A simple strategy to simultaneously improve the lifetime and activity of classical iridium complex for photocatalytic water-splitting
Abstract
Herein, we design and synthesize a series of oligomers [Ir(ppy)2(dabpy)-ODPA]n (D1-n) by copolymerization of [Ir(ppy)2(dabpy)][PF6] (D2) with 4,4′-Oxydiphthalic anhydride (ODPA), to resolve the problem of simultaneous improvement of stability and activity of classical iridium complex for photocatalytic water-splitting. Fourier-transform infrared spectroscopy, X-ray photoelectron spectroscopy, solid-state nuclear magnetic resonance, and gel permeation chromatography results indicate that the degree of polymerization (n) of D1-n could be tuned by the synthesis method. The best photocatalytic performance is reached by D1-n with n at of 2 and/or 3 (D1-2/3), which exhibits a photocatalytic lifetime up to 676 h and a photocatalytic hydrogen evolution of 162055.1 μmol·g-1. Compared with classical iridium complex D2, the photocatalytic lifetime of D1-2/3 is about 38 times longer and the photocatalytic activity is 1.3 times higher. Further increase of n leads to a decrease in both photocatalytic lifetime and activity. According to the spectroscopic characterizations, photoelectrochemical experiments, and density functional theory calculation, the significantly enhanced photocatalytic performance of D1-2/3 originates from the oligomeric structure. The oligomer chain of D1-2/3 with suitable length acts as a large steric hindrance to reduce the undesired photoinduced decomposition and prolong its lifetime. The possible coupling of adjacent Ir complexes in D1-2/3 lowers the energy gap and increases the utilization of visible light, which overcomes the adverse effect of large steric hindrance and finally improve the activity. This work first provides a simple strategy for constructing oligomeric Ir photosensitizers to simultaneously achieve long lifetime and high activity, it will lay the foundation for the design of highly efficient photosensitizers in the future.
1 INTRODUCTION
Nowadays, hydrogen energy attracts more and more attention as a clean and renewable energy source, and hydrogen production technologies have been widely developed, such as electrolysis, electrocatalysis, and photocatalysis.[1-6] Photocatalytic hydrogen evolution is considered a promising way to obtain hydrogen energy.[7-11] Among reported photosensitizers for hydrogen evolution, Ir complex photosensitizers arouse a lot of interest due to their outstanding photophysical properties.[12-21] However, constructing Ir complex photosensitizers with both long lifetime and high activity is still challenged, which becomes a key issue limiting the application of Ir complex photosensitizers.
In the past decades, extensive research showed that photocatalytic activity and lifetime of Ir complex photosensitizers are determined by different structural factors. Expanding the range of light absorption and promoting electron transfer were important methods of improving photocatalytic activity. For example, Lu et al. modified Ir complexes with Coumarin 6 and Bodipy, which extended the visible light absorption and trigger the electron transfer during photocatalysis. The complex achieved a high TON value of 115840. Regretfully, it only had a lifetime of 18 h.[22] It indicates that the structure modification for improving the photocatalytic activity could not prevent the decomposition of iridium complexes, which leads to the short lifetime of iridium complexes in the photocatalytic process.
To improve the photocatalytic lifetime, an effective approach is introducing a large steric hindrance group to the auxiliary ligand, but too large a steric hindrance results in low photocatalytic activity in theory. Such as in our previous work, [Ir(ppy)2(dabpy)][PF6] (D2) (ppy = 2-phenylpyridine, dabpy = 4,4′-diamino-2,2′-bipyridine) structure was anchored on polypropylene film, the film as a super-steric hindrance effectively inhibited the photodegradation of Ir complexes and resulted in a hydrogen evolution lifetime of 730 h which was the longest photocatalytic lifetime reported so far. Nevertheless, its TON was only 70.[23] Therefore, it is highly desired to propose a strategy that can synergistically improve the photocatalytic lifetime and activity.
