molecular” clusters or “microsphere polymers”. Encouraged by this interesting phenomenon, microsphere clusters of different topological structures were stabilized by the in situ acetal cross-linking chemistry. The photo-induced fusion polymerization of monodisperse polymer microspheres provides a new strategy for designing photo-responsive clusters and allows for control over the mechanical properties of microspheres with high spatiotemporal resolution.
1 INTRODUCTION
Polymer microspheres[1-7] have drawn extensive attention due to their great potential application in chiral recognition materials,[8, 9] drug delivery,[10-12] light-emitting materials,[13] etc. The unique functions of the polymer microspheres are dependent on the parameters such as the size, sized distribution and surface structure. Dispersion polymerization is an attractive technique to prepare the monodisperse polymer microspheres in the range of 1–15 µm, which could provide good modulation of the above parameters.[14-21] In addition, polymer microspheres modified by functional groups could possess the unique properties. Azobenzene (Azo) chromophores have attracted intense interest owing to their unique physicochemical properties and the reversible photoisomerization of the Azo units.[22-37] In the polymer microspheres, unique photo-responsibility could enable microspheres with a wide range of applications.[38-40] Our group successfully achieved supramolecular chirality from achiral polymers to fabricate chiroptical Azo polymer microspheres.[41, 42] Wang et al. used the unique photo-induced mass transfer function of Azo polymers as a powerful platform to manipulate nano/micro-sized Azo polymer spheres, which could regulate the shape of the polymer microspheres by irradiation with linearly polarized light.[43-46] Therefore, the introducing of Azo groups into polymer microspheres could impart unique properties of photo-responsive and shape change to the polymer microspheres.
In the meantime, the shape of the polymer microspheres is also a significant factor that can endow the material with useful functions, since external stimuli can alter the shape of the polymer microspheres.[47] Due to the good spatial and temporal control of light,[48-52] the shapes and size of Azo polymer microspheres could be adjusted in a straightforward and controllable manner.[53-55] In recent years, most of the microspheres clusters are structured by noncovalent bond interactions,[56-58] which are sensitive to external stimuli (such as temperature, pH, solvents, etc.), limiting its further functionalization and applications. The well-defined shape and size change of Azo polymer microspheres could provide a wide range of opportunities for subsequent reactions between the adjacent microspheres, which has potential applications in the construction of different topological conformations and structure-stabilized microsphere clusters. Hence, Azo polymer microspheres are anticipated to solve the above problem.
In this study, we reported the concept of photo-induced fusion that monodisperse Azo polymer microspheres was employed as monomer units to form interconnected microsphere polymers (MPs) under Ultraviolet (UV) light initiator. The “polymerization-depolymerization” transition of microspheres was fully reversible by a trans-cis photoisomerization process. Unlike heat annealing treatment of polymer microspheres, this method is not limited by spatial and temporal constraints, which is more straightforward and controllable. The reversible ability also provides the possibilities for the in situ chemical cross-linking of the polymer microspheres to prepare topological clusters with improved stability. To the best of our knowledge, the findings we describe here represent the first photo-induced fusion polymerization of monodisperse microsphere and microsphere clusters with ultra-high ultrasonic stability, which holds great potential for various materials science applications.
