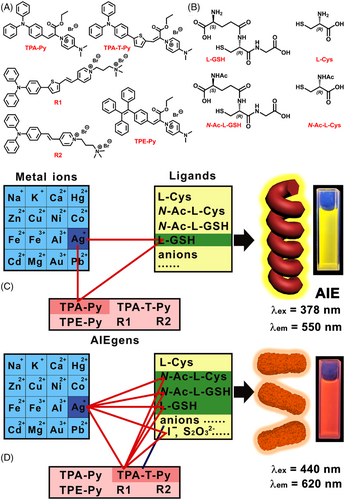
Unique three-component co-assembly among AIEgen, L-GSH, and Ag+ for the formation of helical nanowires
Abstract
Nature has selected, produced, and evolved numerous molecular architectural motifs over billions of years for particular bio-functions. During this process, molecular self-assembly plays a critically important role. Inspired by these delicate assemblies, scientists devote themselves to developing various sophisticated complexes assembled by diverse and numerous structural motifs. By far, most of the examples focus on single-molecule self-assembly or co-assembly between two molecules, seldom works report the co-assembly among three building blocks due to the substantially increased complexity and decreased controllability. Here we report a novel three-component co-assembly among L-glutathione (L-GSH), AgNO3, and an aggregation-induced emission luminogen (AIEgen), namely triphenylamine–pyridinium (abbreviated to TPA-Py). TPA-Py, L-GSH, and Ag+ co-assemble into numerous long and ordered nanowires with left-handed helices, which show remarkable AIE properties that can be observed by naked eyes. This effect is only detected in this system, the replacement of TPA-Py with other AIEgens, L-GSH with other cysteine derivatives or anions, Ag+ with other metal ions, could not lead to the unique AIE effect, which endows this system with high specificity and controllability. This work provides new insight into the design of biomolecule recognition systems and discloses that ternary co-assembly could become a facile route to build sophisticated nanobiomaterials.
1 INTRODUCTION
Molecular self-assembly is a ubiquitous process in nature where it plays numerous important roles and underlies the formation of a wide variety of complex biological structures from protein folding, transmembrane channels, DNA double helix, cell membranes, to tissue, and organ. The ingenuity and complexity of the assembly process are far beyond our imagination.[1] Self-assembly is an effective bottom-up way to construct new substances beyond the molecular levels.[2-4] Precise regulation of molecular assembly and the realization of highly controllable, functionally integrated assembly systems are the biggest challenges in molecular self-assembly research.[5-7] “How far can we push chemical self-assembly?” has been listed by Science as one of the top 25 scientific questions to be solved in the 21st century.[8]
Natural self-assembly systems are complicated in structure while usually need the participation of multiple molecules, which work cooperatively to perform elegant functions.[9] Their diversity in terms of structure and function increases exponentially with the growing number of different building blocks integrated into a single system by non-covalent interactions.[10] This paves a highly promising route toward artificial nanoarchitectonics with great potential for application.[11] Numerous assembled behaviors between the different building blocks have been studied, and most of them focus on the study of co-assembly between two components. Specific molecular recognition and interactions drive the formation of diverse nanostructures, which facilitate many exciting applications in bio-sensing,[12-16] drug delivery and release,[17-19] tissue engineering,[20, 21] organic semiconductors,[22] energy transfer,[23, 24] light-harvesting,[25] and materials nanoarchitectonics,[2, 6] etc.
By comparison, the reports about three-component assemblies are relatively rare because it is difficult to screen out the optimal composition of the building blocks. Meanwhile, the assembled complexity increases exponentially and the precision decreases sharply.[26] Only several cavernous building blocks, such as cucurbituril,[27, 28] calix [4] resorcinarene,[29] aryl macrocycle,[30] and cyclodextrins[31, 32] have been reported for constructing three-component assemblies, based on the excellent preorganization of their cavity structures. However, they often require elaborate design and multi-step synthesis with tedious purification, and most of the assembled behaviors were conducted with the assistance of organic solvents, which is far from simulating the complex assembly systems of the living body.[33-35]
Water-soluble aggregation-induced emission luminogens (AIEgens) with functionalized groups are a class of ideal building blocks to study multi-component co-assembly. In contrast to conventional fluorophores often encountering aggregation caused quenching effect, the aggregation process, which usually refers to the assembly of different components, could be direct visualization by the introduction of AIEgens, which might greatly facilitate the screening of building blocks.[36, 37] However, three-component co-assembly based on an AIEgen is seldom reported before.