The simultaneous improvement in photocatalytic activity and lifetime demands the structure simultaneously meet the requirements of fast electron transfer and large steric hindrance in auxiliary ligands based on the aforementioned reports. Inspired by the phenomenon of clusteroluminescence in nature, that natural luminophores only fluoresce in a clustering state and not single-molecule species,[24] we believe that polymerization could be a perfect strategy to fulfill these structure requirements. Because the clustering of Ir complexes could obtain multiple active sites within the molecule and the flexible polymer structure could act as a large steric hindrance. Till now, it is a pity that no systematic research has been carried out in this field.
In this research, we first design a series of oligomers [Ir(ppy)2(dabpy)-ODPA]n (D1-n) as photosensitizers for photocatalytic water-splitting by theoretical calculations. D1-n are synthesized by copolymerization of D2 with 4,4′-Oxydiphthalic anhydride (ODPA), and n is tuned by the synthesis method. Under the optimum conditions (n is 2 and/or 3), the hydrogen evolution of D1-2/3 reaches 162055.1 μmol·g-1 (TON is 365) in 676 h, which is superior to most metal-organic complexes and polymeric photosensitizers. It is because the oligomer chain with suitable length acts as a large steric hindrance to endow D1-2/3 with a significantly prolonged lifetime, and the possible coupling of adjacent Ir complexes accelerates the electron transfer which overcomes the adverse effect of large steric hindrance on activity. This is the first time to achieve a simultaneous improvement of the lifetime and the photocatalytic activity by polymerization of classical Ir complex photosensitizers, which will lay the foundation for the design of highly efficient photosensitizers in the future.
2 RESULTS AND DISCUSSION
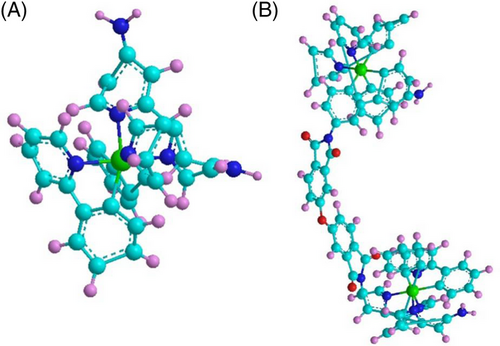
First, we synthesized pure [Ir(ppy)2(dabpy)][PF6] (Figure 1A) according to previous reports[23] (details in Experimental Section 1, Sections S1 and S2, and Figure S1) and the molecular structure was determined by 1H nuclear magnetic resonance (1H NMR) and Matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS) (Figures S3 and S4). Then, [Ir(ppy)2(dabpy)][PF6] solution was mixed with ODPA and stirred at room temperature to obtain amidic acid. The solvent was removed by heating, and the amidic acid occurred imidization at 250°C to obtain D1-2/3 (details in Figure 1B and Experimental Section 2 and Figure S1). Moreover, D1-n with a higher polymerization degree (n > 3) was synthesized in steps by tuning the ratio of D2 and ODPA (details in Experimental Section 3 and Figure S2). The FT-IR and solid-state NMR results certified the synthesis of D1-n (Figures S5 and S6).
The molecular structure of D1-2/3 was determined by Fourier-transform infrared spectroscopy (FT-IR), solid-state NMR, MALDI-TOF MS, and gel permeation chromatography (GPC). Compared to D2 and ODPA, the FT-IR spectra of D1-2/3 (Figure 2A) show the typical peaks of imide structure at 1780, 1720, 1380, and 725 cm-1, which determines the polyimide structure of D1-2/3 (detailed analysis in Section S2). Then according to the results of solid-state NMR of D1-2/3, the peak at 166.54 ppm is mainly attributed to C in the imide structure of C=O (Figure S7). The FT-IR, X-ray photoelectron spectroscopy (XPS), and solid-state NMR results certify that D2 and ODPA are covalently bound via imide structure. The MALDI-TOF MS of D1-2/3 shows the strongest peak of 979.1, attributed to the soluble fragments of D1-2/3 (Figure S8). The molecular weight of D1-2/3 is measured by GPC (Figure S9), which can be inferred that D1-2/3 is a mixture of dimers and trimers. Based on the GPC result, the synthesized structure is labeled D1-2/3. These results confirm the formation of the D1-2/3 structure. Moreover, changing the synthesis method only changes the degree of polymerization of D1-n and the product remains an oligomer, as shown in Figures S5 and S6.
