Nanoparticles as contrast agents for photoacoustic brain imaging
X.L. and Y.D. contributed equally to this work.
Abstract
Photoacoustic (PA) imaging has emerged as a promising technique for real-time detection and diagnosis of brain-related pathologies, due to its advantages in deep penetration of ultrasound imaging and high resolution of optical fluorescence imaging. We herein provide an overview on the latest developments of nanoparticles as contrast agents specifically designed for PA imaging of brain tumor, and brain vascular and other brain-related diseases. Five design considerations of high-performance PA contrast agents for brain-related disease diagnosis are discussed, which include (1) strong absorption in NIR or NIR-II window, (2) good biocompatibility, (3) high photothermal conversion efficiency, (4) precise nanostructure control, and (5) specific targeting capability. Challenges and perspectives of developing more robust and universal contrast agents for enhanced PA imaging are discussed at the end.
INTRODUCTION
Unveiling the elusive composition and functions of human brain has always been a relentless challenge for researchers. In clinical settings, patients with neurologic symptoms would receive careful examinations to identify the lesion location and the pathophysiologic causes, which often involve diseases related to brain tumor, brain vasculature, and brain infection. Brain tumor, especially malignant glioma (78% of all malignant brain tumors), remains one of the most challenging cancers due to difficulties in the early stage detection and potential impairment for the adjacent essential structures for normal brain functions.1 According to the World Health Organization, the definition and classification of central nervous system tumors should be determined upon integration of imaging identification, histologic features, immunohistochemistry information, as well as genetic and molecular profiles.2
Imaging of brain and brain-related pathologies has been drawing enormous interests due to the inherent difficulty of the blockage from the scalp and skull. As a consequence, many imaging tools have been recognized as inappropriate and not applicable for brain imaging.3 Today, a number of invasive and noninvasive imaging tools are available to provide systematic information on the assessment of brain structure, which could be utilized for visualization of brain vasculatures, monitoring and prediction of brain tumor status and progression, and so forth. The first advancement in modern brain imaging was contrast agent-enhanced computed tomography (CT), followed by the breakthrough application of magnetic resonance imaging (MRI) in neuroimaging, which still serves as a routine diagnosis tool till today.4 For example, the current diagnosis of brain vascular pathologies is based on imaging results either taken by noninvasive MRI or CT, or invasive catheter angiography. Taking brain tumor as another example, the standard procedure of applying these imaging tools is to preoperatively diagnose and classify brain tumors, to intraoperatively navigate surgical operations, and to monitor and assess posttreatment response and effect on normal brain functions.5 During this standard procedure, imaging techniques such as CT and MRI often serve as the front-line prescreening and tumor location tool, through which the use of contrast agents could allow a better visualization of the tumor cross-sections.6 Both CT and MRI could give anatomic localization of brain tumors at different stages and potentially detect the mass effect from certain brain tumors and calcification within certain brain tumors, for instance, meningioma and glioblastoma multiforme (GBM).7
Rapid qualitative and quantitative evaluation of brain structures has been enabled by the development of perfusion CT technique, which is seen as an alternative imaging modality for clinical indications such as stroke, head trauma, as well as brain tumors.8 CT might be the first imaging tool being prescribed for a patient with central nervous system symptoms while MRI has gradually taken over as the primary modality for lesion location identification, treatment/biopsy planning, and mass effect on brain and vascular structural evaluation.9 For example, with regard to MRI for brain tumor imaging, blood–brain barrier (BBB) breakdown could allow the contrast enhancement for the localization of brain tumors and other mass lesions. It has been established that the contrast signal intensity is associated with the grade of brain tumor, that is, high-grade glioma would have a stronger signal enhancement. Except for CT and MRI, positron emission computed tomography (PET) based on glucose metabolism using (F-18) fluorodeoxyglucose can be used to provide compelling and unique information of brain function under healthy states and in disorder situations.10 Single photon emission computed tomography (SPECT) using 99mTc-exametazime (HMPAO) and 99mTc-ethyl cysteinate dimer (ECD) allowed for evaluation of the extent of the ischemic attack, stroke, and vascular anomalies.11 PET (SPECT) coupled with CT is able to provide a global view of the anatomical reference of PET (SPECT) images.12 Unlike conventional ultrasound techniques, the newly developed functional ultrasound (fUS) is capable of breaking the temporal and spatial resolution barriers and has recently been used for whole-brain microvasculature dynamics imaging13, 14 and neuroimaging15.
PHOTOACOUSTIC IMAGING (PAI)
Recently, PAI has emerged as a promising technique for the real-time detection and diagnosis of brain-related pathologies. A typical PAI setup for in vivo brain tumor imaging is demonstrated in Figure 1(A). An integrated real-time PAI platform includes laser system, ultrasound transducer (UST) and transducer controller, optical components including fiber bundle which aid in laser alignment, as well as the animal disease model with the administration of contrast agents.16 The mechanism of photoacoustic (PA) signal generation is described as following. When endogenous chromophores (e.g., hemoglobin and lipids) or exogenous contrast agents are excited under a nanosecond pulsed laser, the absorbed photon energy would be transformed into heat through photothermal effect, leading to temporarily elevated temperature of the excited region. With the change in temperature, the thermo-elastic expansion and relaxation of the nanomaterials would cause pressure changes. This results in the generation of acoustic (or PA) wave signals, which are recorded and transformed into images by transducers. Hence, in a typical PAI system, a nanosecond laser illuminates the biological sample (rat brain). Absorption of laser pulses by hemoglobin and exogenous contrast agents results in the generation of PA waves, which can be detected from multiple angles around the sample using one or more USTs. High-resolution PA images are obtained by applying various reconstruction algorithms on the acquired PA signal. Contrast-enhanced MRI and CT have both been routinely used in clinical cancer diagnosis with the advantages to provide clarity and definition of tissues of interest at different depth. However, the scanning operation and data acquisition processes are cumbersome and time consuming, which often result in the discomfort of patients.17, 18 Being a hybrid imaging modality, PAI offers the advantages of high optical contrast and good acoustic resolution. In comparison with optical fluorescence imaging or ultrasound imaging, PAI exhibits both the advantages of deep penetration depth up to a few centimeters and high resolution of several micrometers. In addition, PAI is considered much less hazardous than MRI or CT since no magnetic or ionizing radiation is involved. Further, PAI assisted by PA contrast agents is considered promising for the imaging of brain-related diseases due to its advantages of providing real-time and high-resolution images with a relatively deep penetration depth, which could provide intraoperative information for neurosurgeons.