Here, we report a unique ternary co-assembled system among L-glutathione (L-GSH), AgNO3, and an AIEgen, namely triphenylamine–pyridinium (abbreviated to TPA-Py). The fluorescent screening experiment indicated that a mixture of TPA-Py, L-GSH, and Ag+ emitted intense fluorescence, which was 33-fold of TPA-Py alone. Such dramatic AIE effect was not observed when TPA-Py was mixed with Ag+ and other anions, or mixed with L-GSH and other metal ions (Scheme 1C). Detailed mechanism analysis disclosed that Ag+–L-GSH coordination polymer was formed through Ag+–Ag+ bonds, and strong binding of the polymer with TPA-Py further promoted the co-assembly, which generated numerous long and ordered nanowires with left-handed helices, emitting bright fluorescence. Interestingly, insertion of a thiophene interval between TPA and Py units results in a new AIEgen TPA-T-Py, multiple AIE responsiveness could be found among TPA-T-Py, Ag+, and multiple anions (Scheme 1D), which correspond to typical AIE enhancements induced by electrostatic adsorption, highlighting the uniqueness and specificity of TPA-Py. A detailed analysis of the underlying mechanism revealed that TPA-Py with a rigid and multi-phenyl structure plays an indispensable role in the construction of the ternary assembled structure. This work provides a new perspective to construct a biomolecule recognition system and will promote the development of supramolecular assembly in a more precise direction.
2 RESULTS AND DISCUSSION
2.1 Screening of ternary co-assembled system
This finding originated from a series of screening experiments including three subsets (Scheme 1): the first one included sixteen kinds of metal ions, the second one included L-GSH, L-cysteine (L-Cys), and their N-acetyl protected derivatives (Scheme 1B), as well as 19 kinds of anions, the third subset contains five kinds of AIEgens, such as TPA-Py, TPA-T-Py, TPE-Py, and two typical AIEgens (R1 and R2) reported in the literature (Scheme 1A).[38] It is worth noting that L-GSH receives more attention in this study, which is a tripeptide composed of glycine (Gly), Cys, and L-glutamic acid (L-Glu) (Scheme 1B) and widely present in most mammalian tissues.[39] L-GSH can act as a free radical scavenger, an antioxidant, and a detoxifying agent, function as a key factor for the enzyme glutathione peroxidase, as well as participate in the uptake of amino acids and the synthesis of leukotrienes.[40] TPA-Py, TPA-T-Py, and TPE-Py were designed and synthesized through a one-step Knoevenagel condensation reaction (Scheme S1 in Supporting Information [SI]) and characterized (Figures S1–S7 in SI), R1 and R2 were synthesized according to the method reported in the literature.[38]
Fluorescence test using H2O (low viscosity)–glycerin (high viscosity) (Figure 1A) or acetone (good solvent)–n-hexane (poor solvent) (Figure S8 in SI) mixture indicated that TPA-Py was an AIE active molecule.[41] Glycerin fraction–dependent fluorescent intensity increase curves further confirmed that TPA-T-Py, R1, and R2 had similar AIE properties to TPA-Py (Figure 1B). Meanwhile, compared to these AIEgens derived from triphenylamine reported in this work, the magnitude of fluorescence enhancement of TPE-Py (Figure S9) is much less. Then the responses of TPA-Py to various anions or metal ions were investigated by performing fluorescence titration experiments,[36] respectively. As shown by red columns in Figure 1C,D, TPA-Py had no response to these anions or metal ions, including the individual L-GSH or Ag+ (NO3− worked as a counter anion and is abbreviated in the following description). After the addition of L-GSH to the mixture of TPA-Py with corresponding cations (blue columns in Figure 1C), only the fluorescent intensity of the mixture with Ag+ increased substantially, from 28 to 625 a.u. On the contrary, when Ag+ was added to the mixture of TPA-Py with corresponding anions (blue columns in Figure 1D), only the mixture with L-GSH occurred an evident increase in the fluorescent intensity, which illustrated a unique relationship among TPA-Py, Ag+, and L-GSH.