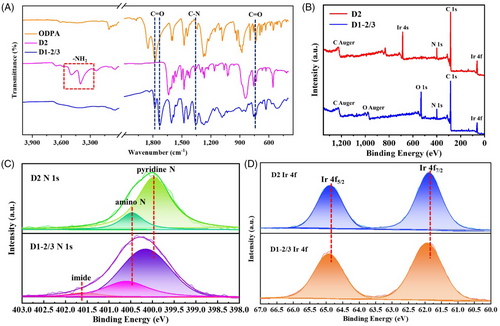
XPS further reveals the changes in bonding and the electronic environment in the molecular chain during D1-2/3 synthesis. In XPS spectra of D1-2/3, an obvious O 1s feature peak appears compared with D2, and the Ir 4s peak disappears (Figure 2B).[25] In the N 1s spectra of D1-2/3, the peak at 401.65 eV is attributed to the N in the C-N bond of imide (Figure 2C). The positive shift of pyridine N (from 400.00 to 400.15 eV), amino N (from 400.50 to 400.65 eV), and the decrease in intensity of amino N in the D1-2/3 spectrum indicate the change of their electronic environment and the amino groups and ODPA are involved in the synthesis of D1-2/3.[26] The above changes confirm the formation of an imide bond between D2 and ODPA. Figure 2D shows the Ir 4f peaks of D2 and D1-2/3. Compare with D2, the Ir 4f peaks of D1-2/3 shift positively (from 64.90 and 61.85 eV to 64.95 and 61.95 eV), which is related to the change in the electronic environment of Ir. The change in the electronic environment of Ir endows D1-2/3 with different photoelectric properties and different photocatalytic performance from D2.
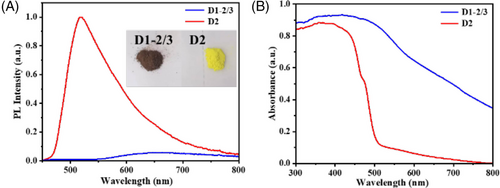
Additionally, D1-2/3 glows orange and D2 glows yellow-green. Compared with D2, the fluorescence emission spectrum of D1-2/3 has a certain degree of redshift, and the emission intensity is weakened (Figure 3A). The weaker fluorescence emission intensity indicates that D1-2/3 possesses higher photoinduced electron migration ability.[27] As shown in Figure 3B, the ultraviolet-visible (UV-vis) absorption spectrum shows that D1-2/3 has a wider light response range compared to D2. According to the UV-vis diffuse reflectance spectra of D1-2/3 and D2, their energy gaps are 1.94 and 2.50 eV, respectively (Figure S10A). Considering the currently reported studies on the energy gap of water-splitting photocatalysts, the energy gap of photocatalysts needs to be in the range of 1.8–2.2 eV while taking into account the energy loss during electron transfer and reasonable solar energy utilization efficiency.[28, 29] It can be inferred that the D1-2/3 structure is more favorable to the photocatalytic hydrogen evolution process than the D2 structure. The lifetime of the excited state of D2 and D1-2/3 is 101.31 and 61.21 ns, respectively, as shown in Figure S10B.