2 RESULTS AND DISCUSSION
2.1 Dispersion polymerization
The Azo polymer microspheres were prepared by the typical dispersion polymerization in ethanol[59, 60] (Scheme 1). The number average molecular weight (Mn) of Azo polymer microspheres and their molecular weight distribution (Mw/Mn) were determined from the gel permeation chromatography (GPC) measurement (Figures S2 and S3), indicating the dispersion polymerization successful occurred. Microspheres with different diameters could be achieved by changing the polymerization parameters. The control over the size (Dn) and uniformity (size distribution: Dw/Dn) of Azo polymer microspheres is of extreme importance for their potential applications. The detailed polymerization parameters of polymer microspheres prepared from Azo monomer (AzoMS) are presented in Table 1. As shown in Figure 1A–C, the average diameters of Azo polymer microspheres increased with the increase of monomer loading (entries 1–3, from 1.45 µm increased to 1.55 µm). Monodisperse Azo microspheres with smooth surfaces can be obtained (Dw/Dn = 1.01) at 30 mg/mL concentration of monomer. The content of surfactant and initiator can significantly affect the polymerization rate of the system, thereby controlling the dispersity of polymer microspheres. We also explore the effect of surfactant (entries 2, 4–6) and initiator (entries 2, 7, 8) concentrations to understand the preparation process. Poly(vinyl pyrrolidone) (PVP) surfactant could prevent the precipitated Azo particles from agglomerating and thus help to form stable dispersion. The initiator in the dispersion polymerization has an influence on the instantaneous concentration of growing oligomeric radicals and the coagulation rate of the unstable primary Azo nuclei. Inappropriate monomer loadings can lead to the generation of polydisperse Azo microspheres, possibly due to the long nucleation period or secondary nucleation. Therefore, monodisperse Azo polymer microspheres can only be obtained with moderate concentrations of initiator and surfactant (entry 2), as shown in Figure 1D–G.
| Entry | AzoMS (mg) | D-AzoMS[a] (mg) | AIBN (mg) | PVP (mg) | Time (h) | Dn[b] (µm) | Dw/Dn[c] |
|---|---|---|---|---|---|---|---|
| 1 | 60.0 | 0 | 0.7 | 3.0 | 20 | 1.45 | 1.08 |
| 2 | 90.0 | 0 | 0.7 | 3.0 | 20 | 1.50 | 1.01 |
| 3 | 120.0 | 0 | 0.7 | 3.0 | 20 | 1.55 | 1.30 |
| 4 | 90.0 | 0 | 0.7 | 1.5 | 20 | 1.80 | 1.23 |
| 5 | 90.0 | 0 | 0.7 | 6.0 | 20 | 1.28 | 1.10 |
| 6 | 90.0 | 0 | 0.7 | 20.0 | 20 | 0.90 | 1.36 |
| 7 | 90.0 | 0 | 0.3 | 3.0 | 20 | 0.98 | 1.06 |
| 8 | 90.0 | 0 | 1.4 | 3.0 | 20 | 1.58 | 1.22 |
| 9 | 90.0 | 0.4 (0.5%) | 0.7 | 3.0 | 20 | 1.47 | 1.03 |
| 10 | 90.0 | 0.9 (1%) | 0.7 | 3.0 | 20 | − | − |
| 11 | 90.0 | 1.8 (2%) | 0.7 | 3.0 | 20 | − | − |
| 12 | 90.0 | 4.5 (5%) | 0.7 | 3.0 | 20 | − | − |
- [a] Azo-containing cross-linker, and the ratio was based on monomer weight.
- [b] The average diameter of the Azo microspheres was measured by SEM and determined as follow, where Di is the mean diameter of the ith Azo microspheres.
(1)
- [c] The size distribution of the Azo microspheres was determined as follow, where Di is the mean diameter of the ith Azo microspheres.
(2)

The growth feature of the microspheres was further elucidated to obtain monodisperse Azo polymer microspheres. Taking entry 2 experiment as an example, the transparent solution became a slightly turbid at a polymerization time of about 0.5 h, indicating that particle nucleation occurred in this period. The microspheres growth as a function of polymerization time was traced by Scanning Electron Microscope (SEM) (Figure 1I–L). Several small and irregular particles can be found after 1-h polymerization (Figure 1I), indicating that the particles have been precipitated out from the original homogeneous polymerization system. As the polymerization time was prolonged to 5 h (Figure 1J), the polymer particle sizes become larger and the particles tend to be spherical in morphology. With the polymerization proceeding on, more polymer spheres formed, gradually grew larger to reach the micron size, and finally becoming monodisperse in morphology (Figure 1K,L). The yields of the polymer microspheres also increased significantly, from 6.1% to 67.4%, with the increase of the polymerization time.
