Aggregation-induced emission polymer systems with circularly polarized luminescence
Hewei Yan and Youling He contributed equally to this work.
Abstract
Functional materials with circularly polarized luminescence (CPL) have attracted tremendous attention due to their promising applications in three-dimensional displays, chiral recognition and catalysis, photoelectronic devices, contrast imaging, information encryption, and other fields. Among various CPL-active materials, polymeric systems with aggregation-induced emission (AIE) have emerged as excellent candidates because of their efficient aggregate-state fluorescence, large solid-state dissymmetry factor, excellent processibility, diversified self-assembly behaviors, and readily switchable CPL properties. This review summarizes and discusses the recent progress as well as future perspective of diverse AIE polymer systems with CPL, including CPL-active covalent AIE polymers, CPL-active supramolecular AIE polymers, and AIEgen/polymer composites with CPL. According to the location or introduction method of AIEgen in polymer structures, this review further divides CPL-active covalent AIE polymers into three categories, including polymers with AIEgen in main chains, polymers with AIEgen in side chains, and CPL-active polymers with clusterization-triggered emission. CPL-active supramolecular AIE polymers are discussed based on the driving force for the formation of supramolecular polymers, including host–guest interactions, metal coordination, and other non-covalent interactions. Moreover, examples on the construction of CPL-active AIEgen/polymer composites by physically mixing AIEgens with chiral (supra)polymers are also presented. This review is anticipated to provide readers with an overall view on the design strategies of CPL-active AIE polymers, and facilitate further research on the development of CPL materials and AIE polymers with advanced applications.
1 INTRODUCTION
Chirality is a ubiquitously basic feature of matter in natural world, which is widely observed in molecules and materials that are non-superimposable on their mirror images. Circularly polarized light can also be observed in nature. For example, Jeweled beetles can spontaneously produce circular polarized iridescence.[1] When a non-racemic chiral luminescent system emits left- and right-handed circularly polarized light with different emission intensities, circularly polarized luminescence (CPL) would be generated. Circular dichroism (CD) spectroscopy is a powerful tool to measure the difference in the absorption of left and right circularly polarized light, which reads the information of the electronic ground state of chiral materials in thermal equilibrium. CPL spectroscopy can be regarded as the luminescence of CD spectroscopy, and the collected CPL reflects the chiroprical properties of the chiral luminophores in the excited states. In the past years, functional materials with CPL properties have attracted tremendous interest due to their wide application prospect in three-dimensional displays, chiral recognition and catalysis, optical data storage, photoelectric devices, contrast imaging, and other fields.[2-9] Traditionally, the generation of CPL often relied on the use of linear polarizer and quarter-wave plate, which can consume up to 50% of the emitted light. By contrast, the direct generation of CPL from chiral luminophores could avoid the energy loss that occurred during the transition between plates.[10] Till now, a wide variety of CPL materials have been developed, including lanthanum ion complexes,[6, 11, 12] liquid crystals,[13-15] organic small molecules,[16, 17] conjugated polymers,[18-20] and supramolecular assemblies.[21, 22] To meet the requirements of practical applications, the design and development of CPL materials with high luminescence dissymmetry factor (glum) and high fluorescence quantum yield (QY) are in great demand. As a critical index to evaluate the performance of CPL materials, the glum, which is defined as glum = 2(IL − IR)/(IL + IR), is employed to indicate the difference between left- and right-handed CPL.[23] In this formula, the IL and IR represent the emission intensity of the pure left and right CPL, respectively, and the glum value ideally ranges from −2 to 2. The glum value depends on the electric transition dipole moment and magnetic transition dipole moment as well as the angle between them.[24] Blocking the electric transition and allowing the magnetic transition are expected to increase the glum value, and the glum value could reach the maximum when the angle between these two dipole moments is 0° or 180°.
In addition to glum, QY is another key factor for the design of CPL materials. Traditional fluorescent molecules could show high QY in dispersed states such as in dilute solutions. However, in aggregate states such as at high concentrations or in solid state, their QY often becomes significantly weak or even completely annihilated due to the aggregation-caused quenching effect.[25-27] This phenomenon greatly limits the performance and application scope of CPL materials. In 2001, a new concept termed as aggregation-induced emission (AIE) was proposed by Tang's group to describe a class of fluorescent materials that can emit strong fluorescence in aggregate state but show weak or no fluorescence in dilute solutions.[28, 29] The efficient aggregate-state luminescence of AIE materials endow them with enormous potential in diverse applications as solid emitters, fluorescent sensors, imaging agents, and so forth. One of the most popular working mechanism for AIE luminogens (AIEgens) is the restriction of intramolecular motions (RIM), predominantly including the restriction of intramolecular rotation and the restriction of intramolecular vibration. Generally speaking, AIEgens often contain rotatable or vibratile structural moieties. According to the RIM mechanism, in the dispersed state, the active intramolecular motions of AIEgens will consume the excited-state energy, leading to the non-radiative decay of excitons. Upon aggregation, the intramolecular motions are restricted to open the radiative pathway, consequently making the luminogens show enhanced fluorescence.[30-38] Chiral AIE materials not only possess high QY but also could emit efficient left and right CPL with high glum in aggregate states.[39-42] In practical applications such as security tags, CPL-based organic light-emitting diodes, and other devices, most of CPL materials need to be prepared into different aggregate forms like solid thin films. Therefore, AIEgens with excellent solid-state luminescence properties could be designed as ideal candidates for the construction of efficient CPL materials, especially those used in solid states.
Among various chiral AIE materials, CPL-active AIE polymers have received much attention in the recent decade. Apart from the inherited properties from functional monomers, the inherently complex and multidimensional structures of polymers allow them to show synergistic amplification effects and integration of multiple functionalities.[43-45] For example, as a typical type of natural polymer, proteins with three-dimensional structures could exhibit specific advanced functions compared to their monomers (i.e., amino acids). The combination of AIE and chirality into polymer systems is a win–win design strategy toward functional polymeric materials with CPL.[46-48] Compared to small molecular AIE counterparts, CPL-active AIE polymers have the advantages of excellent processability, good stability, supra-amplification sensing effect, diversified self-assembly behaviors, rich functionalities, and so forth. The good film-forming ability and high glum, efficient aggregate-state QY, and readily switchable CPL properties of CPL-active AIE polymers are conducive to the real-life applications in various scenarios, endowing them with great application potential in the fields of chiral catalysis, chiral recognition, chiral separation, sensing, stimulus response, and biomedicine.[26, 49, 50]
Attracted by the above-mentioned merits of CPL-active AIE polymer systems, researchers have devoted great efforts to developing such materials. Remarkable progress has been achieved in the past years.[39-42, 51] In this review, we will summarize the recent progress in this field by dividing diverse CPL-active AIE polymer systems into three parts, including CPL-active covalent AIE polymers, CPL-active supramolecular AIE polymers, and AIE luminogen (AIEgen)/polymer composites with CPL. From the discussion of the representative examples, we hope to provide readers with an overall view on the commonly used design strategies, working mechanism, materials performance, and application scenarios of CPL-active AIE polymer systems. At the end of this review, we will briefly discuss the chanlenges and perspectives in this field with the aim of facilitating further research on the design and applications of CPL materials and AIE polymers.
2 COVALENT AIE POLYMERS WITH CPL
One of the most commonly used strategies for the construction of CPL-active AIE polymers is covalently linking or attaching AIEgen in chiral polymer chains. According to the location or introduction method of AIEgens in polymer structures, CPL-active AIE polymers can be roughly divided into two categories, including CPL-active polymers with AIEgen in main chains and CPL-active polymers with AIEgen in side chains. Combined with the design strategy of chiral covalent polymers by introducing chiral moieties in polymer main chains or side chains, the structural diversity of CPL-active covalent AIE polymers could be abundant. Futhermore, CPL-active polymers with clusterization-triggered emission (CTE) have also been developed recently. Different from traditional AIEgen-containing fluorescent chiral polymers, the non-conjugated chiral CTE-based polymer systems with abundant electron-rich atoms such as N, O, S, P, and Si could result in extended delocalization of the lone pair electrons, and the consequent through-space conjugation and conformation rigidification eventually endow them with efficient aggregate-state fluorescence properties.
