Wide-range color-tunable afterglow emission by the modulation of triplet exciton transition processes based on buckybowl structure
Yongfeng Zhang and Chenchen Xiong contributed equally to this work
Abstract
Buckybowl structures as non-uniform electrostatic potential distributions of polycyclic aromatic materials show a unique photoelectric performance. In this work, OTC was utilized for dynamic modulation of triplet exciton transition processes. Five host molecules with different functional units were selected, thus providing different intermolecular interactions in the host/guest systems. Therefore, the delayed emissions were regulated from 536 to 624 nm via the tuning of the triplet exciton transition processes of OTC in different hosts. Experimental data and theoretical calculations revealed that the varied triplet transition behaviors resulted from the competition between the intersystem crossing (ISC) process of OTC-monomer and the reverse intersystem crossing (RISC) process of OTC-aggregates. This work proves the superior structure of buckybowl-based luminophore for controlling triplet exciton transition processes and supplies a new perspective for persistent afterglow luminophore design.
1 INTRODUCTION
Pure organic room temperature phosphorescence (RTP) materials have become one of the most promising luminescence materials, owing to their high potential applications in security,[1] bioimaging,[2] lighting and display,[3] organic solid-state laser,[4] and nonlinear optics.[5] Various effective strategies have been developed to achieve high RTP performance, such as crystallization, host/guest interaction, and matrix rigidification.[6-25] Accordingly, numerous applications have been achieved by employing the regulation of triplet exciton transition processes, such as the energy/electron transfer of triplet exciton for photodynamic therapy[26, 27] and photoredox reactions.[28, 29] In addition to transition decay and energy/electron transfer, triplet excitons can also return to the lowest singlet state (S1) from the lowest triplet state (T1) through the reverse intersystem crossing (RISC) processes, followed by delayed fluorescence emission. A material can show RTP or thermal activated delayed fluorescence (TADF) through precise regulation of intersystem crossing (ISC) and RISC processes, exhibiting potential applications in dynamic displays, efficient OLEDs, and high-resolution bio-imaging.[2, 30-32] Some studies have been developed on efficient ISC or RISC processes, which mainly focus on the diverse stacking models in crystal, planar/twisted intramolecular charge transfer, and dopant-matrix strategy, which provides the conditions for realizing high-performance RTP or TADF.[33-40] However, tunable multi-emissive RTP and TADF formation in a single organic material remain a challenge.
The buckybowl-based structure has been used as the core component in constructing polycyclic aromatic compounds with diverse structures and functions.[41-44] Compared with the planar conjugated molecules with the symmetric electrostatic potential (ESP) distribution on two surfaces (Figure 1A), buckybowl molecules’ concave and convex surfaces have different electron cloud distributions on two surfaces (Figure 1B). Moreover, buckybowl-based molecules mainly adopt the columnar stacking of the concave-convex-concave model at the aggregated state because of the geometric configuration and intermolecular dipoles.[44, 45] Hence, diverse emissions can be obtained when buckybowl molecules were doped into the host matrices, due to the formation of various intermolecular interactions between the external electronic donor/acceptor hosts and bowl-like guests. However, limited investigations of luminescence properties, particularly the triplet excited state emission, have been reported for the modulation of triplet exciton transitions.
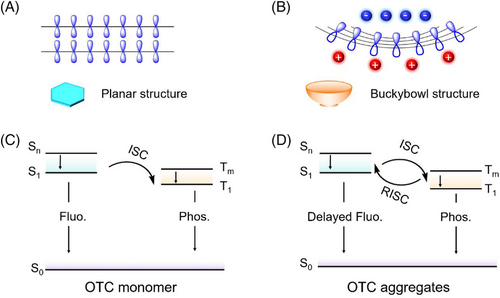
Herein, a representative buckybowl-based luminophore (oxazole-fused (S, S-dioxide) trithiasumanenes-chlorobenzene compound, OTC, Figure 2A and Scheme S1). Interestingly, distinguishable triplet exciton transition behaviors were observed between the monomer and aggregate. In addition, the transition processes of triplet exciton in the host/guest systems could be precisely modulated by the external hosts due to the formation of different intermolecular interactions, such as donor-acceptor and hydrogen-/halogen bonding interactions between the host and guest (Figure 1C,D). The most extended persistent lifetime and delayed quantum yield reached up to 483.5 ms and 16.4%, respectively, and the delayed emission peaks of OTC were regulated from 536 to 624 nm, with the tunable room temperature afterglow from green to red. Experimental data demonstrated that OTC was molecularly dispersed in the electron-donating host while forming the aggregates in the electron-withdrawing host. The buckybowl-based structure holds tremendous potential for the triplet exciton transition processes modulation and the persistent afterglow (RTP or TADF) luminophore design.