2.2 Photo-induced fusion of polymer microspheres
Next, we investigate the reversible photoisomerization behavior of the monodisperse Azo polymer microspheres in the dispersion state and dry state. The trans-cis-trans photoisomerization of Azo polymer microspheres was investigated upon 365 nm UV light and 435 nm visible light irradiation. As presented in Figure 2A and Figure S5, after being irradiated with UV/visible light about 1 h, the monodisperse Azo polymer microspheres could reach the photostationary state (PSS). Meanwhile, the morphology of the microspheres was also monitored by transmission electron microscopy (TEM) and SEM during the process of photoisomerization. When the dispersed microspheres in EtOH were irradiated with 365 nm UV light followed by 435-nm visible light, the microspheres were still in a highly separated state due to the high-speed Brownian motion,[55] while the size changed from 1.71 µm to 2.76 µm and then 1.96 µm. This process was also performed with Zeta potential in EtOH, but only the cis-Azo microspheres showed a slightly increased ζ value (Figure S6).
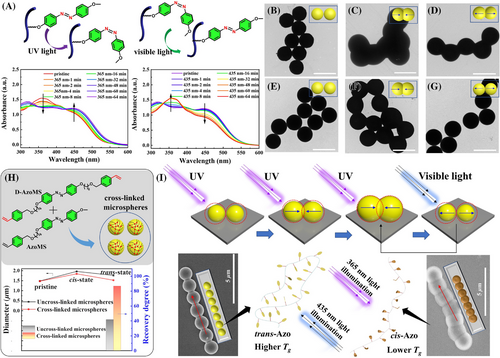
Interestingly, irradiating the microspheres film with 365-nm UV light about 1 h produced the phenomenon of cohesion and fusion of microspheres (Figure 2B–D), in other words, the isolated microspheres could act as monomer units (Microsphere Monomer Units, MMUs) to form locally interconnected and fused topological superstructures, similar to the polymerization process. Furthermore, visible light irradiation gradually separated adjacent Azo polymer microspheres and restored spherical MMUs, resulting in depolymerization-like behavior. For the complete “depolymerization”, we shorten the UV light irradiation time (30 min) to reduce the degree of fusion of adjacent microspheres. As shown in Figure 2E–G , the photo-induced fusion still took place in adjacent polymer microspheres after 365-nm UV light irradiation for 30 min. The adjacent polymer microspheres could also be completely defused after visible light irradiation for 30 min and subsequently separated by ultrasound. To further unveil the mechanism of the photo-induced fusion of Azo polymer microspheres, time-dependent morphology change under the irradiation of UV light were traced by TEM (Figure S8). The fusion degree between microspheres increased with the extension of 365f-nm UV light irradiation, and the maximum volume of overlap was reached at PSS. As reported in the literature, the glass transition temperature (Tg) of cis-Azo polymers is much lower than that of trans-Azo polymers and may even be lower than room temperature to cause photo-induced solid-liquid transition and the changing of material shape.[61-63] The occurrence of fusion indicates that the Azo polymer segments undergo chain motion or chain relaxation under the irradiation of UV light due to the large spatial resistance and lower Tg of cis-azobenzene. In this aspect, we characterized Tg of the trans-Azo and cis-Azo polymers by differential scanning calorimetry (DSC). The results demonstrated that Tg of trans-Azo polymers was about 55°C, while that of cis-Azo polymers was −8°C, which is much lower than room temperature (Figure S9). In the meantime, the GPC efflux curve also indicates that the hydrodynamic volume of cis-Azo polymer becomes smaller after the cis-isomerization, in which lower molecular weight polymer play a primary role according to the results of multi-peaks Gaussian fitting (Figure S10). Cis-isomerization of Azo units is more influential in the low molecular weight polymer chains, indicating the dominance of low molecular weight chain segment motion in the changes of polymer microspheres. After UV light irradiation, the polymer segments of cis-Azo polymers can move and relax at room temperature due to the lower Tg and the larger spatial resistance than tran-Azo polymer.[63, 64] As a result, the volume, chain stiffness, and interchain cohesion of polymer chains are different in cis- and trans- configurations, photo-induced fusion might be induced by the lower Tg and movement of cis-Azo polymer sidechain in the microspheres. At lower levels of cis-isomerization, the polymer chains are not entangled at the interfacial fusion of the adjacent microspheres, so separation could occur after trans-isomerization and ultrasonic treatment.