2.1 CPL-active polymers with AIEgen in main chains
As shown in Scheme 1, the construction of CPL-active polymers with AIEgen in main chains can be achieved by polymerizing chiral monomers and AIEgen-containing monomers. The chiral moieties could locate in polymer backbones or as pendants in side chains. Numerous functional polymers with good fluorescence and chiral optical properties have been developed based on this strategy.[52-56] For instance, our group has synthesized a kind of conjugated poly(enaminone)s with high molecular weights through a one-pot three-component sequential polymerization method of alkyne, carbonyl chloride, and amino ester salt. The introduction of optically active chiral amino ester as a side group endowed the polymer products with helical rotating backbones, and the incorporation of tetraphenylethene (TPE) moieties in the polymer main chain allowed the polymer to show obvious AIE behavior, eventually making the resulting polymers exhibit excellent CD and fluorescence signals in aggregate states.[54]
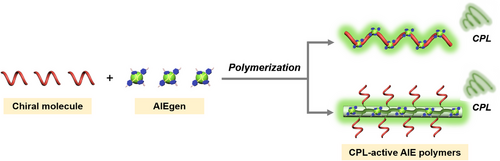
In the past years, Wang et al. have developed a series of CPL-active AIE polymers in which both the AIEgen and chiral moieties were placed in polymer backbones.[57, 58] In 2018, they synthesized one CPL-active AIE polymer based on the one-step polymerization reaction of chiral 1,1′-binaphthyl-containing diene monomer and TPE-containing difunctional azide monomer.[59] As shown in Figure 1A, a pair of (S)-/(R)-P1 polymer enantiomers were obtained by this click polymerization. As evidenced by the spectra shown in Figure 1B,C, the produced polymer P1 not only exhibited typical AIE properties with blue aggregate-state fluorescence but also showed strong aggregation-induced circularly polarized luminescence (AICPL). The underlying mechanism for the AICPL response of (S)-/(R)-P1 could be explained by the different self-assembly morphologies in the dispersed and aggregate states. As depicted in Figure 1D, when the water content in THF/water mixtures was increased from 40% to 90%, the self-assembly morphology obviously changed from sphere nanoparticles to left-handed helical nanowires, thus leading to a stronger CPL signal. The glum of the nanoaggregates and film of (S)-/(R)-P1 were measured to be up to 6.97 × 10−3 and 1.45 × 10−2, respectively. Furthermore, (S)-/(R)-P1 self-assembled the same left-handed helical nanowires. The formation of the same left-handed helical nanowires could be attributed to the cooperative effects of the π–π interactions influenced by binaphthyl chirality and the presence of multiple weak intermolecular hydrogen bonding as well as the steric effect.
In addition to CPL-active AIE polymers with blue fluorescence, the same research group also developed AIE covalent polymers with deep red AICPL.[57] As depicted in Figure 1E, a four-component chiral polymer was synthesized via a one-pot and two-step method based on the Pd-catalyzed Sonogashira reaction and Suzuki reaction of monomers incorporating the (R)-/(S)-1,1′-binaphthyl, 4,7-di(thiophen-2-yl)-2,1,3-benzothiadiazole (DTBT), TPE, and fluorene moieties. The molar ratio between the DTBT unit and TPE unit in the obtained polymer (P2) was determined to be 1:19. In this conjugated polymer, the binaphthyl component acts as the chiral source, while the TPE component serves as the AIE-active group and the Forster resonance energy transfer (FRET) relay converter. The DTBT and fluorene components function as the first FRET donor and the second FRET acceptor. It was anticipated that this chiral polymer can undergo two FRET processes with fluorene-TPE as the first FRET pair and TPE-DTBT as the second FRET pair to eventually exhibit deep-red CPL. Indeed, polymer P2 exhibited obvious chirality transfer process from the chiral binaphthyl moiety to the conjugated polymer main chain through the acetylenic bond, and the occurrence of two successive FRET processes was clearly demonstrated due to the appropriate spectra overlapping between the donor and acceptors (Figure 1F). The PL spectrum of the model polymer without DTBT chromophore further verified the occurrence of the FRET process from TPE moiety to DTBT moiety. Benefiting from the twisted polymer backbone and the successive FRET mechanism, P2 showed obvious AIE behavior with bright red fluorescence in the aggregate state. Moreover, the CPL intensity of (S)-P2 and (R)-P2 was also greatly enhanced upon aggregation, showing an AICPL emission signal in the deep red region (Figure 1G). The glum of AICPL can reach up to ±2.0 × 10−3 in aqueous mixtures with high water contents.
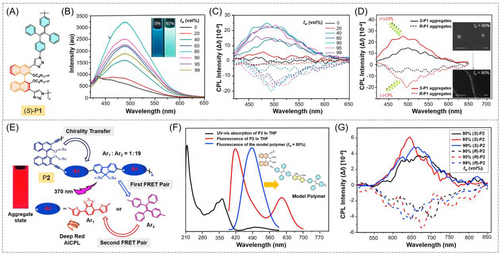
Regarding the CPL-active AIE polymers in which the AIEgen locates in main chains, but the chiral moieties locates as pendants in side chains, the reported design strategies were more diversified. For instance, Liu et al. synthesized a linear polymer (P3) with TPE in polymer backbone and a chiral L-alanine derivative as pendants by click polymerization.[55] As depicted in Figure 2A, P3 can self-assemble into various morphologies ranging from vesicles, helical nanofibers to microfibers by regulating the water contents and polymer concentration in THF/water mixtures. The formation and transition of the self-assembly processes were clearly characterized by SEM (Figure 2B1‒E1). Taking advantage of the efficient aggregate-state fluorescence of this AIE-active chiral polymer, its self-assembly morphologies can also be observed by a fluorescence microscope (Figure 2B2‒E2), and the microscopic morphology and chirality of P3 can be readily correlated with the visual fluorescence intensity. The rational coexistence of AIEgen and chiral moieties further endowed P3 with obvious aggregation-induced CD and AICPL behaviors. Later on, Liu et al. further designed a series of CPL-active polymers carrying TPE-containing backbones and chiral L-phenylalanine pendants in 2020.[60] These polymers also showed excellent AIE and AICPL responses and can form helical assemblies with the increase of water contents due to the intermolecular interactions. The obtained CPL-active AIE conjugated polymers were demonstrated to have great potential for applications in miniature optoelectronic devices. Besides, Liu and coworkers have also constructed CPL-active covalent polymers with TPE units in backbones and chiral moieties in pendants from the one-step Sonogashira cross-coupling polymerization of 1,2-bis(4-ethynylphenyl)-1,2-diphenylethene and 3′,5′-diiodo-N-α-tert-butoxycarbonyl-O-octyl-L-tyrosine methyl ester.[61] The glum values of the obtained CPL-active AIE polymer can be remarkably regulated from 0.44 to 0.08 by increasing the water content in THF/water mixtures because the glum value of the polymer might be mainly related to the electronic interaction of the isolated molecules and it was beneficial for the higher glum values in THF solution.
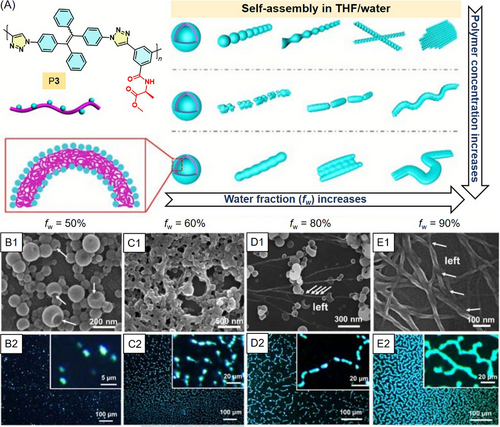
Apart from the aforementioned CPL-active AIE homopolymers with chiral moieties closely attached to the AIEgen-containing main chains, Hu et al. designed two conjugated block copolymers (P4 and P5) with electron-donating poly(carbazole-ran-acridine) backbones, electron-withdrawing dibenzothiophen-2-yl(phenyl)methanone (DBT-PM) substituent, and chiral alanine moieties at the end of side chains (Figure 3A).[62] Due to the presence of DBT-PM and the large steric hinderance of side chains, the obtained two polymers with different molar ratios of monomer units exhibited excellent aggregation-induced delayed fluorescence (AIDF) characteristics. Both the decay lifetime and the corresponding ratio of delayed fluorescence of P4 and P5 were increased after adding water as shown in Figure 3B,C. Moreover, obvious CPL signals were observed at around 550 nm in the film state of P4 and P5 (Figure 3D), and the corresponding glum values were determined to be −2.01 × 10−3 and −1.39 × 10−3, respectively. The good chiroptical properties of these two polymers indicated that the chirality successfully transferred from the alanine pendants in side chains to the conjugated polymer main chain, and the chirality of the polymers could be efficiently triggered in the excited state. Based on the AIDF and CPL properties, polymers P4 and P5 were further employed as solution-processed emitting layers for the fabrication of organic light-emitting diodes (OLEDs). Based on the device structure shown in Figure 3E,F, both nondoped and doped OLEDs were fabricated. The constructed OLEDs emitted green electroluminescence with maximum brightness of 1477 cd/m2 and maximum current efficiency of 2.52 cd/A. The work provides an effective design strategy to achieve AIDF polymers with CPL activities.