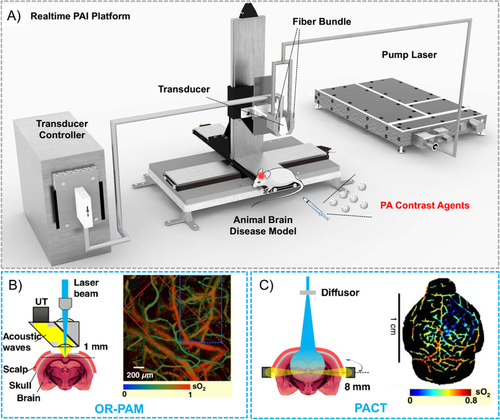
PAI systems are generally categorized into two major types of implementations: scanning-based photoacoustic microscopy (PAM)19, 20 and reconstruction-based photoacoustic computed tomography (PACT)21. PAM can be further divided into optical-resolution PAM (OR-PAM, Figure 1(B)) and acoustic-resolution PAM (AR-PAM). PAM could generate PA images with a resolution in the range of hundreds of nanometers to sub-100 μm with a penetration depth of 1–3 mm. Macroscopic biological imaging often sets a more stringent requirement for a greater imaging depth of centimeters and the transverse scanning of PAM is thus not suitable for such applications. In comparison, PACT employs higher energy pulses and wide-field scanning and is capable of imaging at a depth of up to several centimeters with a lateral resolution of hundreds of micrometers (Figure 1(C)), offering relatively better chances for clinical applications. Hence, PACT has been widely exploited in the imaging of brain-related diseases.
Based on the two major types of PAI systems discussed above, the advantages of PAI such as deep penetration depth and high spatial resolution have been demonstrated by many clinical and preclinical applications.22 In the past decades, PAI systems have been rapidly developed with imaging capabilities to visualize not only cardiological, dermatological, gastroenterological, ophthalmological conditions, but also brain vasculatures, brain tumors, neuron activities, as well as other brain-related diseases such as strokes and Alzheimer.23-26 Although the current presence of PAI in clinics is not as prevalent as other traditional imaging methods discussed above, much effort has been devoted in the development of clinical PAI for monitoring certain health indicators such as glucose level or some superficial medical conditions such as skin and oral diseases.27, 28 To image a deeper depth, PA contrast agents with high thermo-elastic expansion and photothermal coefficients are often required to enhance the contrast difference between targeted area and the surrounding tissues.29 Common PA contrast agents include some organic small molecules and conjugated polymers, and inorganic carbon, gold, copper sulfide, molybdenum disulfide nanomaterials.30 PAI assisted by PA contrast agents is considered promising for the imaging of brain-related diseases due to its advantages of providing real-time and acceptably high-resolution images with a relatively deep penetration depth.
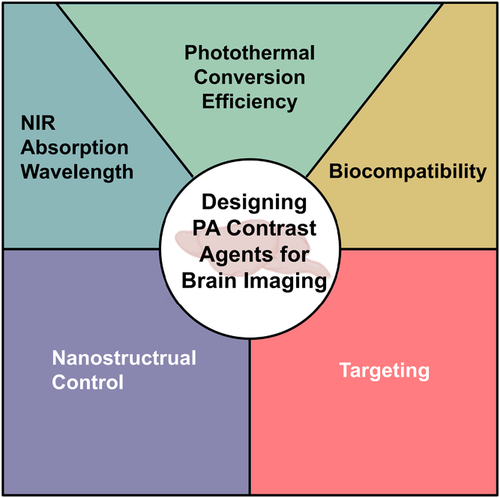
So far, several previously published reviews had summarized the progress of PA contrast agents for tumor visualization.31-36 We herein aim to provide an overview on the latest developments of nanoparticles (NPs) as PA contrast agents specifically designed for imaging of brain tumor, brain vascular, and other brain-related diseases. The photophysical properties and applications of those NPs are summarized and compared in Table 1. The series of general design considerations and unique requirements of high-performance PA contrast agents for brain-related disease diagnosis include: (1) strong absorption in NIR or NIR-II window, (2) good biocompatibility, (3) high photothermal conversion efficiency (PCE), (4) precise nanostructure control, and (5) specific targeting capability (Figure 2). The rationale and common practice of applying these five design considerations are, respectively, discussed and illustrated with state-of-art examples in the field. Finally, challenges and perspectives of developing more robust and universal PA contrast agents for enhanced brain imaging are discussed.