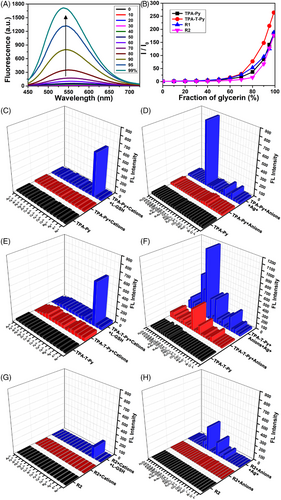
Further, the responses of TPA-T-Py to these metal ions or anions were studied. As shown in Figure 1E, TPA-T-Py has no response to various metal ions, only when L-GSH was added to the mixture of TPA-T-Py and Ag+, the fluorescent intensity increases remarkably, which was similar to that observed on TPA-Py. Differently, TPA-T-Py also displayed moderate fluorescent responses to I–, S2–, SO32–, ATP, and P2O74– (Figure 1F red columns). Upon the addition of Ag+ to the mixtures of TPA-T-Py and various anions, evidential increases in the fluorescent intensity were detected on I–, S2O32–, SO32–, N-Ac-L-Cys, L-GSH, N-Ac-L-GSH (Figure S10 in SI), ATP, and P2O74–, which indicated multiple relationships among TPA-T-Py, Ag+, and these anions. Similar multiple responses were detected when other AIEgens, such as R1 (Figure S11 in SI) and R2 (Figure 1G,H) were studied, but their fluorescent intensity changes were substantially smaller. When TPE-Py was studied, owing to its strong AIE capacity, the initial fluorescent intensity of TPE-Py in either H2O or C2H5OH/H2O (v:v = 1:4) was larger than 100 a.u., the addition of both cations and L-GSH, or of both anions and Ag+ did not induce evidential changes in the fluorescent intensities, as shown in Figure S12 in SI.
2.2 AND logical gate illustrating the relationship among Ag+, L-GSH, and TPA-Py
The above screening experiments provided a clue that TPA-Py had specific interaction with Ag+ and L-GSH, which was further validated by detailed fluorescence titration (Figure 2A). The fluorescent intensity (at 552 nm) of TPA-Py (10 μM) mixed with L-GSH (80 μM) increased gradually from 28 to 940 along with the addition of 8 equiv. of Ag+. Instead, fluorescence titration performed using the mixture of TPA-Py with Ag+ titrated by different molar ratios of L-GSH indicated that the fluorescent intensity markedly increased only after the addition of 3 equiv. of L-GSH, suggesting that the addition sequence of L-GSH and Ag+ could affect the formation of the complex.
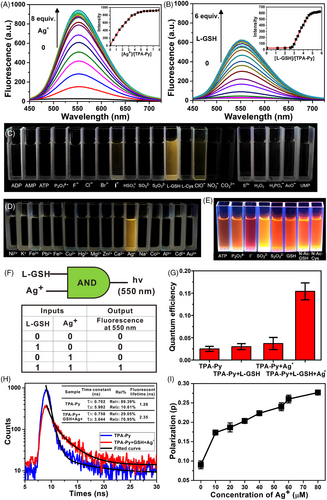
The remarkable enhancement of the fluorescent intensity and the unique responses of TPA-Py, Ag+, and L-GSH could be observed by naked eyes directly (Figure 2C,D). Only cuvettes containing TPA-Py, Ag+, and L-GSH emitted strong yellow fluorescence, other mixtures were dark. By comparison, cuvettes containing TPA-T-Py, Ag+, and eight kinds of anions (i.e., ATP, P2O74–, I–, SO32–, S2O32–, L-GSH, N-Ac-L-GSH, N-Ac-L-Cys) emitted bright orange fluorescence (Figure 2E), which highlighted the uniqueness of the TPA-Py, Ag+, and L-GSH system. An AND logic gate was constructed based on the Boolean logic idea and the aforementioned fluorescence behavior of the mixture of TPA-Py with L-GSH and Ag+. As shown in Figure 2F, Ag+ and L-GSH were used as inputs and the emission of TPA-Py at 550 nm as an output. The presence or absence of L-GSH or Ag+ was assigned a value of “1” or “0”, respectively. The emission of TPA-Py “ON” and “OFF” at 550 nm was assigned an output value of “1” and “0”, respectively. Among four possible input conditions, only when two inputs were present (1/1), the emission of TPA-Py at 550 nm was switched on and the output was “1”. While in other cases, no evidence of fluorescence fluctuation was observed, and the output value was “0”. The AND logic gate indicated the indispensable roles of L-GSH and Ag+ in the AIE effect of TPA-Py.
The findings of a fluorescent quantum yield (QY) test (rhodamine 6G as a standard reference) demonstrated that the QY of the TPA-Py solution (10 μM) was 0.025, which slightly increased to 0.03 or 0.037 when 8 equiv. of L-GSH or Ag+ was added, respectively (Figure 2G). The QY increased to 0.154 when 8 equiv. of both L-GSH and Ag+ were added. Time-resolved fluorescence spectra (Figure 2H) revealed that the fluorescence lifetime of TPA-Py and the L-GSH–Ag+–TPA-Py complex were 1.26 and 2.35 ns, respectively. The higher quantum efficiency and longer lifetime of the complex provided solid evidence for the AIE effect. In addition, the fluorescence polarization value of the L-GSH–Ag+–TPA-Py complex increased from 0.17 to 0.27 when 8 equiv. of Ag+ was added finally (Figure 2I). The increase in isotropy indicated that the free rotation of TPA-Py might be restricted by the complex, which was consistent with the AIE mechanism.