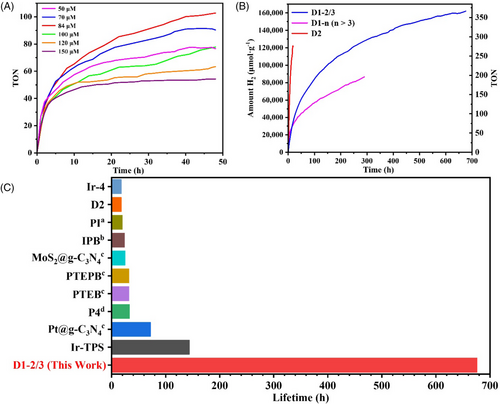
The performance of photocatalytic water splitting of D1-2/3 is evaluated with the condition of K2PtCl4 as catalyst (3 μmol), triethylamine (TEA) (20 mL) as sacrificial reductant, DMF/H2O (80 mL, v/v = 3:1) as a solvent and stirring under visible-light irradiation (λ > 420 nm) (details in Experimental Section 6, Figures S11 and S12). First of all, the photocatalytic hydrogen evolution performance of D1-2/3 with different concentrations (50-150 μM) was tested. The results show that D1-2/3 has the highest photocatalytic activity at 84 μM (Figure 4A). The following photocatalytic experiments were conducted under this amount of investment. The hydrogen evolution of D1-2/3, D1-n (n > 3) and D2 were measured, as shown in Figure 4B. The hydrogen evolution kinetic curve of D1-2/3 exhibits a hydrogen evolution amount of 162055.1 μmol·g-1 and a TON of 365, its lifetime reaches an astonishing 676 h. The hydrogen evolution of D1-n (n > 3) is 86568.1 μmol·g-1 and the lifetime is 290 h, which are both lower than D1-2/3. This result shows that too long a molecular chain is not conducive to improving the activity. The hydrogen evolution amount of D2 is 122298.8 μmol·g-1 with a TON of 298, and its lifetime is only 18 h. The lifetime of D1-2/3 is about 38 times the D2, and the hydrogen evolution is about 1.3 times the D2. According to these experimental results, the formation of oligomers (D1-2/3) by copolymerization of D2 with ODPA is an effective way to prolong the hydrogen evolution lifetime of D2 and improve the activity. In addition, we compare the photocatalytic performance of D1-2/3 with the previously reported iridium complexes and polymer photocatalysts. The lifetime of D1-2/3 is superior to most reported photocatalytic systems (Figure 4C), and the activity of D1-2/3 exceeds the reported polymeric photocatalysts (Table S2).[30-34]
The possible photocatalytic mechanism for D1-2/3 is investigated. The fluorescence emission spectra of D1-2/3 before and after the addition of TEA are compared in Figure 5A. The fluorescence emission intensity of D1-2/3 decreases significantly after the addition of TEA due to TEA supplying electrons to the photosensitizer to participate in the fluorescence quenching process, indicating that the photocatalytic process is a reduction quenching process. The possible mechanism of D1-2/3 photocatalytic hydrogen evolution is illustrated in Figure 5B. During the photocatalytic process, the phenylpyridine group of photosensitizers first captures photons to enter the excited state. The excited state photosensitizer obtains an electron from the sacrificial agent TEA. Then, the electron transfers to the pyridine group and ODPA through the Ir center. Subsequently, the electron transfers to the Pt catalyst via ODPA to reduce water and produce hydrogen. Finally, the photosensitizer returns to the ground state.
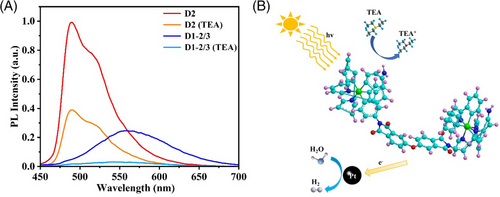
Photoelectrochemical tests were performed to further investigate the origin of the enhanced photocatalytic activity of D1-2/3 than D2. As shown in Figure 6A, the result of electrochemical impedance spectroscopy (EIS) reveals D1-2/3 has a smaller radius than D2, showing a lower impedance of D1-2/3 than D2. Photocurrent measurements also indicate D1-2/3 has a stronger photocurrent response (Figure 6B). These results suggest that electrons in D1-2/3 could transfer more efficiently than in D2, thus facilitating the photocatalytic process.