To test our conjecture, we prepared Azo microspheres with cross-linked polymer chains by adding different proportions of D-AzoMS (crosslinker) with polymerization parameters being held constant (Table 1, entries 9–12). It can be seen clearly from Figure S11 that narrow disperse cross-linked Azo polymer microspheres were obtained with a mass ratio of D-AzoMS to AzoMS less than 0.5%, while only coagulum particles were formed when the mass ratio ranged from 2% to 5%. Compared to uncross-linked Azo polymer microspheres, the degree of photo-induced fusion was lower. The microspheres could almost recover to the initial state after 435-nm visible light irradiation (Figure 2H). We hypothesized that the cross-linked parts limit the movement of polymer chains in cis-Azo polymer microspheres at room temperature. After 365-nm UV light irradiation, the polymer chain segments were not “frozen” at room temperature. And the coplanar trans-Azo units can be isomerized into curved cis-structure with larger spatial steric hindrance. As a result, the polymer chain segments could stretch accordingly to reduce the energy of the system. Since the Tg of cis-Azo polymers was lower than room temperature, the stretched polymer chain segments can be intertwined with each other to reduce the spatial resistance effect, resulting in the expansion of the Azo polymer microspheres and the fusion between adjacent Azo polymer microspheres (Figure 2I).
From above results, we demonstrated the fusion of microspheres can be physically fused to form regular linear arrays upon the UV light (Figure 2I). If there is an advanced microsphere arrangement technology, thousands of MMUs have the potential to be polymerized by fusion polymerization to form “MPs”. In addition, the ordered arrays can also be disassembled into MMUs by using visible light, similar to the process of “depolymerisation”. However, for the physically crosslinked MPs with intertwined chains, a certain degree of mechanical force will still make the microsphere separate obviously, such as ultrasonic force.
2.3 In situ cross-linking reaction for cluster stabilization
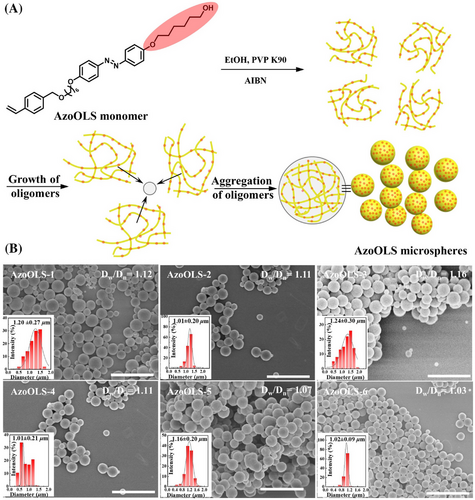
In this case, in situ chemical crosslinking of the fused polymer microspheres could enable maintenance of specific topological structures, for example, microsphere clusters with different geometries. In order to permanently maintain the fused topological clusters, cross-linkable hydroxyl groups were introduced at the terminal of Azo segment side-chains for the following in situ acetal reaction (AzoOLS MMUs). Narrow or monodispersed AzoOLS polymer microspheres with hydroxyl functional group were readily obtained following a similar procedure as that used for the preparation of AzoMS polymer microspheres (Table S1, Figure 3 and Figure S12). The formation process also involves the typical particle nucleation and growth stages as Stöver and co-workers proposed[20, 65] (Figure 3A). The Dw/Dn values of AzoOLS polymer microspheres are less than 1.20, and their average diameters are in the range of 1.0 to 1.2 µm (Figure 3B). Furthermore, the 1H Nuclear Magnetic Resonance (NMR) and X-ray photoelectron spectroscopy (XPS) indicated that the existence of -OH in polymer terminal, illustrating that AzoOLS microspheres are indeed hydroxyl functionalized microspheres with uniform sizes (Figure S13). Meanwhile, the adjacent AzoOLS microspheres can be separated by intense sonication after photo-induced fusion polymerization (Figure S14). A reasonable assumption is that if the fused polymer microspheres were further chemically cross-linked, the fused microspheres would have improved stability to provide the possibility for the preparation of colloidal clusters with topological structure and high physicochemical stability. Hence, chemical cross-linking of fused AzoOLS polymer microspheres was carried out by an efficient in situ acetal reaction. After photo-induced fusion and chemical cross-linking by acetal reaction of microspheres, the AzoOLS polymer microspheres still remained cohesive aggregates without separation under intensity sonication (Figure S15). The advantage of the result can offer is their versatility in preparing well-defined colloidal clusters with different topologies, which allows them to remain highly stable under further external stimuli.