2.3 Morphology of co-assembly
Dynamic light scattering (DLS) experiments were performed to study the size distributions of the co-assembly in water (Figure S13 in SI). The average diameter of binary system of L-GSH-Ag+ was determined to be 952 nm. While for the ternary system of TPA-Py, Ag+, and L-GSH, the average hydrodynamic diameter increased to 1659 nm, exhibiting Tyndall effect visually observable (Figure S14 in SI). Morphologic observations through scanning electronic microscopy (SEM), laser scanning confocal microscopy (LSCM), and transmission electron microscopy (TEM) provided direct evidence for the co-assembly of the ternary complex. The sample was prepared by dropping one droplet of the complex solution (TPA-Py concentration of 0.1 or 1 mM) onto a silica wafer or a microslide surface. As shown in Figure 3A, numerous nanowires with an average length of 690 nm and a width of 50 nm could be observed through SEM in the field of view. Part of these nanowires exhibited an exclusive left-handed twist with an average helical pitch of 170 nm (Figure 3B). It is worth noting that the size of the aggregated nanoparticles measured in the solution by DLS did not match that on the substrate measured by SEM, which may be due to the volume shrinkage caused by solvent volatilization, as well as the inaccuracy of the DLS calculation model, especially for the structure of nanowires.
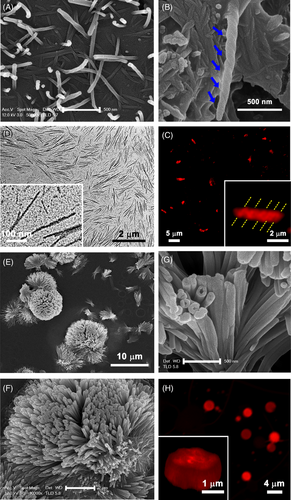
Because of the AIE effect of the complex, these long nanofibers were also observed through LSCM (Figure 3C) and exhibited clear red emission (λex = 405 nm, λem = 505 ± 20 nm). Moreover, LSCM could detect some nanowires with left-handed helical structures (Figure 3C inset). Morphological observations through SEM and LSCM both indicated that the molecular chirality of L-GSH was transferred and amplified into the supramolecular helicity level in the assembly. Furthermore, TEM observations both at a large scale and 300-nm scale (Figure 3D) demonstrated the highly ordered self-assembly of the complex. Because of the good conductivity of Ag+, these nanofibers could be observed clearly, thus confirming the participation of Ag+ in the assembly.
When the concentration of TPA-Py was increased from 0.1 to 1 mM with the addition of 8 equiv. of both L-GSH and Ag+, the co-assembly was more evident. Many flower-shaped nanostructures with a size of 9.4 μm were observed through SEM (Figure 3E). Amplified SEM images (Figure 3F,G) indicated that these micro-flowers were composed of numerous thin nanowires; this finding is consistent with the assembled behaviors observed at the low concentration. Similarly, LCSM revealed many bright red balls because of the strong AIE effect, and fluorescent three-dimensional reconstruction (Figure 3H inset) exhibited the profile of the “flowers”.
2.4 Driven force for the ternary co-assembly
The force that drives the co-assembly remains unclear, thus a series of spectroscopic tests were performed. First, the interaction between Ag+ and L-GSH was examined through UV-vis spectra. As shown in Figure 4A, no adsorption of L-GSH was observed between 250 and 500 nm. Upon the addition of the different equivalents of Ag+ to the L-GSH solution, two strong absorption peaks centered at 277 and 360 nm appeared and increased gradually. The characteristic adsorption at 360 nm suggested the onset of Ag+–Ag+ interactions.[42] A plot of absorbance at 360 nm as a function of the molar ratio of Ag+ to L-GSH (Figure S15 in SI) indicated a 1:1 interaction stoichiometry between Ag+ and L-GSH, indicating the formation of either the 1:1 complex or (1:1)n coordination polymers.
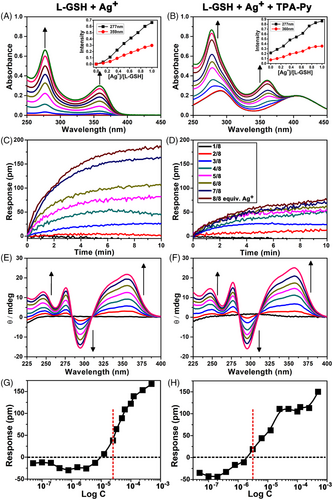
The coordination behaviour was investigated by performing an aggregation assay procedure on a sensor surface by using label-free Corning Epic technology (Figure S16 in SI), which is particularly suitable for studying molecular aggregation.[43] As shown in Figure 4C, the time dependence of the Epic response changed after the addition of L-GSH solution mixed with different molar ratios of Ag+. The formed aggregates are deposited on the sensor surface, resulting in signal response. The addition of more Ag+ induced more remarkable response changes, indicating that Ag+ could promote the formation of the L-GSH–Ag+ aggregates. Atomic force microscopy (AFM) displayed the self-assembled morphology of L-GSH–Ag+, featured with some short and thin nanofibers (Figure S17 in SI) with an average length, width, and height of 240, 22, and 3 nm, respectively.