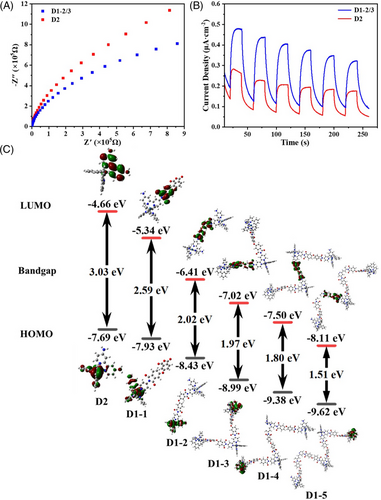
For gaining in-depth information regarding the photophysical behaviors of D1-2/3 and D2, density functional theory (DFT) calculation was performed using the Gaussian 09 program via the B3LYP/LANL2DZ method.[38, 39] Figure 6C depicts the highest occupied and lowest unoccupied molecular orbitals (HOMO and LUMO) for D2 and D1-n with n at 1-5 (the molecular formulae for D1-1∼D1-5 are given in Table S1). With n increasing from 1 to 5, the HOMOs of D1-n are all localized on the D2 part, while the LUMOs gradually transferred to ODPA. Compared with D2 whose LUMO is located on the dabpy part, the shift in the LUMO position of D1-n reveals a substantial alteration of the intramolecular electron distribution, implying the electron interaction of adjacent Ir complexes via ODPA. Meanwhile, it is clearly seen that the energy gap of D1-1 decreased to 2.59 eV, and for D1-2 it further decreased to 2.02 eV compared to D2 (3.03 eV). This result is supported by the broader UV-vis adsorption of D1-2/3 than D2. Furthermore, as the degree of polymerization increases, the energy gap gradually decreases. The narrower energy gap leads to lower energy for electron excitation and improvement of the utilization of visible light, which will be conducive to photocatalysis.[28, 40] On the other hand, although D1-n (n > 3) has a relatively narrower energy gap and lower emission intensity than D1-2/3 (Figure 6C and Figure S14), the change of terminal groups, the variation of the driving force between D1-n and Pt catalyst with the increase of n and the reduced coupling effect between Ir complexes (due to longer molecular chains) decrease the activity during the photocatalytic process. Therefore, a suitable length of molecular chain is necessary for improving the activity.
The causes of the activity decay of D1-2/3 during photocatalysis are further elucidated by analyzing the composition of the D1-2/3 photocatalytic system after photocatalysis. The results of gas chromatography (GC) show that the proportion of DMF/H2O/TEA (v/v/v) in the solution is 71.6%, 26.1%, and 2.3%, respectively (Figure S15). Compared with the proportion of DMF/H2O/TEA (3:1:1) in the solution before photocatalysis, a large amount of TEA is lost during the photocatalytic process, and the main loss paths include degassing and volatilization during xenon lamp irradiation. For evaluating the loss of TEA during degassing, the proportion of DMF/H2O/TEA (v/v/v) after degassing was measured, which is 67.48%, 25.59%, and 6.93%, respectively. The TEA content after degassing is still about 3 times higher after the photocatalytic reaction, demonstrating the large loss of TEA during photocatalytic which may be an important factor that leads to the activity decay of the D1-2/3 photocatalytic system. Then, solutes in the system after photocatalysis were collected through spin-drying and detected by MS (Figure S16) and FT-IR (Figure S17). For comparison, a blank control experiment in the absence of light was carried out (details in Experimental Section 7) and the solute in a blank control system was also detected. The MS result of solute in the D1-2/3 photocatalytic system after photocatalysis shows that the strongest peak is attributed to the D2 and no signals of higher molecular weight fragment attributes to D1. In contrast, the MS of the blank control group shows strong signals corresponding to different fractions of dissolved D1-2/3. The FT-IR result of the solute in the D1-2/3 photocatalytic system after photocatalysis shows a lack of obvious characteristic peaks of imide structure. This may be related to the structural decomposition of D1-2/3 during the photocatalytic process. And it is important that the blank control group shows obvious C=O characteristic peaks of imide structure at 1780 and 1720 cm-1. This unavoidable decomposition may be another important reason for the decay of the photocatalytic activity. Therefore, although the large steric hindrance forms the oligomer structure to improve the lifetime of D1-2/3 by reducing its photoinduced decomposition, the great loss of sacrificial agents during the long time photocatalysis process and the slow decomposition of some D1-2/3 leads to the final activity decay.[36] Moreover, the reduction of a photocatalytic lifetime by increasing n of D1-n is attributed to the complicated decomposition mechanism of macromolecular and the reduced structural integrity (as reflected from solid-state NMR).