2.2 CPL-active polymers with AIEgen in side chains
CPL-active polymers can also be constructed through the introduction of AIEgen in side chains of polymers, which could avoid the design and synthesis of difunctional AIEgen-containing monomers. The chirality of the obtained AIE polymers could arise from polymer main chains or from the chiral moieties in side chains (Scheme 2).
Optically active poly(isocyanide)s with single-handed helical conformation have attracted extensive attention due to their important applications in molecular recognition, photoconductivity, liquid crystal, asymmetric synthesis, and so forth. In the past years, diverse functional luminescent poly(aryl isocyanide)s (PAIs) have been synthesized and their chiroptical properties have been carefully investigated.[63, 64] For instance, Yan et al.’s research team and Wu et al. have synthesized a series of TPE-containing PAIs under different polymerization conditions.[65-67] In these optically active AIE polymers, the TPE unit was attached in the side chains and the chirality stemmed from the helical characteristics of the PAI backbones. Diverse types of catalysts were demonstrated to be effective for the homopolymerizations of TPE-containing monoisocyanide monomers, including a series of half-sandwich rare-earth dialkyl complexes[66] as well as different transition metal catalysts such as AliBu3 and Pd-based complexes.[65, 68] Considering the negative effects of metal residues for the stability and materials performance of PAIs, metal-free initiators have also been adopted for the synthesis of functional fluorescent PAIs. As shown in Figure 4A, Yan et al. have synthesized several TPE-containing PAIs under the catalysis of commercially available, metal-free, and highly efficient [Ph3C][B(C6F5)4].[67] The homopolymerization of TPE-containing isocyanide produced P6, while the copolymerization of TPE-containing isocyanide and isocyanide substituted with chiral ester groups (D/L-IMCI) afforded D/L-P7. Unlike typical AIE polymers, the PL intensity of P6 and P7 first increased as the water content rose and then fell down (Figure 4B), which might be related to the formation of aggregate states with looser packing at high water contents. The CD spectra of D/L-P7 showed obvious mirror symmetry with the positive and negative CD signals at 364 nm (Figure 4C), demonstrating that they had good single-handed helical conformations and had great potential in developing CPL signals.
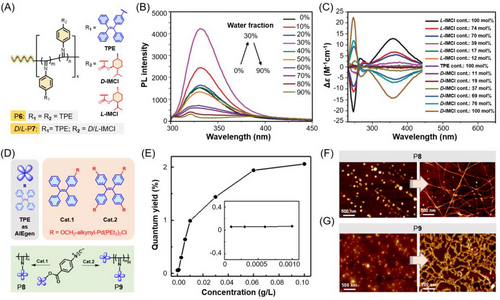
Recently, He et al. synthesized a linear PAI (P8) and a four-armed PAI (P9) that were functionalized with TPE pendants. These two conjugated polymers were prepared by the living polymerizations of the same TPE-functionalized phenyl isocyanide monomer but in the presence of different TPE-based Pd(II) catalysts (Figure 4D).[68] The resulting PAIs showed typical AIE behaviors (Figure 4E), and their fluorescence properties can be facilely tuned by varying the concentration or molecular weights of the polymers. Moreover, different self-morphologies were observed at different conditions. As shown in Figure 4F (left), the linear polymer P8 indicated uniformly distributed spherical nanostructures at low molecular weight with a degree of polymerization (DP) of 14. When the DP value changed from 14 to 65, stable and well-defined helical nanofibrils were formed (Figure 4F, right), indicating that the molecular weights of polymers played a crucial role on the morphology properties and the subsequent chiral optical properties. By contrast, the four-armed polymer P9 can self-assemble into an apparent bumpy “caterpillar”-like assembly at high concentrations (Figure 4G, right), which was very different from the morphology obtained at a low polymer concentration (Figure 4G, left). These main-chain helical PAIs with efficient aggregate-state fluorescence are promising to be further designed and developed into optical materials with good CPL properties.
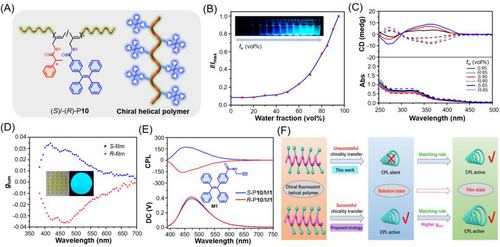
The construction of CPL-active polymers with both the AIEgen and chiral moieties in side chains has less limitation for the polymerization types. For instance, recently Lu et al. synthesized chiral fluorescenthelical polyacetylenes based on the copolymerizations of chiral-substituted acetylenic monomers and an achiral acetylenic monomer with TPE pendant.[69] As shown in Figure 5A−C, the obtained copolymers with chiral side chains and AIEgen-containing side chains showed excellent AIE performance and chiroptical properties. Obvious CD signals were detected at high water fractions from 85% to 95%, but the CD intensities were gradually decreased with the increase of water content. The aggregation-attenuated CD effect might be due to the disruption of the predominantly single-handed helix of the polymer backbone by the strong polarity of water to some extent. No CPL emission was observed in copolymer dispersions. However, the CPL activity of P10 in composite film state was greatly amplified due to the more ordered arrangement of polymer chains in the solid films. As depicted in Figure 5D, by doping a small amount of P10 into the poly(methyl methacrylate) matrix, the obtained fluorescent composite films exhibited strong positive CPL signals, with the maximum glum value of ±3.6 × 10−2. Besides, strong CPL signals can be achieved by simply blending a chiral molecule with the fluorescent polymer (Figure 5E). Different from the common chirality transfer mechanism for CPL-active materials, the underlying CPL generation mechanism of P10 in the composite films was attributed to the “matching rule” between fluorescent moieties and chiral helical polyacetylenes. As illustrated in Figure 5F, no efficient chirality transfer occurred from the helical main chain to the fluorescent side chains, thus the copolymers showed no CPL emission in a solution and dispersed state. When the copolymer was transformed to the film state, ordered arrangement or self-assembly was initiated to endow the composite films with strong Cotton effect in the emission region of P10. The overlapping between the CD and PL spectra at such conditions made P10 show remarkable CPL activities. Through rational structural design to synergistically utilize the chirality transfer and matching rule, the CPL performance could be further amplified. This work provides new perspectives for the design of CPL materials with glum values in the solid state.