Narrow or monodisperse microsphere clusters with designed functional topologies have attracted considerable interest; however, the noncovalent interactions that ensure adhesion between microspheres are very sensitive to environmental changes, such as sonication, which could cause irreversible disintegration of the clusters. Many efforts have been devoted in this respect and some cross-linked microsphere clusters containing specific bonding capabilities have been prepared. Introducing a certain amount of chemically bonded particles into the cluster system may also lead to the conglutination of microspheres without specific orientation and, and even the formation of bulk aggregates due to the charge density mismatch between the formed clusters.
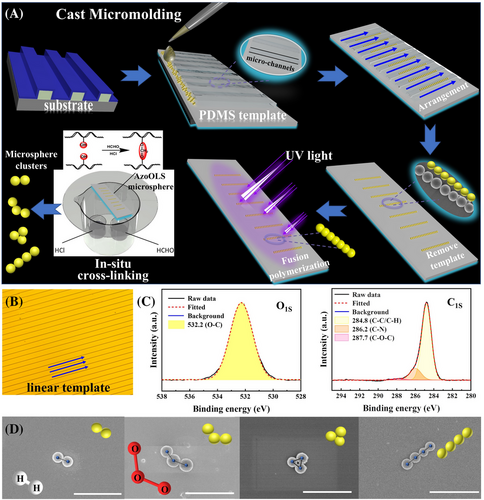
Combining the advantages of photo-induced fusion of microspheres and in situ chemical cross-linking, Azo microsphere clusters can maintain high stability under strong chemical environmental stimuli. As presented in Figure 4A, AzoOLS polymer microspheres can be arranged into different topological structures by linear (or circular) photolithography templates (Figure 4B and Figure S16), also known as template assisted technology. Uniform or monodisperse AzoOLS polymer microspheres are first injected into PDMS inverted moulds, which could be obtained from photoresist templates. The polymer microspheres can be automatically arranged into specific topologies in the micro-channels, microsphere clusters including molecular configurations of oxygen and ozone, as well as triangles and tetramers, can be prepared after the PDMS template is removed. Subsequently, microsphere clusters can easily undergo mild fusion with each other through UV light-induced fusion polymerization while maintaining their own topologies. After in situ cross-linking between adjacent AzoOLS microspheres and removal of the photolithography templates by ultrasonic treatment, the AzoOLS microsphere clusters with different topological structures and improved stability could be successfully obtained. The cross-linking of the microspheres was confirmed by XPS and Fourier Transform Infrared (FT-IR). Both the O-H peak at 533.9 ev in the O1s spectrum and the C-OH shoulder at 285.7 ev in C1s disappeared after acetal reaction in the XPS spectra(Figure 4C). FT-IR spectra show complete disappearance of the stretching vibration peak of hydroxyl functional group at 3332 cm−1 after cross-linking reaction, illustrating that the cross-linking reaction is very complete (Figure S17). Compared with the unchemically cross-linked clusters, these novel clusters maintained their original shape without disintegration under intensity sonication (Figure 4D), revealing the improved stability of the topological clusters and thus the potential to apply these clusters in more complex and harsh environments.
3 CONCLUSION
In conclusion, we reported the first concept of photo-induced fusion polymerization to prepare microsphere clusters and the potential to construct high molecular weight “MPs” with microspheres as monomer units. The fusion process of narrow or monodisperse and functionalized Azo polymer microspheres with changeable segment mobility can be easily controlled by adjusting the reversible trans-cis-trans photoisomerization. Taking advantage of the photo-induced fusion of microspheres, Azo polymer microsphere clusters with high stability and different topological structures were constructed by the in situ acetal reaction for chemical cross-linking, which was not limited by spatial and temporal constraints. The microsphere clusters with improved stability can maintain their original topologies in extreme conditions, such as intense ultrasound. Following the proposed methodology, it is hoped that more microsphere clusters will be designed due to their desirable endurance and potential applications in display materials, drug delivery and molecular catalysis. We are currently continuing our efforts in this attractive research direction.