In addition, the CD titration experiment explained the origin of helical chirality. Both L-GSH and AgNO3 did not exhibit CD signals between 250 and 400 nm (Figure 4E). With a gradual increase in the Ag+ concentration, two positive Cotton effect peaks at 276 and 350 nm, and a negative Cotton effect peak at 295 nm appeared, and their intensities increased gradually. This finding indicated that the point chirality of L-GSH was successfully transferred to helical chirality of the L-GSH–Ag+ aggregates.[42, 44] After examining the interaction between L-GSH and Ag+, we could reasonably speculate the complexation among L-GSH, Ag+, and TPA-Py. TPA-Py might be adsorbed on the L-GSH–Ag+ aggregates through hydrogen bonding interaction between COOH of L-GSH and pyridinium of TPA-Py. UV-vis, Epic aggregation, and CD spectra (Figure 4B,D,F) proved this presumption. It is worth mentioning that CD signals in the ternary L-GSH–Ag+–TPA-Py system are very similar to that of L-GSH–Ag+ binary system, probably due to the site of TPA-Py and L-GSH–Ag+ acting away from their chiral centers, inducing no further chiral change. Differently, according to concentration-dependent Epic response change curves (Figure 4G,H, Figure S18 in SI), L-GSH–Ag+–TPA-Py had a lower critical aggregation concentration (2 μM) than did L-GSH–Ag+ (12.5 μM), indicating a stronger aggregation capacity of the ternary system.[43] The nanofibers formed by the ternary system (Figure 3A) were also thicker and stronger than that of the binary system (Figure S17 in SI).
Further analysis indicated that the chiral assembly of L-GSH–Ag+–TPA-Py had gab values of +0.001 and +0.003 at wavelengths of 275 and 360 nm, respectively. Control experiments performed using fluorescence, UV-vis, and CD spectra showed no changes in the interaction between TPA-Py and Ag+ or L-GSH (Figures S19 and S20 in SI), confirming that the formation of the aggregates resulted from the interaction of TPA-Py with the L-GSH–Ag+ complex rather than the individual Ag+ or L-GSH. The effect of pH on the co-assembly of TPA-Py, L-GSH, and Ag+ has been investigated (Figure S21 in SI). Compared to the emission of TPA-Py, a significant enhancement was observed for its three-component assembly under weak acidic solution (3.0 < pH < 6.0). The isoelectric point (pI) of L-GSH is 5.93, and no significant emission enhancement was observed when pH value was more than 6.0 in our case. The explanation is that the repulsion of the negative charged carboxylate among the GSH residues in the side chains probably weakens or even breaks the Ag+–Ag+ interaction, thereby leading to the un-aggregated state of TPA-Py with no significant emission enhancement.
2.5 Different co-assembled behaviors between TPA-Py and TPA-T-Py in complexation with L-GSH and Ag+
After clarifying the driving force for the co-assembly among TPA-Py, L-GSH, and Ag+, an interesting question was raised: what is the assembly mode among TPA-T-Py, L-GSH, and Ag+? The only difference between TPA-Py and TPA-T-Py is the thiophene interval unit, but TPA-T-Py had multiple responses to some anions or anions added with Ag+. The fluorescence spectrum (Figure 5A) displays that TPA-T-Py also occurs a remarkable fluorescent enhancement after the addition of both L-GSH and Ag+. But in the UV-vis spectrum (Figure 5B), the peaks at 277 and 360 nm that are attributable to the Ag+–Ag+ bond are not obvious, which indicated that only a small amount of L-GSH–Ag+ coordination polymers formed when they interacted with TPA-T-Py. CD spectra further proved this presumption, as shown in Figure 5C, the three characteristic Cotton effect peaks at 276, 295, and 357 nm detected in the TPA-T-Py, L-GSH, and Ag+ system are substantially weaker than that detected in the TPA-Py ternary system, revealing that only part of L-GSH point chirality signal was transferred into the helical chirality of the assembly.