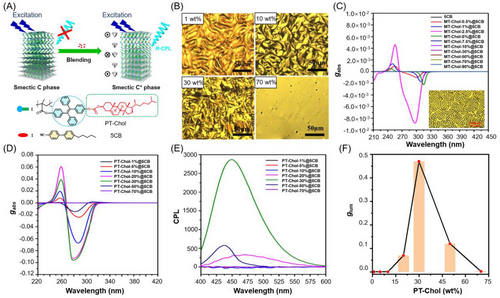
The commonly used olefin polymerizations are also suitable for the design and synthesis of side chain-type CPL-active AIE polymers. For example, Luo et al. recently reported the highly efficient CPL from a kind of chiral luminescent liquid crystalline polymer with AIE properties.[70] This polymer was synthesized by the radial polymerization of a methacrylate monomer carrying both TPE moiety and the chiral 4-cholesteryl ester (MT-Chol) group in pendants. As illustrated in Figure 6A, the obtained AIE-active chiral liquid crystalline polymer (PT-Chol) was demonstrated to possess a layered structure. Although the pure polymer did not show CPL behavior in the solid state, the blending mixture of the polymer with a specified amount of 4-cyano-4′-pentyl biphenyl (5CB) exhibited highly efficient CPL. This phenomenon could be explained by the formation of a chiral nematic liquid crystal or smectic C* phase in the mixture of the polymer and 5CB at low doping concentrations. The polarized light microscopic images shown in Figure 6B suggest that blending concentration had a critical effect on the phase structure, and an obvious fingerprint texture was found in the polymer-5CB mixture at low concentrations of PT-Chol. Both the TPE-containing monomer (MT-Chol) and the polymer (PT-Chol) showed enhanced PL intensity with the increase of their concentration in 5CB, and their chiroptical behaviors showed an interesting supramolecular structure-dependent effect. As shown in Figure 6C, with the increase in the content of MT-Chol in 5CB, the absorption dissymmetry factor (gabs) of the resulting mixture first dramatically increased and then gradually decreased. The maximum gabs value of −9 × 10−3 was collected at 2.5% MT-Chol content due to the formation of the fingerprint texture at this composition. Similar tendency was also observed for the gabs values of polymer-5CB mixtures (Figure 6D). Nevertheless, the blending mixtures of MT-Chol and 4CB cannot induce CPL behavior, but obvious CPL signals can be detected from the polymer-5CB mixtures, with the PT-Chol content of higher than 20% (Figure 6E). And the highest glum value of +0.45 was achieved at 30% PT-Chol content (Figure 6F). These results suggested that the CPL property was derived from the polymer structure, and the formation of PT-Chol aggregates in this liquid crystal hybrid system can simultaneously enhance the luminescence efficiency and CPL performance. The presence of 5CB molecules acted as plasticizers to facilitate the helical ordered packing of the polymer chains. The obtained CPL-active composite material showed excellent stability, which was conducive to applications in optoelectronic devices.
2.3 CPL-active polymers with clusterization-triggered emission
AIE materials generally possess rigid and conjugated aromatic structures, extended π-conjugated systems, or unsaturated heterocyclic systems.[30] However, a large number of non-conjugated molecules or polymers with abundant electron-rich atoms such as N, O, S, P, and Si were also found to be efficient AIE materials. Zhang et al. and Lai and coworkers proposed that such an unconventional luminescent behavior could be due to the CTE mechanism.[32, 71-73] That is, the clusterization of these electron-rich atoms could result in extended delocalization of the lone pair electrons, and the consequent through-space conjugation and conformation rigidification eventually endow such materials with efficient fluorescence properties. The non-conjugated CTE polymers have the advantages of facile synthesis, good water solubility, low biological toxicity, and so forth, and are attractive alternative materials of the conventional conjugated AIE polymers for diverse applications.
In addition to the covalent AIE polymers with typical AIEgen in the polymer structures, CTE-based polymer systems have also been applied for the generation of CPL through the ordered self-assembly between non-conjugated chiral CTE polymers and the interacting molecules (Figure 7A).[74-76] For instance, recently Liao et al. reported a purely organic CTE system that can emit strong CPL based on the strategy of solid-phase molecular self-assembly of cationic α-poly-L-lysine (PLL) and oleate ion (OL).[77] As illustrated in Figure 7B, the ionic assembly between PLL and OL can form cross-linked vesicles in aqueous solution, where PLL displayed typical CTE behavior but no CPL was detected. After applying a mild mechanical pressure to make the composite form a well-defined structure, the vesicle networks could precipitate out upon centrifugation and transform into planar bilayers to show obvious CTE-based CPL. Figure 7C suggested that the mixed system showed dramatically increased turbidity with the increase in OL fraction and became whitish at a molar ratio of 1:1 due to the formation of vesicular clusters at such conditions. However, the formation of such vesicles could not contribute to the well-defined folding of the PLL chains as no CPL was detected. Under mechanical pressures beyond 5 MPa, the cross-linked vesicles can be transformed into a flexible and self-supporting transparent film with strong blue fluorescence, and the CTE QY was up to 5.56%. Moreover, the film exhibited strong CPL signal with a high absolute glum value of 0.016. Interestingly, the CTE-based CPL intensity and the glum value were responsive to humidity. The increasing humidity can cause significant decrease in glum values due to the reduced supramolecular chirality in the solid film (Figure 7D). To further tune the CPL color, a FRET strategy was adopted by doping a yellow-emissive commercial dye (fluorescein sodium, FS) as the energy acceptor into the PLL-OL film. As depicted in Figure 7E,G, the emission of the PLL-OL⊃FS film gradually became stronger and red-shifted with the increasing doping amount of FS. Benefiting from the presence of strong interchain hydrogen bonding, the through-space conjugation of orderly aligned electron-rich O and N atoms leads to CTE-based blue CPL, which is capable of transferring energy to FS acceptor via the FRET process (Figure 7F). By changing the doping amount of acceptors, color-tunable CPL signals were readily achieved. As shown in Figure 7H, compared with PLL-OL, the CPL spectrum of PLL-OL⊃FS film showed brighter and yellower right-handed CPL with the peak at 575 nm, indicating the efficient complexation of FS with the folded PLL chains. This seminal work demonstrated that the chain folding state of CTE-based polymers is critical for the efficient generation of CPL.
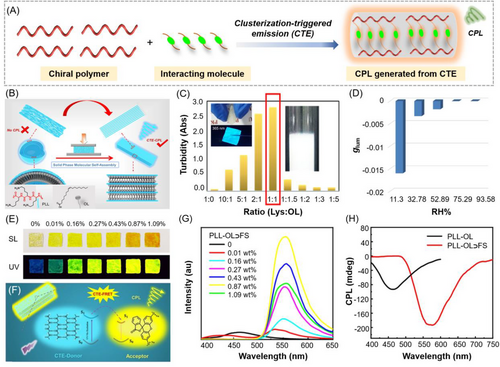
Despite of the economic and sustainable advantages of the CTE-based CPL materials, examples on the development of CPL-active polymers based on CTE mechanism are still very limited. One possible reason might be the heterogeneity of clusteroluminogens in a CTE system, which is undesirable for the generation of CPL. The solid-phase molecular self-assembly strategy proposed by Yan and Tang et al. provides an expedient method to transform the CTE materials from random clusters to regular arranged superstructures. With more efforts for the development of CTE-based CPL polymers, the biological applications of CPL materials could be further expanded due to their good biocompatiblity and water solubility.
3 SUPRAMOLECULAR AIE POLYMERS WITH CPL
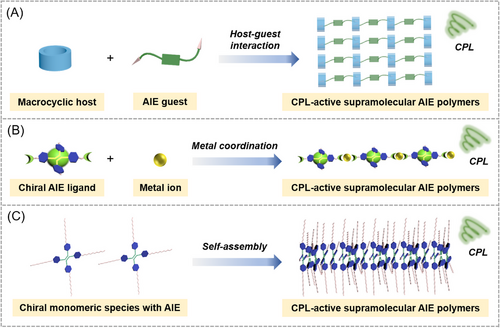
Supramolecular polymers are formed by the assembly of supramolecular monomers via reversible and highly directional non-covalent interactions, such as host–guest interaction, hydrogen bonding, metal coordination, electrostatic interaction, hydrophobic interaction, and π–π interaction.[78-84] Compared with covalent polymers, the non-covalent bonds in supramolecular polymers are dynamic and reversible, which lays an important foundation for smart supramolecular polymer systems, such as stimuli-responsive polymers, self-healing polymers, and degradable polymers.[85-89] The transition from single-molecule chirality to high-level macro-helicity can be achieved during the supramolecular assembling processes, which can contribute to the chiral transfer and amplification. Therefore, supramolecular polymers are widely used in the CPL field. On the other hand, AIE effect can effectively solve the problem of solid-state luminescence quenching of traditional fluorescent molecules, and provide convenience for the construction of CPL materials with high brightness.[39, 42] The combination of chiral supramolecular polymers and AIE not only can simultaneously improve the fluorescence and chirality performance but also endows the materials with potential stimuli-responsive properties, providing an effective strategy for developing smart CPL materials with high dissymmetric factors. Based on the driving force for assembly, the commonly used strategies for the construction of CPL-active supramolecular AIE polymers can be classified into three types (Scheme 3), including (A) the host–guest interaction between the macrocyclic building unit (host) and AIEgen (guest), (B) the metal coordination interaction between chiral AIE ligand with coordination sites and metal ions, and (C) self-assembly process of AIE-active chiral monomeric species via other non-covalent interactions.