| Contrast NPs | Size (nm) | Absorption Peak (nm) | Encapsulating matrix | Application |
|---|---|---|---|---|
| SPN447 | 46 | 748 | DSPE-mPEG | Subcutaneous tumors PAI |
| P1RGD NPs52 | 50 | 1064 | DSPE-mPEG | Orthotopic brain tumors PAI and PTT |
| PTD NPs53 | 40 | 1160 | DSPE-mPEG | Cerebral and tumor vasculatures PAI |
| SPN-PT58 | 30 | 1079 | PLGA-PEG | Tumor and brain vasculature PAI |
| HCu59 | 50 | – | – | Orthotopic brain tumors PAI |
| CP-IO75 | 150 | 750 | DSPE-mPEG | 4T1 tumor PAI |
| HALF-cRGD76 | 51 | 685 | DSPE-mPEG | C6 brain tumor PAI |
| cRGD-CM-CPIO89 | 74 | 730 | PLGA | Brain tumor PAI |
| PBPs94 | 45 | 701 | – | Traumatic brain injury PAI |
DESIGN CONSIDERATIONS OF PA CONTRAST AGENTS FOR BRAIN IMAGING
Absorption wavelength
During the early days of PAI, green light at 532 nm was employed with the aim to image blood vasculatures since blood absorbs strongly in the visible region.37 Soon it was realized that in vivo PA signal amplitudes would be overwhelmingly affected owing to the strong tissue attenuation between the wavelengths ranging from 200 to 700 nm.38 Compared to visible light, the first near-infrared (NIR-I) light (700–1000 nm) is capable of penetrating biological entities with less light scattering. Therefore, the pulsed laser wavelength of PAI progressively evolved from the visible region to the NIR region between 700 and 1000 nm.39-41 As such, majority of standard commercial PAI systems were by default equipped with pulsed lasers of wavelengths in between 680 and 1000 nm. Accordingly, the first generation of inorganic and organic PA contrast agents were developed with high mass extinction coefficients and absorption wavelength in the NIR-I window in correspondence to this laser range and exploited for various in vivo experimental validations, including brain imaging.42 Primarily, the design and selection of suitable exogenous PA contrast agents for imaging brain-related diseases should be first determined upon the optical absorption spectral differences as compared with endogenous optically absorbing compounds such as hemoglobin, melanin, water, lipids, collagen. High mass extinction coefficient at specific absorption wavelengths could be carefully designed and selected to maximize the image contrast of PA against the background endogenous absorbers since the latter have distinct absorption ranges between 700 and 1100 nm. Typical inorganic PA contrast agents with surface plasmon resonance absorption in the NIR-I window include noble metal, transition metal, and copper-based nanomaterials.43 Noble metals such as gold and palladium could be processed into nanomaterials, which may absorb in the NIR region. Among others, gold nanomaterials including gold nanorods, gold nanostars, and hollow gold nanospheres have been mostly experimented for the visualization of brain tumors and brain vasculature.44 Transition metal dichalcogenide such as two-dimensional molybdenum disulfide (MoS2) nanosheets have broad UV-vis absorption spectra with the major peak near 450 nm and the tail covering and extending till 800 nm, which also showed good potential as PA contrast agents.45 Copper-based nanomaterials such as nanoscale copper chalcogenides (Cu2-xE, E = S, Se, Te, 0 ≤ x ≤ 1) are another important category of PA contrast agents due to their strong localized surface plasmon resonances in the NIR window.46 While these inorganic NPs have demonstrated good optical properties and tremendous potential for in vivo PAI applications, there are concerns with their long-term biological safety and toxicological profile.
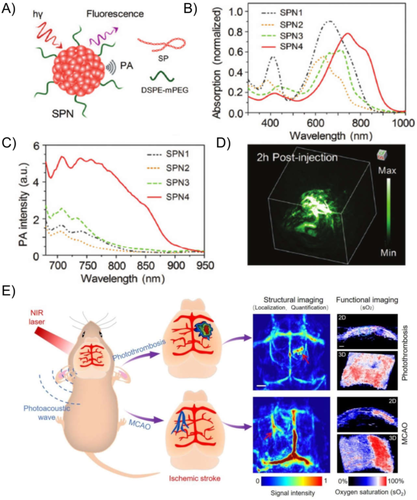
In response to these issues, a large number of organic nanomaterials including small molecules and conjugated polymers have been developed for PAI applications over the past decade due to their excellent biodegradability and biocompatibility (Table 1).30, 31 For example, Pu and Rao reported a series of organic NPs based on conjugated polymers consisting of diketopyrrolopyrrole (DPP) moieties with low bandgap for in vivo tumor PAI.47 As shown in Figures 3(A) and 3(B), the organic NPs SPN1–SPN4 were prepared via the typical nanoprecipitation method with absorption spectra covering the NIR window from 500 nm up to 950 nm. The PA performance of SPN4, which showed the brightest PA signal (Figure (3C)), was validated and demonstrated with a three-dimensional PA image of a mice subcutaneous tumor (Figure 3(D)). Similarly, in vivo brain tumor imaging has been demonstrated with organic NPs encapsulating conjugated polymer designed with delocalized π-electron backbones and donor–acceptor–donor structures.47 Besides, several commercial organic molecules approved by US Food and Drug Administration (FDA) such as indocyanine green (ICG), Evans blue (EB), methylene blue, and Prussian blue have been recognized as suitable agents for PAI in the NIR window for identifying and monitoring brain tumors,48 BBB disruption,49 and stroke.50 For instance, Nie et al. utilized a versatile PAI technique assisted by EB dye to dynamically display the structural and functional features of ischemic stroke on mice brain at a very early stage (Figure 3(E)).50
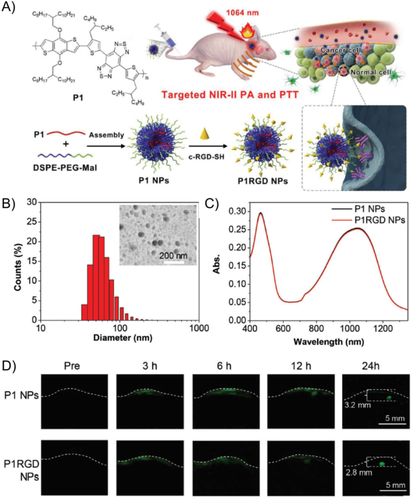
Nevertheless, increasing evidence has recently suggested that NIR window is also associated with some drawbacks such as inadequate penetration depth, significant tissue autofluorescence, and substantial background noise, which may eventually result in decreased contrast and resolution in PA image.51 More importantly, to clearly identify the lesions in the brain and minimize the blockage and scattering of signals, most PAI experiments of mice brain using the NIR window require the removal of scalp and skull, which is time consuming, cumbersome, and could significantly affect the survival of the animals. To overcome these drawbacks, PAI systems with longer laser wavelengths in the NIR-II window between 1000 and 1700 nm have been developed. In comparison with conventional NIR PAI, PAI in the NIR-II window exhibits several merits including (1) reduced light scattering by biological tissues and high maximum permissible exposure, which simultaneously enable deeper penetration depth; (2) diminished background autofluorescence, which allows for higher signal-to-noise ratio; and (3) inexpensive 1064 nm Nd:YAG laser, which promotes the affordability of the NIR-II PAI system. The emergence of NIR-II PAI systems further sparked the development of high-performance NIR-II PA contrast agents, which could effectively enhance the image contrast. Notably, majority of the NIR-II PA contrast agents developed are compatible with 1064 nm lasers. For instance, Liu et al. fabricated conjugated polymer NPs (P1 and P1RGD NPs) with sizes of approximately 50 nm and broad absorbance ranging from NIR-I to NIR-II windows for brain tumor PAI (Figures 4(A)–(C)).52 The conjugated polymer P1 with a planar donor–acceptor structure was designed through the copolymerization of benzodithiophene and benzobisthiadiazole to yield low band gap and strong absorption. High-quality NIR-II PA images of brain tumor with a depth of 2.8 mm below the skull and an excellent signal-to-background ratio (SBR) of 90 were obtained based on laser excitation at 1064 nm, 24 h postinjection of the P1RGD NPs (Figure 4(D)). This unveiled the great potential of PA contrast agents based on organic conjugated polymers for imaging of deep brain tumors.