2 RESULTS AND DISCUSSIONS
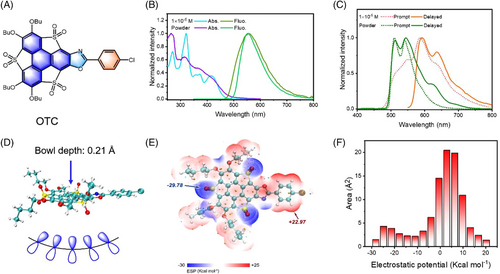
As shown in Scheme S1, compound 1 was prepared according to our previous procedure,[46] followed by a three-component Debus-Radziszewski reaction of compound 1, p-chlorobenzaldehyde, and ammonium acetate to afford compound 2. The oxidation of thiophene on compound 2 with H2O2 afforded OTC (synthetic details and characterization see supporting information, Figure S24-S30).[46] The target compound with electron-withdrawing sulphone units increased the intramolecular donor-acceptor interaction as well as the (π, π*) proportion of T1.[47] Commonly, the introduction of heteroatoms facilitated the ISC process due to the involvement of 1(n, π*)→3(π, π*) transitions.[48] Four absorption peaks were observed at 1 × 10−6 mol/L in CH2Cl2. In Figure 2B,C, the onset absorption of OTC in a solid state displayed an apparent redshift compared to that in dilute solution, indicating the existence of strong intermolecular interactions in OTC solid. Both OTC-dilute solution and OTC-crystalline powder showed fluorescence emission at approx. 560 nm. However, OTC showed different delayed emissions in solution and in solid-state at 80 K. Two prominent delayed emission peaks at 512 and 550 nm were observed in the solid state, while two emission peaks at 592 and 640 nm were found in dilute solution. Compared with the fluorescence emission at room temperature and the prompt spectra at 80 K, the delayed emissions at 512 and 560 nm were attributed to the delayed fluorescence, while the long-wavelength emission peaks at 592 and 640 nm in the dilute solution were related to the phosphorescence emission from the triplet states.
Theoretical calculations were further carried out to gain deep insight into OTC luminophore. The calculated orbital distributions showed that the energy levels of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of OTC were −6.39 and −2.95 eV, respectively (Figure S1 and Table S1). As shown in Figure 2D and Figure S2, OTC exhibited a bowl configuration with 0.21 Å depth in the central conjugated aromatic units.[43] The asymmetric bowl structure led to the non-uniform electron cloud distributions of OTC surfaces, thus providing an opportunity for donor-acceptor interaction in the vertical direction.[41, 45, 46] In addition, the calculated ESP values ranged from −29.78 (O atoms in the sulfonyl group) to +22.97 kcal/mol (benzene ring) (Figure 2E, Figure S3, and Tables S2 and S3). Therefore, the OTC molecule presented a vertical ESP difference from the different bowl surfaces as well as an intramolecular ESP difference from the donor and acceptor units. Furthermore, Figure 2F showed that the total van der Waals surface area was 786.97 Å2, with non-uniform distributions, and the ESP value over OTC was moderately positive (∼40%, around +5 kcal/mol). Therefore, multidimensional interactions existed in OTC molecules, which allowed tuning the optical properties by adjusting the intermolecular interactions.
A series of benzyl cyanide-based host molecules (Figure 3A) were selected to stabilize the triplet excitons at room temperature. All the hosts showed non/weak phosphorescence at room temperature (Figures S4 and S5). The five host molecules displayed distinct positive and negative ESP distributions. From CPAN, DPAN, HPAN, BPAN to IPAN, the positive ESP distributions gradually reduced from 67.67%, 60.75%, 53.25%, 46.88%, to 46.90%. Accordingly, the negative ESP distributions gradually increased from 32.33%, 39.25%, 46.75%, and 53.12%, to 53.10%, respectively (Figure 3B and Figures S6 and S7). Therefore, upon doping OTC into the host molecules, the intermolecular interaction between the host and guest can be varied.