3.1 CPL-active supramolecular AIE polymers based on host–guest interactions
The reported construction strategies of CPL-active supramolecular AIE polymers based on host–guest interactions could be further divided into several groups. One is based on the host–guest self-assembly interaction between chiral macrocyclic host and achiral luminescent guest. For example, Guo et al. recently reported a CPL-active supramolecular polymer system based on the host–guest complexation between chiral bishelic[6]arenes (P/M-BH6) and different achiral AIEgen guests.[90] As depicted in Figure 8A, a pair of amide group-bridged P/M-BH6 was synthesized as the chiral host, while blue-emissive AIEgen (BG) and yellow-emissive AIEgen (YG) with two trimethylammonium pendants were selected as the fluorescent guest molecules. With the increasing concentration of host–guest complex, uniform spherical particles and strips were successively observed, demonstrating the formation of linear supramolecular polymer chains via the host–guest interaction. Two-dimensional networks with sheet-like structures were finally formed at the gelation concentration through hydrogen bonding between amide groups of the host linkers. The PL spectra shown in Figure 8B suggested that the fluorescence intensity of AIEgen (BG or YG) was significantly enhanced after the formation of host–guest complexes, which can be explained by the restriction of intramolecular motions of AIEgens in the self-assembled supramolecular gels. Regarding the chiroptical properties, the AIEgen alone showed no CD signals, while the chiral host (P/M-BH6) alone showed mirrored CD signal ranging from 270 to 300 nm. After mixing the achiral AIEgen with the chiral macrocyclic host, new mirrored Cotton effect appeared in the absorption wavelength region of the AIEgen. The CD signal of the new peak gradually increased with the increase of the proportion of guest molecules (Figure 8C), indicating the occurrence of efficient chirality transfer from the chiral host to achiral guests. The enhanced fluorescence and chiral property of AIE supramolecular gels eventually induced obvious CPL signals (Figure 8D). Compared with the host and guest complexes in solution state, supramolecular gels exhibited much better CD and CPL activity. By adjusting the ratio of the blue and green-emitting AIEgen guests (BG and YG) in the complex, CPL-active ternary supramolecular gels with white light emission were readily fabricated with |glum| values of around 1.3 × 10−3. This work provides a convenient and efficient method for the construction of host–guest supramolecular polymers with CPL activity using chiral macrocyclic hosts and achiral AIEgens.
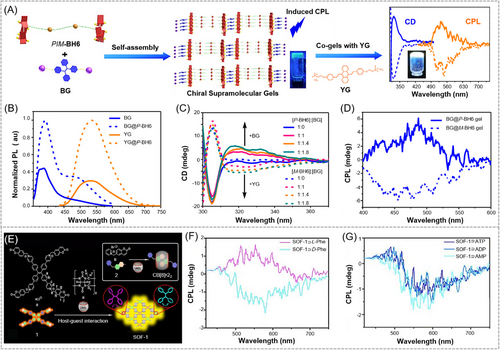
Another strategy is through the host–guest self-assembly interaction between achiral macrocyclic host and latent chiral AIEgen guest. For instance, in 2021, Li and coworkers[91] developed a CPL-active supramolecular polymer system taking advantage of the P/M rotational conformation of TPE. As illustrated in Figure 8E, a tetra(6-coumarinylmethyl-pyridinium) TPE derivative was designed and synthesized as a latent chiral AIEgen guest. By using cucurbit[8]uril (CB[8]) as the host macrocyclic molecule to interact with the AIEgen guest via head-to-tail host–guest complexation, an achiral supramolecular organic framework (SOF-1) can be generated. When L-/D-phenylalanine or a small amount of adenosine derivatives (ATP, ADP, and AMP) are encapsulated into the cavities of SOF-1, the achiral CB[8]-based supramolecular network polymer can be induced to show adaptive chirality (Figure 8F,G). The addition of chiral molecules can create an asymmetric environment in this supramolecular system, which could promote the selective homodirectional P or M rotational conformation of TPE units in the supramolecular polymer skeleton, thereby leading to the efficient through-space chirality transfer (TSCT) from the chiral molecules to SOF-1 framework. Mirror-image CD and CPL can be simultaneously achieved in water. The chiral-molecule-induced dynamic adaptive chirality of this supramolecular polymer system makes it an ideal platform for the applications in determining dipeptide sequences and distinguishing some polypeptides/proteins in water.
Recently, Tu's group[92] ingeniously designed a class of di-pyrene-modified chiral γ-cyclodextrin derivatives that can self-assemble into CPL-active supramolecular polymers via host–guest complexation. Although these monomeric species do not contain any classical AIE group, they exhibit typical AIE properties with efficient aggregate-state fluorescence. The fluorescence quantum yield of the γ-cyclodextrin derivative with butyl amide-linked pyrene substituents was 26.79% in DMF and 59.7% in aqueous suspension with a water content of 99%. The enhanced fluorescence property could be ascribed to the formation of assemblies that avoid collisions with solvents. Moreover, the CD signal of the γ-cyclodextrin derivative was significantly enhanced with the increase of water content. In 99% aqueous solution, the γ-cyclodextrin derivatives can form supramolecular polymers with excellent CPL properties due to the rigid chiral environment created by the hydrophobic complexation-induced aggregation. Significant electronic circular dichroism and CPL with high gabs (up to 4.3 × 10−2) and glum values (up to 5.3 × 10−2) can be achieved. Furthermore, the dynamic characteristic of the host–guest self-assembly system enabled it to possess remarkable stimuli-responsive chiroptical properties. The aggregation behavior and chiroptical performance of this supramolecular system could be readily manipulated by temperature, pH, and competitive guest complexation. As a brief sum-up, the aforementioned examples of AIE-based host–guest self-assembly strategy can simultaneously achieve excellent fluorescence properties as well as efficient chiral transfer and amplification without very complex synthetic produces. However, investigation on the construction of CPL-active supramolecular AIE polymers by host–guest assembly strategy still remains very slow and limited, possibly due to the lack of appropriate AIE guests to induce efficient CPL generation.
3.2 CPL-active supramolecular AIE polymers based on metal coordination
Chiral coordination polymers with AIE property are another type of attractive candidates for the design of CPL-active polymer materials. Compared with the corresponding monomeric complexes, chiral coordination polymers generally display much higher luminescence efficiencies and larger |glum|.[93, 94] As a special group of supramolecular polymers, metal-organic framework (MOF) materials provide a new opportunity to connect chiral units and AIEgens. The dynamic chiral frameworks could enable a different manner of chirality transfer and stimuli-responsive capabilities for CPL materials.
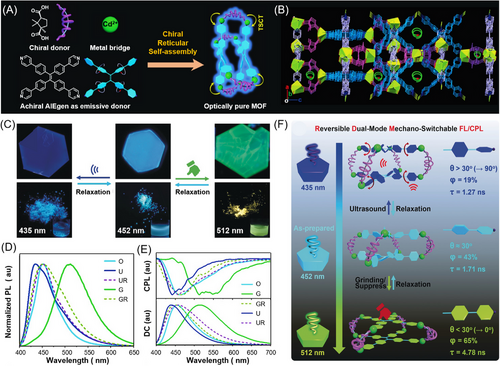
For example, Shang and coworkers reported a chiral reticular self-assembly strategy that combines chiral donors, achiral AIEgens, and metal ions together for the construction of optically pure MOFs with dual mechano-switchable CPL.[95] As illustrated in Figure 9A, the MOF structure was composed of tetrakis(4-pyridylphenyl)ethylene (a typical AIEgen) as the emissive donor, camphoric acid as the chiral donor, and CdII ion as the metal bridge. The formation of the optically pure MOFs with hexagonal structures was clearly verified by the single-crystal X-ray diffraction (Figure 9B). After the chiral reticular self-assembly, the frame-enable emission property and the TSCT from the chiral donor to the achiral AIEgen endowed the resulting MOF material with efficient fluorescence and CPL in solid states. As shown in Figure 9C−E, the MOF crystals formed from D-camphoric acid depicted strong cyan blue fluorescence, with the maximum emission peak at 452 nm and obvious CPL signal with a |glum| value of up to 0.012. After grinding and ultrasonication, the fluorescence of D-MOF crystals shifted to 513 and 435 nm, respectively. Meanwhile, the CPL properties of D-MOF changed correspondingly. All of these altered states could fully recover to their original state by suspending the grinded powders into DMF for relaxation or removing the DMF solvent from the ultrasonication system. Such an interesting dual mechano-switchable fluorescence/CPL could be attributed to the influence of external stimuli on the local or global dynamics of the AIEgen rotors in the MOF structure. As indicated by Figure 9F, the dihedral angle between the phenyl ring and the adjacent pyridine moieties in the prefabricated AIE MOF crystals is measured to be about 30°, which contributes to the strong fluorescence (QY = 43%, life time τ = 1.71 ns) and CPL of the crystalline MOF. Grinding made the distance of AIEgen closer and the dihedral angle became smaller (<30°), which further restricted the movement of the AIE units and eventually led to the red-shifted fluorescence, an enhanced QY (65%), and a longer life time. On the contrary, ultrasound promoted the movement of AIEgen compared with the unprocessed state, and the phenyl conformation became more twisted. The nonrigid chiral donor also plays an important role in the reversible mechano-responsive behavior. The coordination framework was demonstrated to be retained under grinding and ultrasonication stimuli to maintain the microscopic dynamics, which explained the reversibility of the mechano-responsive fluorescence/CPL performance. This example suggested that the chiral reticular self-assembly could be an efficient and versatile strategy for constructing CPL-active supramolecular AIE polymers with stimuli responsiveness.