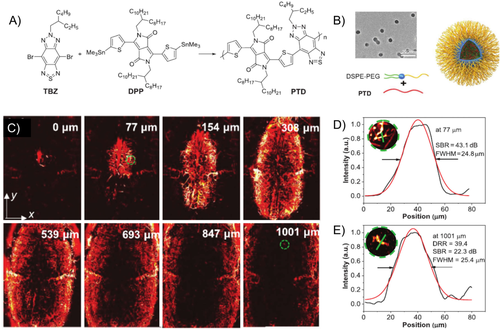
In another example, polymer PTD conjugated by thiophene-substituted DPP and thiadiazolobenzotriazole (TBZ) was designed (Figure 5(A)) and the PTD NPs with a size of 40 nm were obtained via microfluidics nanoprecipitation method (Figure 5(B)).53 The rigid planar backbones and repeating donor–acceptor (D-A) units of PTD facilitate a high extinction coefficient, small bandgap, and bathochromic-shifted absorption to NIR-II range (1160 nm). Then the PTD NPs were used for 3D optical-resolution photoacoustic microscopy imaging (ORPAMI) of whole-cortex cerebral vasculatures layer by layer (Figure 5(C)). The cerebral vasculature imaging had a large imaging area of 48 mm2, good resolution of 25.4 μm, and a high SBR (SBR = 22.3 dB) at depth up to 1001 μm through the intact skull (Figures 5(D) and 5(E). In comparison with the NIR-II fluorescence vascular imaging, this system shows a remarkably enlarged image area with deeper penetration and a higher SBR ratio.
Biocompatibility and biodegradability
Similar as other biological imaging applications, PAI of brain-related diseases requires good water dispersibility, biocompatibility, and targeting capability of the contrast agents, especially mono-dispersed inorganic NPs, which are often fabricated under high temperature in organic solvents. To ensure the water dispersibility of both inorganic nanomaterials and organic compounds, it has been a universal strategy to coat the compounds with a shell layer of amphiphilic block copolymers, which are composed of hydrophobic and hydrophilic blocks.54 Hydrophobic segments such as polyesters could form the lipophilic cores or compartments to encapsulate highly hydrophobic compounds. Hydrophilic segments such as water-soluble polyethylene glycol (PEG) would form a highly hydrated shell layer to reduce the agglomeration of biomolecules and compounds. For example, nanoprecipitation method has been widely applied to produce NPs by encapsulating organic molecules or conjugated polymers.55
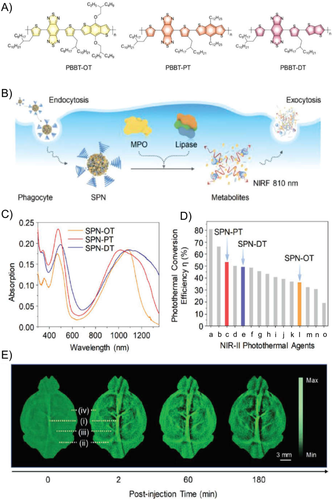
On top of water dispersibility, biocompatibility could be realized via multiple methods, such as elimination of toxic compounds, limiting the dosage, and designing biodegradable or metabolizable contrast agents. Normally, 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, serum biochemical analysis, hematoxylin and eosin (H&E) staining of main organs, and acute inflammation test were the main methods to evaluate the toxicity of NPs.56 For brain PAI, as the nervous system is quite vulnerable to toxins, the exposure to chemicals and heavy metals may result in neurotoxicity. To improve the biocompatibility of PA contrast NPs, PEG coating is a general strategy to reduce the potential toxicity of NPs, while designing NPs with biodegradability is another essential approach. By introducing stimuli-responsive functional groups into the contrast agents, the NPs showed biodegradability when exposed to bio-related signals, like GSH, pH, enzymes.57 For example, metabolizable organic NPs based on conjugated polymers of donor–acceptor structure with BBT acceptors and different donors have been synthesized for NIR-II PAI of subcutaneous tumor and brain vasculatures (Figure 6).58 Upon degradation by intrinsic enzymes such as myeloperoxidase and lipase, which are prevalent in phagocytes, the 30 nm NPs were deformed into ∼1 nm ultrasmall metabolites. Through in vitro validations, the successful biodegradation of the NPs could be confirmed by the NIR fluorescence emitted by the degraded products, which could be effectively circulated out of biological systems with minimal safety concerns (Figure 6(B)).
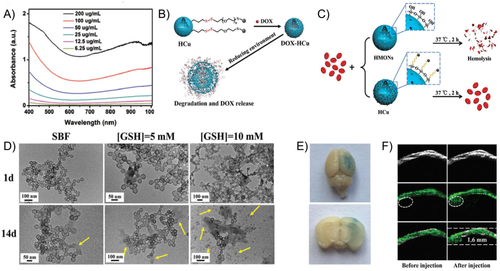
Monodispersed inorganic NPs are often fabricated under high temperature in organic solvents and laborious postsynthesis surface modification is usually necessary to endow them with good water dispersibility and biocompatibility for bioimaging applications. Various types of carriers could be designed to make the best use of inorganic NPs for biological applications. For instance, biodegradable inorganic copper-based NPs have been developed for PAI-guided therapy for brain tumor (Figure 7).59 Herein, hollow mesoporous organosilica NPs (HCu) were utilized as the carrier for Cu2-xSe NPs. With the broad absorption profile from NIR-I to NIR-II regions, the developed HCus exhibit tumor-triggered programmed destruction capability. The responsive disulfide bonds linking the Cu2-xSe NPs and the silica carriers could be cleaved under the reducing microenvironment with GSH, which enables the tumor-specific biodegradation and on-demand release of the therapeutic agents. In combination with focused ultrasound (FUS) technique for BBB opening, orthotopic brain tumor could be clearly imaged with the designed PA contrast agents.