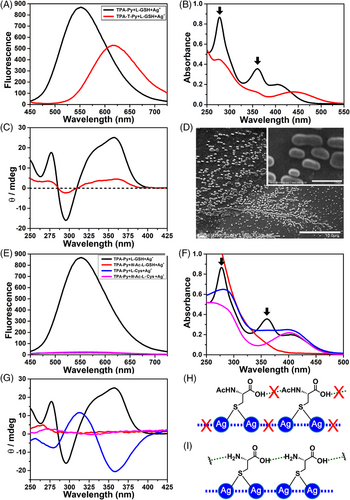
In addition, morphological observation of the TPA-T-Py, L-GSH and Ag+ aggregates by SEM only found numerous disordered elliptical particles with lengths ranging from 600 nm to 1.7 μm. Therefore, we presumed that the complexation of TPA-T-Py, L-GSH, and Ag+ only generated some disordered aggregates (Figure S22 in SI), which was different from the helical assembly observed on the TPA-Py ternary system. Similar phenomena were also detected when TPA-T-Py, Ag+, interacted with N-Ac-L-Cys or N-Ac-L-GSH (Figure S23 in SI), or when R2, Ag+, interacted with N-Ac-L-Cys, L-GSH or Na2S2O3 (Figure S24 in SI). Their detailed binding modes would be discussed in following molecular mechanism part.
2.6 Different assembly behaviors between L-GSH and L-Cys derivatives in complexation with TPA-Py and Ag+
TPA-Py could co-assemble with Ag+ and L-GSH and emitted strong fluorescence but had no response to the mixtures of Ag+ and other L-Cys derivatives (Scheme 1B, Figure 5E), which motivated us to investigate its assembly modes with these L-Cys derivatives. When TPA-Py, Ag+ were mixed with N-Ac-L-Cys or N-Ac-L-GSH, in which the terminal α-amine of L-Cys or L-GSH was protected by acetyl, neither the characteristic UV absorption peak at 360 nm nor the Cotton effect peaks at 276 and 357 nm could be observed in UV-vis and CD spectra (red and violet lines in Figure 5F,G), which revealed that N-Ac-L-Cys–Ag+ or N-Ac-L-GSH–Ag+ coordination polymers did not form in these systems (Figure 5H, Figures S25 and S26 in SI), being incapable of binding TPA-Py and triggering an AIE effect.
For the complexation among TPA-Py, Ag+, and L-Cys, moderate UV adsorption bands around 276 and 360 nm were detected (blue line in Figure 5F), corresponding to the formation of L-Cys–Ag+ coordination polymers. The CD spectrum further confirmed that the point chirality of L-Cys was transferred to the helical chirality of the L-Cys–Ag+ polymers, reflecting as two negative Cotton peaks at 280 and 358 nm and a positive peak at 313 nm in Figure 5G (blue line). Although the formation of the L-Cys–Ag+ coordination polymers, no AIE effect was observed when TPA-Py was mixed with the polymers (Figure S27 in SI). We presumed that carboxylic acid (COOH) of L-Cys interacted with an amine of the neighboring L-Cys, which largely shortened the distance between two Ag+ and promoted the argentophilic interaction (Figure 5I). Under this condition, no redundant COOH of L-Cys could bind with TPA-Py, which was incapable of adsorbing onto the L-Cys–Ag+ polymers. Thus, the AIE effect was not observed. These results highlighted the dispensable role of L-GSH in the ternary co-assembly, two COOH groups and N-terminal α-amine of L-GSH are the key groups for the complexation. It is worth noting that the CD peak shape of L-Cys–Ag+–TPA-Py (blue line in Figure 5G) is nearly opposite to that of L-GSH–Ag+–TPA-Py (black line), which indicated that the helical chirality of the latter one was mainly controlled by the L-Glu unit rather than L-Cys in L-GSH.
2.7 Mechanism analysis
X-ray photoelectron spectroscopy (XPS) was used to analyze the chemical composition of the assembly system. The sample was prepared by collecting the precipitation of TPA-Py–L-GSH–Ag+. As shown in Figure 6A, sharp peaks belonging to Ag3d, C1s, N1s, O1s, and S2p are detected. According to molar ratios among Ag, N, and S calculated from the areas of peaks, a relative ratio of 4:4:1 was determined among L-GSH, Ag+, and TPA-Py. A Job's plot of fluorescent intensity as a function of the molar ratio of L-GSH-Ag+ to TPA-Py further proved this result (Figure 6F). Two Ag3d signals at 368(Ag3d 5/2) and 374(Ag3d 3/2) eV (Figure 6B) and two Ag3p signals at 573(Ag3p 3/2) and 604(Ag3p 1/2) eV could be detected, thus confirming that Ag participated in the assembly. NaBH4 reductive experiment (Figure S28 in SI) further identified that the Ag species existed in the state of +1 rather than zero.[45] The spectrum of C1s (Figure 6C) was deconvoluted into multiple peaks, assigned to C–C (284.7 eV), C–O (285.7 eV), and C=O (288.5 eV) functional groups. The deconvolution of the N1s spectrum (Figure 6D) exhibited multiple peaks including N+ in pyridinium (401.3 eV), C–NH2(400.2 eV), N–C=O (399.7 eV), and N–Ar (399.2 eV). In addition, the appearance of the S2p peak (161.9 eV, Figure 6E) validated the participation of L-GSH in the complexation.