In addition to the MOF-type chiral coordination polymers, AIE-active metal organic clusters formed through metal coordination have also been rationally designed and developed to show excellent CPL properties.[76, 96] For example, Wang et al. successfully constructed novel AIE-active chiral gold clusters using alkynyl alcohol enantiomers.[97] The fluorescence of the obtained chiral gold clusters was strongest at 60% water content in DMF/water mixtures, and the solid QY can reach up to 73%, which was demonstrated to be conducive to the generation of CPL with high glum in the aggregate state. Zhang's group has also prepared diverse chiral metal clusters with both AIE and CPL properties and applied them to biological imaging.[74] Compared to metal organic clusters, the development of CPL-active supramolecular AIE polymers based on metal coordination still remain very slow. Although coordination polymers have been extensively studied and widely used, examples on coordination polymers with both AIE and CPL properties are rare and there is still a large room to be further explored.
3.3 CPL-active supramolecular AIE polymers based on other interactions
In addition to host–guest interaction and metal coordination interaction, other non-covalent interactions such as hydrogen bonding interaction and electrostatic interaction have also been utilized for the construction of CPL-active supramolecular AIE polymers. One of the representative examples is hydrogen bonding-based supramolecular AIE polymers with finely controlled CPL. Recently, Lee et al. reported a kinetically controlled mechano-responsive CPL supramolecular polymerization system, where a chiral enantiomer (L-1/D-1) was prepared with TPE moiety as the emissive core and the chiral alanine derivative as the branch side chains (Figure 10A).[98] The presence of TPE moiety not only can endow the resulting supramolecular polymer with AIE properties but also could promote the helical self-assembly process through the C‒H···π stacking interactions of TPE planes in addition to the intermolecular hydrogen bonding of amide groups. As shown in Figure 10B, when a clockwise rotating mechanical force was applied to the THF solution of the monomeric species L-1, its luminescence color remarkably changed from green at 500 nm to blue fluorescence at 455 nm, and the luminescence intensity increased by 500 times. The enhanced luminescence could be explained by the activation of AIE effect during the formation of the supramolecular polymer, while the blue-shifted fluorescence might be due to the more twisted conformation and stacking of TPE moieties in the polymer framework. The supramolecular polymerization was drastically accelerated by increasing the rotation speed (Figure 10C), which was directly related to the mechano-promoted dissociation of the intramolecular hydrogen bonds formed in the monomer L-1. The dissociation of the hydrogen bonding in monomer species consequently facilitates the rapid growth of the supramolecular polymer. Chirality in the target molecule is transferred and amplified during supramolecular polymerization. The time-dependent morphological changes of the self-assemblies under mechanical stimulation suggested that external mechanical stimulus played a key role in the supramolecular polymerization as a helical inducer and accelerator. By finely controlling the duration and strength of the external mechanical stimulus (rotation time and speed), dual enhancement in both fluorescence and chirality can be achieved, which eventually increased the CPL signals and |glum| values (Figure 10D,E). As shown in Figure 10E, the CPL intensity rapidly increased with time at a rotation speed of 1000 rpm, and the |glum| value can reach up to 2.2 × 10−2. This mechanical stimulus-accelerated chiral supramolecular polymerization provides a distinctive design strategy for the construction of hydrogen bonding-based AIE supramolecular polymers with CPL activity. Recently, Zheng and coworkers developed a series of CPL-active supramolecular self-assemblies of AIEgens using TPE as the parent nucleus.[99-101] For instance, they have designed and prepared a TPE-containing macrocyclic system.[102-104] After being modified based on the alkyl chain engineering, such macrocyclic TPE derivatives could possess excellent self-assembly ability to form a variety of CPL-active AIE supramolecular polymers through different intermolecular interactions. These research results provide a new idea for developing novel AIE supramolecular polymers with CPL activity.
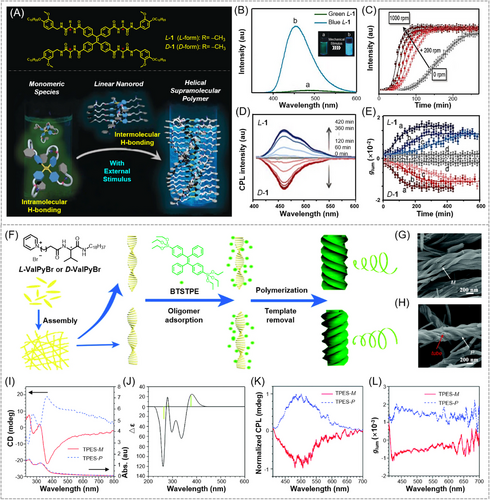
Moreover, temperature-responsive AIE supramolecular polymers with CPL activity have also been developed taking advantage of the dynamic feature of non-covalent interactions. For instance, Wu et al. recently constructed an AIE supramolecular polymer system with temperature-stimulated CPL response by self-assembly technology.[105] Such AIE supramolecules could form gel through weak interaction forces like hydrogen bonding at room temperature. In this state, the movement of AIE molecule was effectively restricted, which was conducive to enhancing the fluorescence properties of the material. Meanwhile, hydrogen bonding interaction was beneficial to chirality transfer and can thus enhance the chiral properties. Therefore, the obtained supramolecular polymers showed excellent CPL properties in gel state. When the temperature was raised, the intermolecular hydrogen bonds were broken, and the polymer changed from a gel (aggregated state) to a solution (dispersed state), which resulted in a decrease in both chirality and fluorescence intensity, and ultimately decreased the CPL signal.
Besides the above-mentioned examples, in 2019, Cai et al. employed electrostatic interaction for the construction of CPL-active supramolecular helical nanotubes based on TPE-bridged polybissilsesquioxane (TPES).[106] A supramolecular templating approach was used for the fabrication of such luminescent helical inorganic/organic hybrid nanomaterials. As shown in Figure 10F, a pair of chiral cationic low-molecular weight gelators (L-ValPyBr and D-ValPyBr) were used to form supramolecular assemblies (nanofibers) with single-handed helicity. Then TPE-containing bis(triethoxysilane) (BTSTPE) was dropped into the supramolecular systems. Under basic conditions, the triethoxyl groups of this AIEgen precursor were hydrolyzed to form hybrid silica oligomers, which can be adsorbed by the pyridinium rings of the gelators via electrostatic interactions. With the nanofibers as templates, the oligomers further underwent polymerization on the nanofiber surfaces, and the helical nanostructures of the AIEgen-containing inorganic/organic hybrid assemblies (TPES-M and TPES-P) were finally obtained by removing the templates. The SEM images shown in Figure 10G,H clearly demonstrated that TPES-M and TPES-P possessed left-handed and right-handed screw senses, respectively. The CD analysis further verified that the chirality of the chiral supramolecular templates was transferred to the AIE scaffolds (Figure 10I,J). Combined with the excellent fluorescence efficiencies of the solid TPES-M and TPES-P (QY = 26.2% and 21.2%, respectively), their hybrid nanotubes exhibited CPL activities with good glum values of −0.6 × 10−3 and +1.6 × 10−3, respectively (Figure 10K,L).
Based on the above-mentioned design strategies for the construction of supramolecular AIE polymers with CPL, it is anticipated that diversified AIE polymer systems with more efficient CPL, higher QY, and more intelligent functionalities could be developed in the near future.