Photothermal conversion efficiency
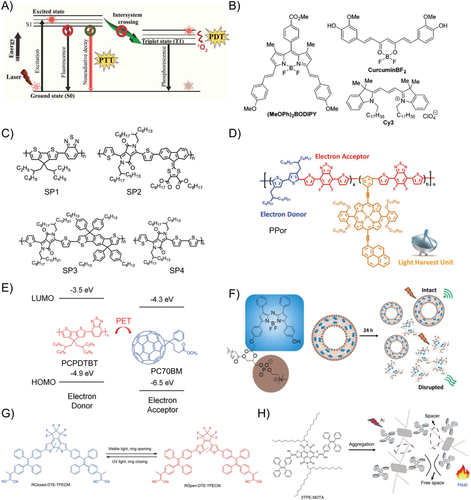
Apart from light absorption capability and the Grüneisen coefficient,60 PCE of exogenous contrast agents is also a critical factor for achieving high-performance PAI in biological systems. By definition, photothermal efficiency refers to the ratio of heat generation to the total incoming radiation input.61, 62 In organic agents, the excitation energy from photons absorbed would be primarily dissipated via three channels: radiative decay pathway (fluorescence), nonradiative decay pathway (heat generation), and intersystem conversion (e.g., phosphorescence; Figure 8(A)).63
With reference to the PA signal generation process described above, in addition to enhancing light absorption affected by mass extinction coefficient and decreasing light scattering, heat generation from organic compounds is primarily determined by the nonradiative decay pathway, which competes with the radiative decay pathway to drive the thermal expansion process and further affects the PA signal amplitude. In this regard, adjusting the dissipated energy through the nonradiative decay pathway is appealing to improve PCE. Several molecular and nanomaterial engineering approaches have been developed to promote the nonradiative decay pathway.
Molecular design is the most direct way to regulate the molecular photophysical property. Rochford et al. compared series of BODIPY, curcumin, cyanine, and crystal violet fluorophores by optical and PA tests. They found that a long-lived S1 excited state and large excited-state absorption extinction coefficient could facilitate the rapid Sn →S1 nonradiative decay and enhance the PA intensity (Figure 8(B)).64 Other parameters, such as the nonradiative decay efficiency, affect the PA intensity. Pu et al. systematically analyzed the structures and optical properties of a series of semiconducting polymers and found that SPN4 exhibited the highest PA signal due to the strongest electron-donating ability and narrowest bandgap, which favors the nonradiative deactivation more than that of SP1–SP3 (Figure 8(C)).65 In another example, semiconducting polymer NPs copolymerized with electron donor and acceptor monomers have emerged as a new class of PAI agents. By introducing a light-harvesting unit side chain to the D–A polymer backbone, Lee et al. reported a high PCE of 62.3% under irradiation of 635 nm laser (1.4 W cm−2) (Figure 8(D)).66 Apart from chemical conjugation of a light-harvesting unit into the polymer chain, Pu and coworkers have also introduced an intraparticle molecular orbital engineering approach by incorporation of electron-withdrawing fullerene into the SPNs. Enhanced nonradiative heat generation was achieved through quenching the fluorescence of SPNPs due to intraparticle photoinduced electron transfer between semiconducting polymer (PCPDTBT) and fullerene within NPs (Figure 8(E)).67
The photophysical processes in small molecules can also be adjusted by modulating their energy pathways through spatial arrangements within a nanostructure or by intramolecular structure changes. Strong π–π stacking, such as J-type or H-type aggregates, are often reported to increase the nonradiative decay pathway by quenching the fluorescence. For instance, Zheng et al. reported an aza-BODIPY-lipid that can self-assemble into a BODIPYsome vesicle structure with optically stable NIR J-aggregation.68 J-dimerization in BODIPYsomes provided a large extinction coefficient (128 mM−1 cm−1) and high efficiency in fluorescence quenching (99.70 %), which allowed the BODIPYsome to serve as a notably efficient PA agent (Figure 8(F)). As PA and fluorescence are always the competing processes, Ding et al. developed a function-transformable optical agent for PA or fluorescent imaging as needed.69 The ring-closing and ring-opening isomers of DTE-TPECM were reversibly switchable when triggered by external UV-vis light irradiation. The absorbed excitation energy can be photo-switched to the route of thermal deactivation for PAI in the ring-closed state where the absorbed energy of TPECM was intramolecularly transferred to the nonemissive ring-closing DTE core. This led to the fluorescence quenching of TPECM for PAI, or fluorescence imaging in ring-opened state when the intermolecular interactions were hindered (Figure 8(G)).