4 EXPERIMENTAL SECTION
4.1 Materials
1-Chloro-6-hydroxyhexane (Acros, 95%), poly(vinyl pyrrolidone) (PVP K90, J&K), 4-amino-anisole (Aladdin, AR), 4-aminophenol (Sinopharm Chemical Reagent Co., Ltd, CP), phenol (Aladdin, AR) were used without further purification. 4-Vinylbenzylchloride (Macklin, 90%) was used after removing inhibitor by passing through neutral alumina oxide column. 2,2′-Azobis(2-methylpropionitrile) (2,2′-Azobis(2-methylpropionitrile) [AIBN], Aldrich) was recrystallized from methanol.
4.2 Characterization
1H NMR spectra were recorded on a Bruker nuclear magnetic resonance instrument (300 MHz, Brucker, Kalsruhe, Germany) using CDCl3 and DMSO-d6 as solvent and tetramethylsilane as the internal standard at 25°C. GPC measurements were conducted on the TOSOH HLC-8320 GPC, equipped with refractive-index and UV detectors using two TSKgel SuperMultiporeHZ-N (4.6 × 150 mm, 3.0 µm beads size) columns arranged in series. Polymers can be separated in the molecular weight range of 500-190 kDa. Tetrahydrofuran (THF) was used as the eluent with a flow rate of 0.35 mL/min at 40°C. The number average molecular weight (Mn) and molecular weight distribution (Mw/Mn) of sample was calculated with polystyrene standards. SEM images were taken with Field Emission Scanning Electron Microscope (Hitachi, SU8000). TEM images were obtained using a HITACHI HT7700 electron microscope operating at an accelerating voltage of 120 kV. And Optical Microscope (OM) images were examined under Optical Microscope (Olympus Corporation, BX51-P). The UV-Vis spectra were recorded on a UV-2600 spectrophotometer (Shimadzu (Nakagyo-ku, Kyoto, Japan)). Dynamic light scattering measurements were performed at 25°C (Brookhaven, Holtsville, TX, USA, the sample concentration was 0.5 mg/mL). Zeta potential (ζ) was measured on a Malvern Zetasizer Nano-ZS instrument (Malvern, UK) at 25°C (the sample concentration was 0.5 mg/mL). The thermal behaviors and glass transition temperatures of Azo polymer microspheres were measured using a TA instrument DSC 250 (New Castle, DE, USA). The tans-/cis-azobenzene Tg were tested according to the literature reported.[62] Difference in binding energy of polymer particles was measured using XPS (Thermo Fisher Scientific ESCALAB 250XI, A1 KR source). FT-IR spectra were obtained on a Bruker TENSOR-27 FT-IR spectrometer by mixing the polymer into potassium bromide and pressing the tablet, then dried under an infrared light before measurement.
4.3 Synthesis of monomer AzoMS
6-(4-((4-Methoxyphenyl)diazenyl)phenoxy)hexan-1-ol was synthesied as literature reported.[66] The Azo-containing monomer 1-(4-methoxyphenyl)−2-(4-((6-((4-vinylbenzyl)oxy)hexyl)oxy)phenyl)diazene (AzoMS) was obtained as Scheme illustrated (Scheme S1). 4-Vinylbenzylchloride was dissolved in 50 mL anhydrous THF, next adding NaH into the flask at 0°C and stirring at room temperature for 30 min. 6-(4-((4-Methoxyphenyl)diazenyl)phenoxy)hexan-1-ol was dissolved in 250 mL anhydrous tetrahydrofuran at 25°C, then poured into the flask, refluxed and stired at 50°C for 24 h. The reaction solution was cooled to room temperature and filtered. The filtrate was washed with water and dried with anhydrous Na2SO4. Then removed solvent and the product was separated by column chromatography, finally recrystallized in methanol (yield: 41.7%). 1H NMR (CDCl3, 300 MHz) of AzoMS, (δ, ppm): δ 7.98 (dd, 4H), 7.39 (d, 2H), 7.29 (d, 2H), 7.00 (dd, 4H), 6.71 (dd, 1H), 5.74 (dd, 1H), 5.23 (dd, 1H), 4.49 (s, 2H), 4.04 (t, 2H), 3.90 (s, 3H), 3.48 (t, 2H), 1.82 (q, 2H), 1.66 (p, 2H), 1.47 (m, 4H).