4 AIEGEN/POLYMER COMPOSITES WITH CPL
Integrating chiral and luminescent elements into (supramolecular) polymers often requires ingenious design and complex synthetic procedures, which could increase the preparation costs for CPL materials. “Simplicity and generality” is a goal that scientists have always been pursuing. In this regard, physically mixing AIEgens with chiral (supra)polymers is supposed to be a simple yet powerful strategy to construct AIE polymeric systems with CPL. As shown in Scheme 4, CPL-active AIEgen/polymer composites are generally prepared with small molecular AIE dyes as dopants and chiral covalent polymers or supramolecular polymers as host matrices. The phenomenon of TSCT from chiral polymers to AIEgens often occurs in such systems. By adjusting the proportion and variety of the dopants (AIEgens), the chirality and fluorescence properties can be finely regulated, which is beneficial for the achievement of full-color-tunable CPL with high dissymmetry factor. In this section, recent progress on the development of diverse CPL-active AIEgen/polymer composites will be introduced with discussion on representative examples.
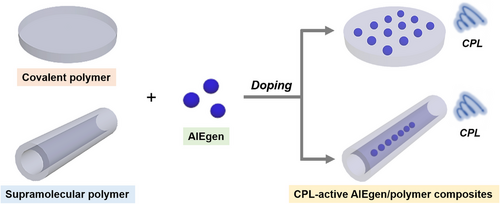
Luminogens with polymorphism can show different emission properties as microenvironment changes due to the differences in their molecular assembled structures. Recently, Khorloo et al. reported a polymorphic AIEgen (TPE-EP).[107] By doping this AIEgen into a chiral covalent polymer (poly(L-lactide), PLLA), remarkable CPL activity can be achieved from the resulting composites. As shown in Figure 11A, after mixing with the semi-crystalline polymer of PLLA, the thermodynamic stable polymorph of TPE-EP with green fluorescence is stabilized in the amorphous PLLA phase, while a metastable polymorph with yellow fluorescence is confined in the crystalline PLLA phase. The polymorphism-dependent emission of TPE-EP not only benefits the direct visualization of microstructures and spatial morphological arrangement of PLLA, but also provides a connection between the microscopic morphological information and macroscopic polarized optical signals. As indicated by the fluorescence microscopic image in Figure 11B, the crystalline PLLA film is composed of spherulites with alternating bright- and dark-yellow emissive spirals. The formation of such specific anti-clockwise (ACW) spiral spherulites could be explained by the twisting of the crystalline lamellar and the subsequent radial growth of the helical lamellae during the slow evaporative crystallization process. Benefiting from the chiral structured spherulites with yellow emission, the AIEgen-embedded polymer film exhibited intrinsic CPL generation capability. A negative CPL response with a glum value of around −1.6 × 10−3 was observed and the CPL spectrum peaked at about 540 nm. It is noteworthy that the CPL response can be switched to positive by flipping the film sample while the enantiometric symmetry was kept (Figure 11C). The induced CPL spectrum of the reversed film with clockwise (CW) spiral showed a mirror image to the ACW spiral, indicating that the macroscopic chiroptical activity of the AIEgen/polymer crystalline films can be directly modulated by the corresponding microscopic spiral morphology.
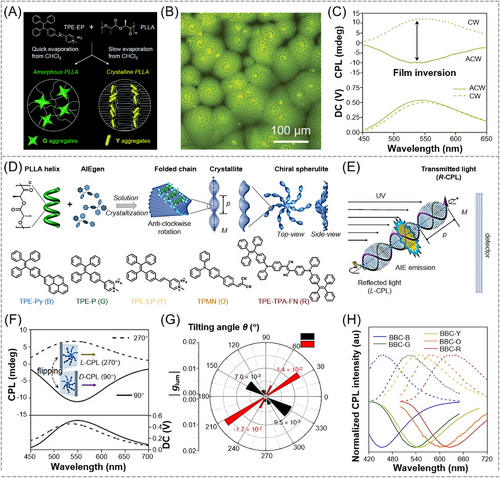
Later on, Khorloo et al. conducted in-depth study about the influence of film tilting angle on the handedness of CPL.[108] A variety of AIEgen-doped crystalline chiral polymer films were prepared by crystalline-induced self-assembly (Figure 11D), where the crystalline chiral PLLA film with periodic spiral structures acts as a polarizing medium to accommodate achiral AIEgens for CPL generation. There was no chirality transfer from PLLA matrix to AIEgens in these composite films, and the AIEgens were merely physically confined between the twisted crystalline stacks. As shown in Figure 11E, the fluorescence of the embedded AIEgens could propagate along the M-axis under UV irradiation. Due to the left-handed twisted crystallites within the spherulites, the reflected light was collected to show left-handed CPL (L-CPL) signal, and the transmitted light was collected to present the corresponding right-handed CPL (R-CPL) component. Therefore, a single or opposite-handed CPL component could be readily achieved by selectively collecting the reflected or transmitted emission light. With the variation in the tilting angle (θ), which is defined as the angle of the M axis to the horizontal reference level, the CPL spectra of the composite crystalline film showed significant changes. Taking the TPE-EP-doped PLLA film as an example, R-CPL was observed at right-tilted angles, while the opposite CPL signal (L-CPL) was detected at left-tilted angles. Arising from the asymmetric conical-shaped structures of the twisted chiral spherulites, the opposite R-CPL and L-CPL signals remained visible even at normal angles of 90° and 270° (Figure 11F). A detailed polar diagram was plotted to show the variation in glum values as the change of θ. As depicted in Figure 11G, the CPL handedness and glum can be manipulated at the same time. By simply modulating the tilting angle, the absolute value of glum can reach up to 1.7 × 10−2. Moreover, full-color-tunable CPL was easily generated by introducing AIEgens with different emission colors into the polarizing polymer matrix (Figure 11H). The facile and versatile θ-dependent CPL switching approach as well as the rigid and continuous nature of this polarizing system provide a promising way for the design and modulation of chiroptical materials with desired performance.
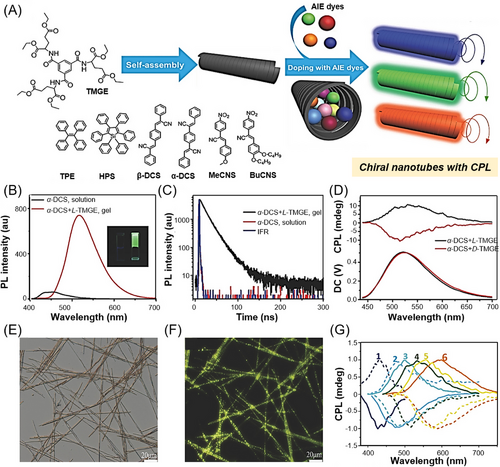
In addition to the use of covalent polymer like PLLA as the polarizing matrix, supramolecular polymer systems have also been employed for the fabrication of AIEgen-doped composites with CPL. For example, in 2017, Han and coworkers reported the construction of multicolored CPL systems by doping achiral AIEgens into supramolecular chiral gel nanotubes to form AIE chiral nanotube composites with CPL activity.[109] As illustrated in Figure 12A, 1,3,5-benzenetricarbonyl D/L-glutamate diethyl ester (D/L-TMGE) was employed as the host gelator to form hexagonal nanotubes through antisolvent gelation based on the stable hydrogen bond interactions among amide groups. A series of classical AIEgens with different emission properties were selected as guest dye molecules. Taking the AIEgen α-DCS as a representative example, the solution of α-DCS alone showed almost no fluorescence, but bright yellow-green fluorescence was observed from the co-gel of the guest AIEgen and the host matrix (L-TMGE) (Figure 12B). The significant increase in fluorescence lifetime after being encapsulated into the L-TMGE gel (Figure 12C) further confirmed that AIEgen has entered the confined space of nanotubes and the non-radiative decay pathway of the AIEgen was suppressed in the aggregates. No CD signal was detected for the individual AIEgen but the co-gel of α-DCS and L-TMGE showed an obvious exciton-type CD signal at the absorption band of α-DCS, indicating that chiral amplification and transfer occurred during the formation of co-gels. The gelation- or assembly-induced fluorescence enhancement and remarkable chirality transfer from the host chiral space to achiral guest AIEgens eventually lead to strong CPL signals of the co-gels with different handedness (Figure 12D). Due to the bright aggregate-state fluorescence of AIEgens, the self-assembly morphology of the AIEgen-doped nanotubes can be directly visualized by fluorescence optical microscopy (Figure 12E,F). All the tested AIEgen/TMGE co-gels showed strong fluorescence and good CPL generation capability (Figure 12G), and the maximum |glum| of these AIEgen-doped co-gels was 1.7 × 10−3. This work provides a simple and general strategy for constructing CPL-active supramolecular polymer materials with different colors.