Recently, it has been reported that the dynamic molecular motion is able to promote energy dissipation through nonradiative decay. Therefore, subtly regulating molecular motions of organic molecules introduced with adequate molecular rotors or vibrators is a promising approach to promote nonradiative decays of molecules in the nanoaggregates.70 Tang et al. proposed a class of near-infrared–absorbing organic molecules with intrinsically active excited-state intramolecular motion inside NPs. Combining naphthalene diimide-fused 2-(1,3-dithiol-2-ylidene)acetonitriles (NDTA) with tetraphenylethylene (TPE), 2TPE-NDTA enables excellent photothermal conversion ability (54.9%) and superior PAI in live mice (Figure 8(H)).71 2TPE-NDTA was designed with far-red/near-infrared absorption, large molar absorptivity, and strongly twisted intramolecular charge transfer effect in the aggregate state within NPs. The efficient intramolecular motion of 2TPE-NDTA in a loose packing state resulted in energy dissipation via thermal deactivation pathway, which generated about 2.0-fold and 2.9-fold higher PA signal than NPs of PCPDTBT (Figure 8(E)) and methylene blue. In addition, in contrast to nonradiative decay, excited chromophore may also induce intersystem crossing from singlet state to triplet state. An effective design approach to reduce intersystem crossing can diminish the production of singlet oxygen and also ultimately enhance the PCE.72
Nanostructure control and arrangements
In addition to wavelength, biocompatibility and PCE, the physical size and morphology of nanomaterials are other key parameters to be considered for optimal PAI performance. For example, the size of nanomaterials plays a dominant role in PA signal amplitude where larger NPs show stronger PA signal owing to the increased heat transfer as compared with small NPs or single molecular probes.73 On the other hand, it is noteworthy that smaller NPs are preferable for brain tumor imaging because they are less likely to be rapidly cleared by phagocytes and the reticuloendothelial system as compared with larger ones. Small size NPs also possess longer blood circulation time and may passively penetrate the BBB and accumulate in brain tumor sites.74 Thus, the size of NPs needs careful evaluation to obtain optimal brain tumor PAI outcomes. Besides, the morphology of nanomaterials may also affect the PA performance. The disparity in morphology leads to distinct absorption spectrum and mass extinction coefficients at different absorption wavelengths. For example, Zheng et al. compared the PA signals between single-layer (S-MoS2), few-layer (F-MoS2), and multilayer (M-MoS2) nanosheets exfoliated under sonication and found that PA signals were inversely associated with the layered nanostructures. The light absorption and elastic properties of the nanomaterial were greatly enhanced by decreasing the nanosheet layers, which greatly amplified the PA signals.45
Besides nanostructures, that is, the physical arrangement of different components within a single nanoagent, could also lead to distinct or superior PA performance due to synergy or enhancement in the photothermal generation capability. For instance, it has been reported that the increased iron oxide ratio of the conjugated polymers PDPP3T/iron oxide coencapsulated NPs could amplify the PA signal by generating additional heat and dissipating the heat produced in a faster manner.75 Furthermore, the distribution of iron oxide NPs and organic molecules in the formed nanostructure was also reported to affect the PA signal (Figure 9).76 The unique nanostructure that physically separates the iron oxide NPs and the organic molecules can efficiently relieve fluorescence quenching, maintain high PAI performance, and magnify the MRI signals. Molecular dynamic simulations of the synthesized TC1 molecules in singular or aggregated molecular states unveiled that the fluorescence could be enhanced when the molecules are processed into NPs. It was found that the PA signal in the NIR-I window was the strongest when the iron oxide NPs were evenly distributed in the nanocomposite, while the fluorescent signal was brighter when the iron oxide NPs were separated from the organic molecules. Hence, it is of crucial importance to consider the physical arrangement of the components when designing nanocomposites for different imaging modalities.
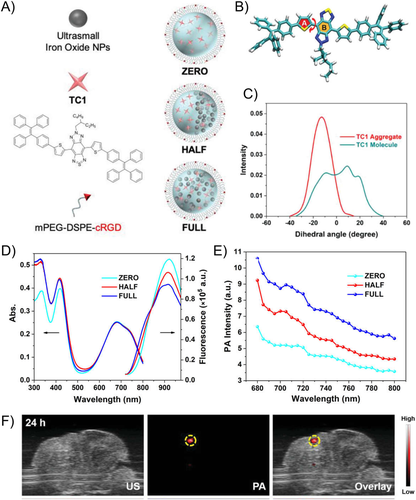
Of equal importance is the nano-structure stability, which is directly associated with the photothermal stability and the long-term PA signal amplitude. For example, gold nanorods have been reported to be more susceptible to laser-induced structural deformation as compared with NPs prepared by organic compounds, potentially leading to a decreased PA signal amplitude after long duration of repeated laser irradiation. In the case of brain tumor imaging, repeated long-duration PAI is unavoidable due to the complex brain structures and the complicated surgical operating procedures. Hence, PA contrast agents with strong PA signal generation performance should be companied with sufficient PA signal stability.
Brain-targeting strategies
The most challenging obstacle that needs to be overcome for effective brain tumor targeted delivery is the well-known BBB. BBB is defined as a protective barrier in brain controlling the transfer of substances between blood vessels and neural tissues.77 The physical components of BBB mainly consist of endothelial cells on the capillary wall, which serve as tight junctions blocking the paracellular passage. Similarly, blood–brain tumor barrier (BBTB) is located between the endothelial cells on the microvasculatures and brain tumor tissues. BBTB would only be formed in high-grade glioma where the brain tumor cells form clusters, invade surrounding normal brain tissues, and damage the original BBB.78 In malignant brain tumors, BBB could be compromised at the core of tumor mass and the abnormality of microvasculatures could improve the permeability of BBTB. However, the infiltrating brain tumor cells at the edge of glioma still strongly hinder the targeted delivery of contrast agents.
The unique characteristics of brain tumor microenvironment include not only the extremely low substance permeability and significant heterogeneity of BBB and BBTB, but also the various brain-resident cell types and complex hurdles of the immune-suppressive environment. Tumor microenvironment typically contains cells including pericytes, fibroblasts, and immune cells, which are also prevalent in brain tumors.79 Further, certain cell types such as astrocytes, microglia, neutrophils, and neurons are uniquely presented in brain tumor tissues. Hence, instead of focusing only on BBB crossing capability, the development of brain tumor–targeted nanomaterials should not neglect the complexity of the brain tumor microenvironment.
The most widely adopted approach to deliver nanomaterials to brain tumors is passive targeting. As mentioned earlier, due to edematous swelling and elevation of intracerebral pressure, BBB could be disturbed to certain extent under pathological conditions, which offers opportunity for passive transport of nanomaterials across BBB. Similar to the delivery requirement of small molecule chemo drugs, the major factors that affect the brain tumor–targeting efficiency are size and lipophilicity. A smaller size and enhanced lipophilicity have been demonstrated to be responsible for an elevated BBB penetration via passive transport mechanism. Nevertheless, it should be noted that enhanced permeability and retention (EPR) effect in brain tumor animal models might not be as efficient as other cancer models such as subcutaneous tumors.80 Thus, the design of nanomaterials that can actively cross BBB and target brain tumors is critically valuable for enhanced controllability and higher delivery efficiency.