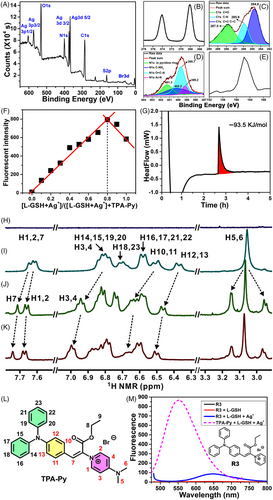
Calorimetric measurement using a C80 Calvet calorimeter revealed that the heat release for the mixing of Ag+–TPA-Py with L-GSH was 93.5 KJ·mol−1 (Figure 6G), which indicated that the co-assembly process was favorable from the point of enthalpy change. Hydrogen nuclear magnetic resonance (1H NMR) titration provided binding details between L-GSH and TPA-Py (Figure 6H–L) in D2O. Characteristic peaks of L-GSH did not appear in these amplified NMR regions (Figure 6H), which avoided interference. When 0.25 or 0.5 equiv. of L-GSH was added to a TPA-Py solution, remarkable chemical shift changes of TPA-Py protons could be observed (Figure 6I–K). For examples, H7 (Figure 6L) shifted from 7.65 to 7.74 ppm, and doublet peaks of H1 and H2 shifted from 7.61 to 7.67 ppm. Because these hydrogen protons are proximal to the N+ atom in pyridinium, they might be directly affected by the interaction with L-GSH. In addition, H protons located in TPA and Py demonstrated downfield shifts, indicating a strong effect of L-GSH adsorption on the charge distribution of TPA-Py (Figure S29 in SI). A single peak ascribed to H5 and H6 split into three peaks, indicating that N(CH3)2 might be another binding site with the COOH of L-GSH. To verify this presumption, a reference compound R3 was synthesized (Figure 6M Inset), in which N(CH3)2 was removed. As shown in Figure 6M, complexation among R3, L-GSH, and Ag+ only led to the increase in the fluorescent intensity from 5 to 85, which was substantially lower than that induced by the TPA-Py ternary system, providing solid evidence for the binding site of N(CH3)2.
13C NMR titration findings revealed that the chiral C and carbonyl C atoms of L-GSH occurred clear shifts (Figure S30 in SI). Thus, we hypothesized that the active COOH and amide groups of L-GSH participated in the complexation with TPA-Py. The NMR peaks of L-GSH or TPA-Py lost their fine structures upon the addition of Ag+ because of its paramagnetic property; therefore, the interaction between TPA-Py and L-GSH–Ag+ complex was not studied through NMR.
Meanwhile, the interaction between TPA-T-Py and L-GSH was studied by 1H NMR titration. Most of the H-protons of TPA-T-Py occurred consistent up-filed shifts (Figure S31 in SI), which could be reasonably ascribed to the impact of acidity induced by L-GSH. This result revealed that TPA-T-Py absorbed onto the Ag+–L-GSH coordination polymers mainly through electrostatic adsorption. This mode was distinct from that detected for the TPA-Py ternary system, in which hydrogen bonding interaction and electrostatic force collaboratively contributed to the complexation.
2.8 Binding model among TPA-Py, Ag+, and L-GSH
Based on the above result of structural analysis, a theoretical model was built using four L-GSH–Ag+ and one TPA-Py to save the computational cost, and details for computations can be found in SI. First, the structure of the binary model (four Ag+ and four L-GSH) was optimized using a GGA-PBE[46] functional and DNP[47, 48] basis sets in DMol3. The core of the metal coordination polymer consists of four silver atoms arranged in a plane with Ag–Ag distances of 2.97, 3.04, 3.23, and 3.31 Å, respectively, whereas the calculated Ag–S distances were all around 2.43 Å (Figure S32 in SI). The calculated absorption spectrum of this model exhibited mainly two bands, and the peaks were located at 274 and 339 nm (Figures S33–S36 in SI, λ339 nm corresponds to the pure electronic transition from HOMO to LUMO, Table S1 in SI), which was in agreement with the experimental data (Figure 4A). Therefore, we presumed that the configuration of this binary model was reasonable. Using the aforementioned GGA-PBE algorithm, an optimized ternary model for TPA-Py–L-GSH–Ag+ was constructed (Figure 7A). Compared with the binary system, the Ag+ metal cluster was unchanged and S atoms from L-GSH provided the rigid skeleton a saddle shape for the ternary model. It is worth noting that Ag+–Ag+ interaction models are plentiful,[49] the Ag+ cluster shown here is only one of the possible models. As shown in Figure 7A, α-COOH located in Glu of L-GSH forms a hydrogen bonding interaction (2.12 Å) with the N(CH3)2 of TPA-Py, two sets of CH–π interactions (3.38 and 3.34 Å) between the methylene of L-GSH and the pyridinium or phenyl further promote the binding of L-GSH with TPA-Py.