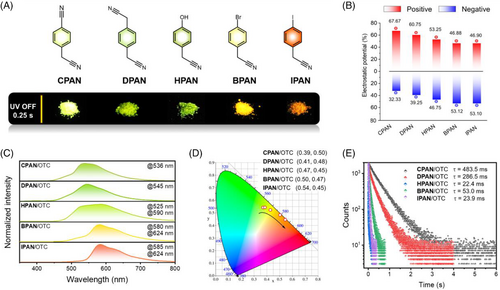
Host/guest doping materials were prepared by the melt-casting method with a mass ratio of 2000:1. All doping materials showed bright and persistent afterglow emissions. The color of the persistent afterglow changed from green to red from CPAN/OTC to IPAN/OTC (Figure 3A). As shown in Figure 3C, only one dominant peak at approx. 536 nm in CPAN/OTC was observed, which slightly shifted to 545 nm in DPAN/OTC. However, after the -CH2CN group of the DPAN host was replaced with the -OH group, two prominent peaks were observed for HPAN/OTC at 525 and 590 nm, respectively. In the case of BPAN/OTC, the dominant peak was redshifted to 580 nm, and a new shoulder peak appeared at 624 nm. Therefore, a yellow-orange persistent afterglow can be observed. In contrast, after replacing the Br atom with the I atom, the peak at about 525 nm became less intense for the IPAN host, and the intensities of peaks at 585 and 624 nm were increased. The Commission International de l'Eclairage coordinates of five delayed emissions changed from (0.36, 0.54), (0.37, 0.52), (0.43, 0.52), (0.51, 0.47), to (0.55, 0.45), respectively (Figure 3D). These results proved that the delayed emissions were tuned after regulating the positive and negative ESP distribution ratio in the external host matrices (Figure S8).
The lifetimes of OTC in different host matrices were further investigated (Figure 3E). The lifetime of all doped materials showed a significant increase compared to OTC molecules, since OTC molecules with negligible phosphorescence. However, the doping materials showed both short-and long-lived species in short wavelength regions. For example, CPAN/OTC showed a lifetime of 2.35 ns and 483.5 ms at 536 nm, respectively, demonstrating the existence of prompt fluorescence and delayed fluorescence of CPAN/OTC (Figure S9 and Table S4). The short-lived decay was not observed in the long emission wavelength region. Notably, the delayed emission lifetime of the doped materials gradually decreased with the increasing negative ESP of host matrices. Two possible reasons existed for the observed decrease in the lifetime: the intermolecular donor-acceptor interactions and the external heavy atoms (i.e., Br, I atoms) promoted the ISC processes; the other is that the hosts with low crystallinity may decrease the stability of triplet excitons, resulting in enhanced non-radiative decay (Figure S10). Accordingly, from CPAN/OTC to IPAN/OTC, quantum yields (Φ) decreased from 16.4% to 3.68% with the increasing negative ESP of the host molecules, which proves that the persistent afterglow emission can be controlled by the varied intermolecular interactions.
The dynamically adjustable afterglow originated from the competition between TADF in aggregates and monomers in RTP. The time-dependent delayed spectra in dilute solution (at monomer state) and powder state were recorded to confirm this speculation. As shown in Figure S11A, our delayed emission bands of OTC monomer appeared at 512, 550, 590, and 640 nm, respectively, with a delayed time of 1 μs. However, the emission intensity at 512 and 550 nm dramatically decreased after the delayed time gradually increased to 100 μs, while the bands at 590 and 640 nm were dominant. On the contrary, as shown in Figure S11B, five delayed emission bands of OTC powder were observed at 512, 560, 590, 650, and 716 nm, respectively, with a delayed time of 1 μs. The dominant delayed emission bands were at 512 and 560 nm. Concentration-dependent delayed spectra at 80 K were also carried out (Figure S12). The long emission wavelengths at 590 and 640 nm were observed in a dilute solution, where the emission at short wavelengths (512 and 560 nm) gradually appeared with increasing concentration. Accordingly, the lifetime decay curves at 512 and 560 nm gradually increased with increasing concentration. A similar phenomenon was also observed in the doping system. As the IPAN:OTC mass ratio increased from 2000:1, 2000:100, 2000:300, to 2000:500, OTC aggregates formed in the host, and the emission at short wavelengths gradually appeared (Figure S13). All these results suggested OTC was inclined to exhibit phosphorescence emission in the molecular dispersion state, while delayed fluorescence in the aggregated state.
Temperature-dependent delayed spectra were also conducted. The delayed emission intensity of OTC in dilute solution with emission peaks at 590 and 640 nm gradually decreased with the increase of temperature from 80 to 300 K, indicating the phosphorescence emission of the long emissive wavelength (Figure 4A and Figure S14). Conversely, the delayed emission intensity of OTC powder with emission bands at 512 and 560 nm increased with increasing temperature from 240 to 270 K (Figure 4B and Figure S15), which demonstrated the TADF properties of short wavelength emission. Similar phenomena were also observed in the temperature-dependent delayed spectra of five host/guest doped materials (Figures S16–S18). Furthermore, the delayed fluorescence from triplet-triplet annihilation had also been excluded, since the maximal intensity of two light source lamps was far less than the inflection point value (Figures S19 and S20).[49, 50] The obtained results showed that OTC exhibited tunable ISC and RISC processes with the change of aggregated states, thus providing an opportunity to tune afterglow emissions.