Active targeting of brain tumors could be further categorized into two types, invasive and noninvasive strategies.3 To circumvent or overcome the difficulty of crossing BBB, many invasive strategies including parenchymal injections, intracavity instillation, and intraventricular or intrathecal administration have been proposed. For instance, during brain tumor surgical resections, direct administration of contrast agents into the extravascular space of CNS has been attempted. While these strategies may be helpful for chemo drugs or contrast agents to reach cerebrospinal fluid (CSF) spaces, they are still hindered by the existence of the CSF–brain barrier. For example, the introduction of parenchymal chemo drugs comprised of biodegradable wafers impregnated with a nitrosourea at the time of brain tumor resection was demonstrated with a slight improvement of 2 months in the median survival (13.9 months versus 11.6 months) for high-grade glioma patients.81 In addition to injection-based strategies, temporal disruptions of BBB tight junctions via chemical or physical stimuli have also been reported. For instance, application of low-intensity FUS in combination with microbubbles has been studied as a reversible approach for BBB opening. Nonetheless, the exact mechanisms underlying the physical BBB opening process remain unclear, with a number of proposed explanations ranging from prolonged stable cavitation of the microbubbles to more violent inertial cavitation, vessel invagination, and microjetting.82
Besides, various noninvasive strategies to tackle BBB and BBTB have been proposed through absorptive-mediated transcytosis, transporter-mediated transcytosis, and receptor-mediated transcytosis.79 First, peptide shuttles with less than 50 amino acids have gradually gained more attention owing to merits such as simple derivation, low immunogenicity, and relatively low production cost. Based on the origin, these protein/peptide shuttles can be characterized into two types: natural penetrating proteins such as transcription-activating factor TAT and VP22, and synthetic peptides such as mTAT (C-5H-TAT-5H-C). However, the drawbacks of absorptive-mediated transcytosis include the potential toxicity and the relatively poor brain tumor–targeting specificity due to nonspecific binding between the cationic peptides and negatively charged cell membrane constituents. Second, BBB being the gateway for brains to gain access to essential ions, nutrients, and hormones, inspires researchers to utilize the existing endogenous transport mechanisms, that is, transporter-mediated transcytosis, to deliver nanomaterials to the brain. Highly selective transporters are able to control the transfer into endothelium cytosol and further to the neural tissues. For instance, a commonly adopted strategy is to increase either lipophilicity, which would further enhance passive diffusion, or positive charge, which interacts with anionic glycocalyx. Third, receptor-mediated transcytosis has been one of the most mature strategies for brain tumor targeting with high specificity, selectivity, and affinity. Targeting at the receptors expressed on the brain capillary endothelium, for instance, transferrin receptor, the low-density lipoprotein receptor and integrin αvβ3 receptor, diverse targeting ligands have been designed and exploited to facilitate the delivery of nanomaterials into brain tumors.83 Another broadly investigated example is arginine–glycine–aspartic acid (RGD) peptide, the ligands targeting integrin αvβ3 receptors, which are overexpressed on both endothelial cells of neovessels and brain tumor cells. Many studies have demonstrated the enhanced brain tumor targeting achieved by the decoration of RGD peptides onto the nanomaterials.84 For instance, RGD-modified NPs containing paclitaxel have been demonstrated with higher accumulation in brain tumor sites and a better inhibition outcome of brain tumor growth. Nevertheless, one common limitation of these targeting ligands is that they may potentially affect the homeostasis and compete with internal natural ligands, which may limit the targeting efficiency.
In addition to the above-discussed active brain tumor–targeting strategies, some emerging technologies such as cell membrane coating and living cell-based delivery have also attracted considerable attention owing to their intrinsic advantages in biomedical applications. Natural NPs possess distinctive intercellular interaction mechanisms and delivery processes, which inspired the integration of these innate advantages of cells or cell components with synthetic nanomaterials.85
Cell membrane coating technology realizes the biomimetic replication of the natural properties of cell membranes, which promote immune escaping and prevent the rapid degradation and clearance from biological systems.86 The coated nanomaterials could not only achieve a prolonged in vivo circulation, but also attain an increased biocompatibility and the capability to cross specific in vivo barriers. Taking advantages of the membrane layers, which are structurally and functionally analogous to those of host cells, the specific surface biomarkers could be useful for homotypic targeting for drug delivery, immunotherapy, vascular and tumor imaging, and so forth.87 Over the past decades, cell membrane coating technology has been exploited to mimic the surface of a broad spectrum of species including red blood cells, leukocytes, platelets, cancer cells, bacteria, and stem cells.85 Various types of inner nanomaterial cores have been explored, such as organic polymeric NPs and liposomes, and inorganic silica, gold, iron oxide, upconversion, and metal-organic frameworks NPs.87 Cancer cells could undergo effective intercellular homotypic adhesion due to the surface adhesion proteins, which could mediate the specific self-recognition.88 For instance, PA contrast agents coated by bioorthogonally labeled brain tumor cell membrane have been reported for targeted diagnosis of glioblastoma (Figure 10(A)).89 Nanocomposites prepared with conjugated polymer PDPP3T, iron oxide NPs, and fluorescent dye DiR on the extracted cell membrane were capable of signal generation through multiple imaging modalities. The surface decoration of brain tumor cell membrane and endothelial integrin receptor-targeting peptide (cRGD) enabled the nanocomposites homotypic targeting and BBB-penetrating ability to the brain tumor microenvironment. Utilizing the broad absorption peak in the NIR-I window, the strong photothermal conversion capability of the conjugated polymer and the designed specific targeting function, multimodal imaging of glioma in the mice model were successfully demonstrated with the designed contrast agents under MRI, PAI, and fluorescence imaging (Figures 10(B) and 10(C)). Cell membrane proteins camouflaged NPs can also maintain the active targeting ability based on homologous binding mechanism. Zheng et al. constructed biomimetic ICG-loaded liposome (BLIPO-ICG) NPs by embedding glioma cell membrane proteins into NPs to actively cross BBB for PA brain tumor imaging at the early stage with an SBR of 8.4 at 12 h postinjection.90
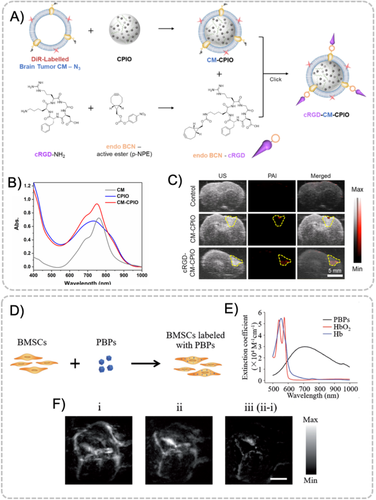
Similarly, living cell-based delivery systems are expected to harness the natural characteristics of cells obtained either directly from the patients or indirectly from external donor cell sources to carry and deliver biotherapeutic agents to desired lesions.91 Many brain-related diseases including Alzheimer's disease, stroke, and traumatic brain injury (TBI) have been exploited with cell-based delivery strategy.92 Among these applications, most cell types including leucocytes, immune cells, cancer cells and bacteria ghosts have been studied as biological carriers of theranostic agents both in vitro and in vivo.93 The main mechanisms of nanomaterial coupling or encapsulation by living cells are internalization or antibody–antigen interaction. The internalization of nanomaterials relies on processes such as endocytosis or phagocytosis.92 For example, bone mesenchymal stem cells (BMSCs) have been exploited to carry Prussian blue particles (PBPs) for the imaging of TBI (Figure 10(D)).94 PBPs were coated by citric acid such that it was endowed with good water solubility and stability. As an excellent PA contrast agent with a broad absorption band from 600 to 900 nm (Figure 10(E)), PBPs were coincubated with BMSCs and then intravenously injected into the mice for further studies. After 6 h incubation, the PBP-labelled BMSCs were found with a relatively high level of the contrast agents. BMSCs were shown to be able to cross BBB with enhanced delivery of PBPs to the brain parenchyma (Figure 10(F)). Most importantly, it was observed in PAT that the BMSCs would transmigrate into the damage regions and repair the ruptured vasculature with a fast wound-healing speed.