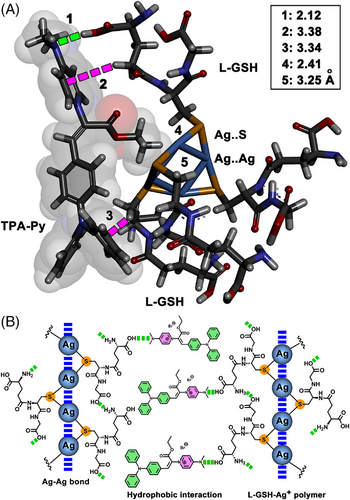
Finally, a possible mechanism was proposed to explain the remarkable AIE effect on TPA-Py–Ag+–L-GSH (Figure 7B). L-GSH binds with Ag+ through the Ag–S bond, which further assembled into the coordination polymers driven by argentophilic interaction.[49, 50] During this process, an intermolecular hydrogen bonding interaction between α-amine (Glu) and neighboring COOH (Gly) considerably shortens the distance between two L-GSH molecules, further prompting the argentophilic interactions. Subsequently, TPA-Py bound with the L-GSH–Ag+ polymer utilizing the hydrogen bonding interaction between the α-COOH in Glu and N-CH3 or pyridinium in TPA-Py, which generates a complex with a hydrophilic head constituted by L-GSH and a hydrophobic tail composed of triphenylamine. The amphiphilicity endows L-GSH–Ag+–TPA-Py with strong self-assembled capacity, resulting in numerous long nanowires in H2O. The adsorption of TPA-Py onto the L-GSH–Ag+ polymer and the self-assembly largely restrict the free rotation of triphenylamine, leading to the remarkable AIE effect. The molecular chirality of L-GSH plays a crucial role in controlling the handedness of the L-GSH–Ag+ polymer, as well as the TPA-Py participated ternary assembly, reflecting strong helical chirality signals in the CD spectra.[51, 52]
3 CONCLUSION
This work first reports three-component co-assembly based on AIEgens. Through a significant fluorescence enhancement effect, the screening process of the key components was greatly accelerated. Compared with the mainstreaming evaluation tools toward molecule self-assembly,[53] such as gelation, 1H NMR, morphological observation by SEM, AFM or POM, fluorescence observation based on the AIEE effect is fast, visual, and convenient, while the output fluorescent signal is easy to be measured, which provides a quick method to screen assembly components and to determine the optimal module combination. This feature will promote the finding of more multicomponent co-assembly systems.
Our ternary co-assembly has successfully realized the accurate recognition of Ag+ and L-GSH. Compared with the traditional host-guest systems that usually have complicated structures (e.g., macrocycle, cavity, tweezer, even helix) to realize the specific binding toward the target biomolecules,[54] the chemical structure of the AIEgen in the ternary assembly is simple, which avoids the complicated chemical synthesis and purification. Particularly, the assembly-induced supramolecular chirality endowed this method with a unique chiral characteristic. The dramatic fluorescent response, high specificity, and unique chirality sense of the ternary co-assembly system provide a new idea for the precise identification of biomolecules.
Finally, there is a huge gap between the artificial assemblies and natural ones in terms of precision and efficiency, the development of ternary co-assembly has just begun! Importantly, the ternary assembly contains more building blocks[10] than the binary assembly, thus it is more likely to achieve stronger maneuverability, which will attract the attention of scientists and promote many exciting applications in nanobiomaterials.[11]
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grant numbers: 21501055, 21922411, and 22174138), DICP Innovation Funding (grant numbers: RC201801 and I202008), Dalian Outstanding Young Scientific Talent (grant number: 2020RJ01). Guangyan Qing would like to thank Prof. Dr. Yonggui Zhou (in DICP) for the valuable discussion and Dr. Yang Yu for helping in measuring heat release of the co-assembly.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
XH and GQ conceived the study, designed the experiments, and wrote the manuscripts. XH, JL, and HT performed most of the experiments. MG and XW conducted the SEM and Epic experiments. XW designed and performed the theoretical calculations. XW synthesized the core molecule TPA-Py. MT conducted the LSCM experiments. FZ and YZ analyzed the CD results. XL analyzed the XPS results.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