4.4 Synthesis of monomer AzoOLS and cross-linker D-AzoMS
Monomer 6-(4-((4-((6-((4-vinylbenzyl)oxy)hexyl)oxy)phenyl)diazenyl)phenoxy)hex-an-1-ol (AzoOLS) and cross-linker D-AzoMS were synthesized through the similar route. 1H NMR (DMSO-d6, 300 MHz) of monomer AzoOLS (yield: 47.1%), (δ, ppm): δ 7.82 (d, 4H), 7.43 (d, 2H), 7.28 (d, 2H), 7.09 (m, 4H), 6.72 (dd, 1H), 5.80 (d, 1H), 5.23 (d, 1H), 4.44 (s, 2H), 4.35 (t, 1H), 4.06 (dt, 4H), 3.41 (p, 4H), 1.73 (q, 4H), 1.58 (q, 2H), 1.39 (d, 10H). 1H NMR (300 MHz, CDCl3) of D-AzoMS (yield: 10.0%): δ 8.05-7.77 (m, 4H), 7.39 (d, 4H), 7.29 (d, 4H), 7.06-6.91 (m, 4H), 6.71 (dd, 2H), 5.74 (dd, 2H), 5.28-5.18 (m, 2H), 4.49 (s, 4H), 4.03 (t, 4H), 3.48 (t, 4H), 1.81 (q, 4H), 1.66 (p, 4H), 1.46 (d, 8H).
4.5 Dispersion polymerization
The side-chain Azo polymer microspheres were obtained by dispersion polymerization, where AIBN and PVP K-90 were used as initiator and dispersant, respectively. The preparation of monodispersed Azo polymer microspheres is described as an example. Monomer AzoMS (90.0 mg, 0.202 mmol), AIBN (0.7 mg, 0.004 mmol), PVP K-90 (3.0 mg), and EtOH (3.0 mL) were added into a 10 mL ampoule bottle. Then the ampoule bottle was flame-sealed under an argon atmosphere after deoxygenated with three freeze-thaw cycles. The polymerization was carried out at 80°C for 20 h. And the yellow polymer product was collected by centrifugation, subsequently washed with EtOH five times. The resulting polymer product was dried in a vacuum oven overnight at 30°C overnight and finally stored in EtOH solution. The preparation of other Azo polymer microspheres with different monomers was kept the same as this procedure.
4.6 Acetal reaction
After irradiation with 365-nm UV light for 30 min, the cross-linking reaction of the arranged Azo polymer microspheres with hydroxyl functional group was carried out when exposed the arranged microspheres to formaldehyde and HCl vapor in the dark at 25°C for 20 h on the substrate.
AUTHOR CONTRIBUTIONS
The authors are grateful for the financial support from the National Nature Science Foundation of China (grant numbers: 92056111 and 21971180), China Postdoctoral Science Foundation (grant number: 2022M722312), Key Laboratory of Polymeric Material Design and Synthesis for Biomedical Function, the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions and the Program of Innovative Research Team of Soochow University.
ACKNOWLEDGMENTS
Wei Zhang conceived and designed the research. Zixiang He, Xiaoxiao Cheng and Su Shen performed on the experiments. The manuscript was written through contributions of Zixiang He, Xiaoxiao Cheng, Tengfei Miao, Gong Zhang, Zhao Wang, Qingping Song and Wei Zhang. All authors have given approval to the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
We agree to share our research data including, but not limited to: raw data, processed data, methods and materials.