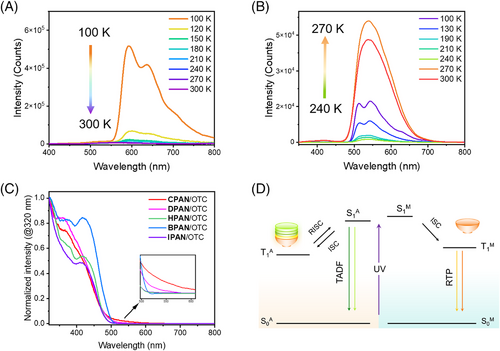
The solid-absorption spectra (Figure 4C) were also recorded to confirm the tunable delayed emission in different hosts at room temperature. The onset peaks of OTC in both CPAN and DPAN systems showed a slight redshift compared to other hosts, indicating the existence of π-π stacking of OTC molecules (OTC-aggregates) in both CPAN and DPAN. This can be attributed to the limited intermolecular interactions between the host and guest, which made OTC difficult to monomeric-level dispersed in the host. On the contrary, the host molecules with both the electron-donating and electron-withdrawing groups can effectively disperse the OTC molecules. In addition, both the hydrogen bonding (between -OH in HPAN and sulphone in OTC) and halogen bonding (between -Br in BPAN/-I in IPAN and sulphone/chlorophenyl substituent in OTC) promoted the intermolecular interaction between the host and guest.[51-53] The multidimensional intermolecular interactions improved the OTC dispersion in the BPAN and IPAN hosts, thus showing RTP with long emissive wavelength.[54, 55]
Theoretical calculations were further conducted to investigate the triplet exciton transitions modulation between OTC-monomer and OTC-aggregates. The initial structure of OTC-dimer and OTC-trimer were optimized at the PM6-DH+ level by using MOPAC2016 (Figures S21 and S22, and Tables S5 and S6). The obtained low-lying energy structures were further optimized and the spin-orbit coupling (SOC) and energy levels were calculated by density functional theory (DFT), TD-DFT, and ORCA program at PBE0/def2-SVP level, respectively (Table S7 and Figure S23). OTC molecule showed the excited S1 of 2.81 eV, and the lowest triplet (T1) of 2.16 eV. The energy gaps between the lowest singlet and triplet (ΔEST) gradually decreased from OTC-monomer (0.65 eV), OTC-dimer (0.61 eV), to OTC-trimer (0.59 eV), indicating that the formation of the aggregates narrowed the ΔEST. Hence, a plausible mechanism was proposed for the modulation of triplet exciton transition processes (Figure 4D). The calculated SOC of OTC results demonstrated the existence of multiple ISC channels from S1 to Tn, which prompted the population of triplet excitons. However, the triplet excitons of OTC-monomer luminophore are hardly reversed from T1M to S1M due to the large ΔEST. Therefore, RTP emission can be observed from the radiation transition from T1 to S0. When OTC aggregate formed, ΔEST was narrowed. RISC was permitted from the lowest triplet state of OTC at aggregated state T1A to S1A; thereby, TADF was dominant.
3 CONCLUSION
In conclusion, owing to the non-uniform ESP distribution of the buckybowl-based luminophore, the external donor/acceptor hosts could regulate the monomer/aggregates state of OTC. Given the multidimensional interactions, we proposed a precise strategy for the modulation of triplet exciton transition processes based on the discrepant intermolecular interactions between OTC and various hosts. The aggregates of OTC were dominant in CPAN and DPAN, while dispersive monomers in BPAN and IPAN were dominant. The modulation of RTP and TADF was achieved by the intrinsic competition between ISC and RISC processes. Therefore, wide-range afterglow emissions were easily tuned by changing the host molecules. This work expands the knowledge of the superiority of buckybowl-based luminophores for modulating triplet exciton transition and supplying a new design principle for dynamic afterglow materials.
ACKNOWLEDGMENTS
The authors acknowledge the National Natural Scientific Foundation of China (Grant Nos. 21975021, 21975020, 21875019, 21871119, 22105019, and 22175023). This work was also supported by Beijing National Laboratory for Molecular Sciences (BNLMS192007), and BIT Research and Innovation Promoting Project (Grant No. 2022YCXZ035).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ETHICS STATEMENT
There are no ethical issues in this work.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