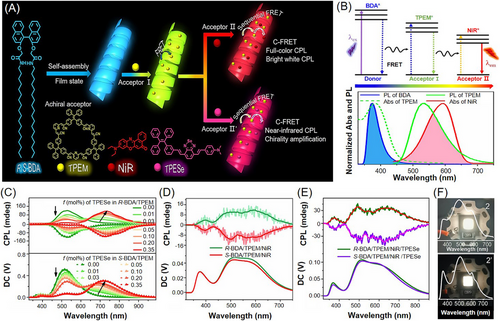
Very recently, Yuan et al.[110] further utilized the efficient and successive circularly polarized fluorescence resonance energy transfer (C-FRET) process for the construction of chiral light-harvesting supramolecular polymer systems with customized color, high QY and high glum. As shown in Figure 13A, blue-emitting chiral binaphthalene di-octadecamidemethoxy (BDA) that can self-assemble into nanohelices was used as the initiator of chirality and light absorbance. An AIE-active green-emitting macrocycle (TPEM) that can carry guest molecules was synthesized as a key conveyor for chirality transmission and energy transfer. Meanwhile, Nile red (NiR) and an AIE-active near-infrared dye (TPESe) were selected as the other terminal acceptors to co-assemble with BDA and TPEM. Investigation on the host–guest interactions between TPEM and NiR demonstrated that NiR is encapsulated into the cavity of TPEM. Benefiting from the close contact and large optical overlap between donors and acceptors at each level (Figure 13B), the aggregates and solid films of BDA/TPEM/NiR triad were confirmed to be efficient chiral light-harvesting systems with a two-step sequential FRET process. Further addition of TPESe into this triad system could result in a much wider range of spectrum outputs via a cascaded FRET process. The energy transfer efficiency of the optimal film systems of BDA/TPEM/NiR and BDA/TPEM/NiR/TPESe can reach up to 98.6%. Regarding the CPL responses, the R- and S-BDA films showed obvious strong CPL signals at 375 nm. After adding the achiral TPEM, a new CPL peak was observed at 514 nm with a significantly higher glum value than that of the chiral BDA assemblies. When the achiral NiR and TPESe was individually added to the BDA/TPEM dyad system, the CPL emission was further extended from 514 to 627 and 733 nm, respectively (Figure 13C). By adjusting the content of each component in the triad and tetrad relaying systems, bright and nearly standard white CPL light (CIE coordinate (0.33, 0.34)) with a high glum value of up to 0.035 and an excellent QY of over 37% was achieved (Figure 13D,E). The CPL white-light spectrum of the BDA/TPEM/NiR/TPESe covered a wide wavelength range from violet to near-infrared region, which is very rare for artificial white-light systems. LED devices that can emit nearly natural white light can be readily fabricated by coating the triad or tetrad system on the UVB LED chip (Figure 13F). Therefore, the C-FRET approach reported in this work could facilitate the development of meaningful CPL-related practical applications in chiroptical physics and chemistry.
The aforementioned examples demonstrated that physically doping AIEgens into chiral (supramolecular) polymer matrix is a simple and relatively versatile strategy for the construction of AIE polymer systems with CPL. With further advances in the fabrication and blending technologies, more diversified AIEgen/polymer composites with efficient CPL would be developed.
5 SUMMARY AND OUTLOOK
This review mainly summarized the recent progress on the design, synthesis, and property investigation of three types of CPL-active AIE polymers, including CPL-active covalent AIE polymers, CPL-active supramolecular AIE polymers, and AIE luminogen (AIEgen)/polymer composites with CPL. The introduction of AIE effect into chiral polymeric luminogens results in a series of advantages, such as high QY in aggregate states, large solid-state glum, diversified self-assembly behaviors, and readily switchable CPL properties. Generally speaking, the most commonly used strategy to construct CPL-active AIE polymers is to simultaneously introduce AIEgen and chiral scaffolds into polymer structures via covalent bonds. Recently, CPL-active covalent polymers with clusterization-triggered emission have also been developed, which can be regarded as a special type of AIE polymers. Compared with covalent polymers, CPL-active supramolecular AIE polymers formed by dynamic and reversible non-covalent interactions, such as host–guest interactions, metal coordination, and hydrogen bonding, lay an important foundation for the design of stimuli-responsive smart CPL materials with high glum. Unlike covalent or supramolecular polymers that often require complex synthetic procedures, physically mixing AIEgens with chiral (supra)polymers is a simple and relatively versatile strategy to construct CPL-active AIE polymers, which can readily achieve full-color or white light-emitting CPL.
Although significant advances have been achieved in the development of CPL-active AIE polymers, this field is still in its infancy. The future development directions of CPL-active AIE polymers might include but not limited to the following aspects. First, continuous efforts are needed for the development of simple, low-cost, and highly efficient preparation methods and strategies toward covalent AIE polymers with CPL. Considering the economic and sustainable advantages yet rare examples of the CTE-based CPL materials, the development of CPL-active polymers based on CTE mechanism could be an attractive research topic in this field. Second, the balance between QY and glum values need to be further improved. In general, QY and glum values affect each other, showing a trend of one decreasing with another increasing. Despite of the excellent aggregate-state QY values of CPL-active polymers in the current studies, their maximum glum values still need to be enhanced to make them ideal materials for practical applications. Third, in-depth mechanism study on the enhancement of CPL activity of chiral AIE polymers is also of great significance to guide the future design of CPL-active AIE polymers. For example, effective and relatively versatile strategies that can achieve efficient chirality transfer and chirality amplification in AIE polymers should be further explored. More efforts are needed to gain insight into the theoretical understanding of the amplified chirality of chiral AIE polymers in aggregate states. Last but not least, examples on the application study of CPL-active AIE polymers still remain very rare and there is a large room for researchers to further explore the advanced applications of these functional CPL materials. One of the reasons that hampers the advancement of CPL materials might be the high cost and poor availability of CPL instruments, which puts forward strict requirements on the research conditions of researchers and limits the development of CPL materials. Therefore, to facilitate the practical applications of CPL-active AIE polymers, it is highly desirable to promote the development of CPL equipments in addition to the creation of novel materials. We cannot address all the opportunities in this wonderland due to the space limitation, but we do hope the present review could help stimulate further interest and efforts from scientists and researchers to achieve more research outcomes on the design and applications of CPL materials and AIE polymers.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (22271197) and the Science and Technology Plan of Shenzhen (JCYJ20220531102601003, JCYJ20190808142403590).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Biographies

Hewei Yan received his PhD degree from Central China Normal University in 2021 under the supervision of Prof. Haibing Li. From 2021 to now, he is conducting his postdoctoral work at Shenzhen University in collaboration with Prof. Ting Han. His current research mainly focuses on AIE supramolecular polymer materials and their applications.

Youling He received her PhD degree from South China University of Technology in 2021 under the supervision of Prof. Ben Zhong Tang. She is currently a post-doctoral fellow at Shenzhen University in collaboration with Prof. Ting Han. Her current research mainly focuses on the design and applications of aggregation-induced circularly polarized luminescence polymers.

Ting Han received her BS degree from Beijing Institute of Technology in 2014, and obtained PhD degree from the Hong Kong University of Science and Technology (HKUST) in 2018 under the supervision of Prof. Ben Zhong Tang. After a postdoctoral study at Prof. Ben Zhong Tang's group in HKUST, she joined the Center for AIE Research at Shenzhen University in 2019 as an assistant professor. She is now a specially appointed researcher at Shenzhen University. Her research interests focus on the design, synthesis, and applications of functional luminescent polymers.

Ben Zhong Tang received his BS and PhD degrees from South China University of Technology and Kyoto University in 1982 and 1988, respectively. He conducted his postdoctoral work at the University of Toronto from 1989 to 1994. He joined HKUST in 1994 and was promoted to chair professor in 2008. He was elected to Chinese Academy of Sciences in 2009. In 2021, he joined CUHK-Shenzhen as the Dean of School of Science and Engineering. His research interests include the exploration of new advanced materials, new luminescent processes, and new polymerization reactions.