According to the above, the simultaneous improvement of the lifetime and activity of D1-2/3 originate from its oligomeric structure: (i) the oligomer chain with suitable length forms a large steric hindrance which reduces the decomposition of D1-2/3 and significantly increases the lifetime, (ii) the possible coupling of Ir complexes in D1-2/3 accelerates the electron transfer process which increases the activity and overcomes the adverse effects of large steric hindrance.
3 CONCLUSION
Here, we have successfully synthesized a series of oligomeric photosensitizers D1-n with high activity and high stability. The best photocatalytic performance is reached by D1-2/3. The lifetime of D1-2/3 is about 38 times of the classical photosensitizer D2, and the photocatalytic activity of D1-2/3 is about 1.3 times of D2 which exceeds most reported polymeric photocatalysts. The excellent photocatalytic performance of D1-2/3 results from a wider light response range, narrower energy gap width, optimal oligomeric structure, and appropriately increased steric hindrance on the auxiliary ligand. This work achieves a simultaneous improvement of hydrogen evolution lifetime and activity by polymerization of classical Ir complex photosensitizers, and it is the first time that the photocatalytic activity of the oligomeric photosensitizer exceeds the complex photosensitizer to the best of our knowledge, which provides a new insight for designing photosensitizers with long lifetime and high activity.
4 EXPERIMENTAL SECTION
4.1 Synthesis of [Ir(ppy)2(dabpy)][PF6] (D2)
6 mmol (2.11 g) IrCl3·xH2O and 15.5 mmol (2.41 g) 2-phenylpyridine are dissolved in a mixed solution of ethylene glycol ether (75 mL) and water (25 mL) and react under 120°C (reflux) in Ar atmosphere for 24 h to obtain [Ir(ppy)2]2Cl2, the yield is 61.6%. 0.9 mmol (0.969 g) [Ir(ppy)2]2Cl2 and 2.25 mmol (0.419 g) dabpy are dissolved in the mixed solution of methanol (45 mL) and chloroform (45 mL) and stirred under 120°C in Ar atmosphere for 24 h to obtain [Ir(ppy)2(dabpy)]Cl, after that 22.5 mmol (3.67 g) NH4PF6 is added and react for more than 0.5 h under room temperature. [Ir(ppy)2(dabpy)][PF6] is obtained by column chromatography for anion replacement, the yield is 56.1% and the purity is 89.74% (calculated from the standard curve determined by ICP-MS, Figure S11).
4.2 Synthesis of [Ir(ppy)2(dabpy)-ODPA]2/3 (D1-2/3)
0.5 mmol (0.416 g) D2 is fully dissolved in extra-dry DMAc, then 0.5 mmol (0.155 g) ODPA is added and stirred at room temperature for 8 h. The solution is spread out on a glass plate and heated at 120°C for 12 h to remove the solvent. Then, the plate is heated to 250°C for 8 h. After the reaction, the plate is cooled to room temperature and taken out. The film is scraped off the plate, and the product D1-2/3 is obtained.