CONCLUSION AND FUTURE PERSPECTIVES
Nanomaterials as PA contrast agents have achieved remarkable progress recently in PA brain imaging due to their unique features and advantages. This review summarized five design considerations for high-performance PA contrast agents: strong absorption in NIR or NIR-II window, good biocompatibility, high PCE, precise nanostructure control, and specific targeting capability.
The emergence of NIR-II PAI systems and the development of high-performance NIR-II PA contrast agents had addressed the shortages of inadequate penetration depth, significant tissue autofluorescence, and substantial background noise in NIR window, which showed great potentials for noninvasive deep-brain imaging through skull and scalp with high signal-to-noise ratios. The biocompatibility of PA contrast agents could be realized via restriction or elimination of toxic compounds, limiting the dosage, coating NPs with PEG to reduce the agglomeration, designing biodegradable or metabolizable contrast agents, and so forth. In general, the photophysical property of PA contrast agents can be tuned under the guidance of the Jablonski diagram. Under this guidance, various NPs as PA contrast agents had been widely used in PA brain imaging with good contrast. Recently, PA contrast agents with excited-state intramolecular motion also showed great potential in regulating the balance between radiative and nonradiative decays. The development of nanofabrication technology and the abundant characterization methods have allowed researchers to prepare NPs of desired sizes and morphologies. Active brain targeting mediated by a receptor or cell membrane with homotypic-targeting ability has enabled the NPs to cross in vivo barriers and target brain tumors. With the assistance of proper exogenous PA contrast agents, various brain functions and disorders can be monitored by PA brain imaging due to the deep penetration depth of up to a few centimeters and high resolution of several micrometers of PAI.
While a number of encouraging PA brain imaging results have been demonstrated, to date, a satisfactory non-invasive strategy with the balance between safety and effectiveness has yet to be achieved. Hence, a deeper understanding, more investigations and innovations are necessary to systematically enhance the brain tumor–targeting efficiency and the PA signal generation of nanomaterials. For brain imaging, the skull and scalp may severely attenuate light and degrade deep brain imaging quality. Increasing tissue penetration depth and reducing scattering of light in the NIR-II window endow the diagnosis of deep-seated brain tissues with enhanced SBR. Another potential strategy to reduce the acoustic distortion from skull is to map the bone profile using some other modalities, such as X-ray or ultrasound CT, and incorporates it into PA tomography reconstruction.
Meanwhile, absorptive and receptor-mediated transcytosis have shown great potential in addressing the low-crossing efficiency of passive diffusion through disordered BBB. In terms of biosafety, the potential toxicity of PA contrast NPs to human health and brain functions is still poorly understood. As the nervous system is quite vulnerable to toxins, the side effect during brain PAI, such as the potential neurotoxicity caused by the chemical or physical agents and the photothermally induced neuronal firing, is the unique challenge in brain imaging that needed to be evaluated. To speed up the preclinical and clinical imaging with high efficiency, it is tactful for material chemists to work with neurosurgeons and neuroscientists to better understand the destination of contrast agents and how the PAI can help in reflecting the structural and functional changes of brain in different brain disorders.
In the near future, the booming development of contrast agents–assisted PAI techniques would further help researchers to understand how the brain functions, monitor the brain physiological indexes (brain glucose level etc.), promote the diagnosis of brain-related diseases, and advance the PA guidance of brain therapy.
ACKNOWLEDGMENTS
We thank Dr. Arunima Sharma for her valuable discussion. The authors acknowledge the financial support from the Singapore NRF Competitive Research Program (grant no. R279-000-483-281) and the National University of Singapore (grant no. R279-000-482-133).
CONFLICT OF INTEREST
The authors declare no conflict of interest
Biographies

Xingang Liu received his Ph.D. in Chemistry from Zhejiang University in 2018 under the mentorship of Prof Guping Tang. Then he joined the Department of Chemical & Biomolecular Engineering, National University of Singapore (NUS), as a postdoctoral research fellow under the supervision of Prof. Bin Liu. His research focuses on developing molecular imaging tools for cancer theranostics and microbial detection.

Yukun Duan is currently a research engineer and Ph.D. candidate under the supervision of Prof. Bin Liu at the Department of Chemical & Biomolecular Engineering, NUS. His major research interest lies in the development of novel organic and inorganic contrast agents for multimodal imaging applications, including fluorescence, photo-acoustic, and magnetic resonance imaging.

Bin Liu received her PhD degree in Chemistry from the National University of Singapore in 2001. After postdoctoral training at the University of California Santa Barbara, she joined NUS where she is currently a Chair Professor in the Department of Chemical and Biomolecular Engineering. Her research focuses on the design and synthesis of functional polymers and organic nanomaterials and exploration of their applications in sensing, imaging and optoelectronic devices.