4.3 Synthesis of the high degree of polymerization of [Ir(ppy)2(dabpy)]n-ODPAn-1 (D1-n, n > 3)
0.5 mmol (0.416 g) D2 is fully dissolved in extra-dry DMAc, then 0.25 mmol (0.076 g) ODPA is added and stirred at room temperature for 8 h. The solution is spread out on a glass plate, and heated at 120°C for 12 h to remove the solvent. Then, the plate is heated to 250°C for 8 h. After the reaction, the plate is cooled to room temperature, and taken out. The film is scraped off the plate and repeated the above steps, reacting with 0.25 mmol ODPA to finally obtain D1-n (n > 3).
4.4 Photocatalytic hydrogen evolution
The photocatalytic hydrogen evolution test is carried out in a 250 mL reactor, which is connected to a closed gas circulation and vacuum system (CEL-SPH2N, CEAULIGHT). The DMF/TEA/H2O solution used in the photocatalysis experiment is 60 mL/20 mL/20 mL (3/1/1, v/v/v), with 3 μmol K2PtCl4. The dosage of D2 and D1-2/3 is 8.4 μmol calculated according to the Ir content calibration curve (details in Experimental Section 5). The solution is completely degassed and irradiated with a 300 W Xe lamp (CEL-HXF300; AULTT) with a 420 nm cut-off filter. The temperature of the photocatalytic solution is maintained at 279 K. The gas is detected by online GC-7900 (CEAULIGHT) using nitrogen as the carrier gas. After finishing the photocatalytic reaction, the solution volume of the system is 79.0 mL.
4.5 Measurement of Ir content
-
0.05 g D2 is added in 300 mL HNO3, heated, and stirred at 80°C for 8 h, then 200 mL of deionized water is added. Subsequently, 20, 30, 40, 50, 60, 70, 80, and 90 mL of the solution are taken out and diluted to 100 mL respectively, and the Ir concentration of these solution are detected by ICP-MS to obtain a calibration curve.
-
0.1 g D1-2/3 is added in 300 mL HNO3, heated and stirred for 8 h at 80°C, then 200 mL of deionized water is added. Subsequently, 10, 30, 50, 70, and 90 mL of the solution are taken out and diluted to 100 mL respectively, the Ir concentration of these solution is detected by ICP-MS to obtain a calibration curve. The same method is used for the determination of the Ir content per unit mass of D1-n.
4.6 Photoelectrochemical tests
Photoelectrochemical tests are studied by using an electrochemical workstation (CHI 660E, Shanghai CH Instruments.) in a conventional three-electrode system. The test uses a platinum sheet as the counter electrode and a saturated calomel electrode as the reference electrode. For the preparation of the working electrode, 5 mg of D1-2/3 is dispersed in 1 mL of DMAc, then 50 μL of Nafion ethanol solution is added to the solution and sonicated for 30 min to form a homogeneous suspension. Note that, 150 μL of the suspension is added dropwise to the conductive surface of the FTO glass (FTO using 2 × 2 cm). FTO glass is dried by IR light for photoelectric testing. EIS is recorded over a frequency range of 5 MHz–100 kHz with an amplitude of 10 mV. For photocurrent measurements, the working electrode is immersed in a 1 M Na2SO4 solution and illuminated from the back by a 300 W Xe lamp (PLS-SXE300+; Beijing PerfectLight Technology Co. Ltd). Mott-Schottky curves are performed in the dark over a range of potentials with an amplitude of 5 mV and frequencies of 0.5, 1, and 2 kHz.
4.7 Blank control experiment
To explore the degradation of D1-2/3 in the absence of light, a blank control experiment is carried out. The composition of the system is the same as depicted in Experimental Section 4. Note that, 8.4 μmol D1 and 3 μmol K2PtCl4 are added to the DMF/TEA/H2O (60 mL/20 mL/20 mL) solution, and the system is kept in dark at 279 K for 500 h. Then, the solution volume is measured, which is 94.0 mL. The composition of the solution and solutes are analyzed by GC, FT-IR, and MALDI-TOF mass spectrum, as shown in Figures S16 and S17.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 51803208) and the Youth Innovation Promotion Association CAS (E2202005).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.




