Entropically favoured through space charge transfer ‘lighted’ photosensitizing assemblies for ‘metal free’ regulated photooxidation of alcohols and aldehydes
Abstract
Strong acceptor-weak acceptor system FN-TPy has been designed and synthesized, which undergoes solvent dependent self-assembly in mixed aqueous media to generate through space intermolecular charge transfer assemblies. The as-prepared entropically favoured assemblies of FN-TPy exhibit excellent photostability and photosensitizing properties in the assembled state to activate aerial oxygen for efficient generation of reactive oxygen species through Type-I and Type-II pathways. The FN-TPy exhibits excellent potential for regulated oxidation of alcohols and aldehydes under mild reaction conditions (visible light irradiation, aqueous media, room temperature) using aerial oxygen as the ‘oxidant’. The present study demonstrates the potential of FN-TPy assemblies to catalyse controlled oxidation of benzyl alcohol to benzoic acid.
1 INTRODUCTION
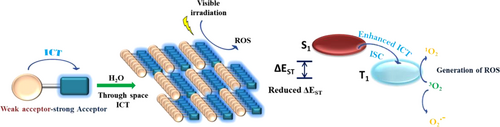
Visible light-driven sensitized oxidation of organic compounds is a sustainable approach for preparation of synthetically important building blocks.[1-3] Photosensitization enables activation of oxygen to generate reactive oxygen species (ROS), which eventually acts as the potent ‘oxidants’ in the oxidative organic transformations. Besides having strong absorption in the visible region, the impeccable photosensitizers are expected to exhibit high photostability, ‘lighted’ and long excited state for efficient catalysis.[4] A variety of metal-based and ‘metal free’ (commercially available and synthetic dyes) photosensitizers have been developed for facilitating the oxidative organic transformations efficiently.[5, 6] Apart from the cost of precious and toxic metal complexes, their less natural abundance and requirement of having supporting co-catalyst makes the metal-based photosensitizers less popular.[7, 8] Among ‘metal-free’ organophotosensitizers, photosensitizers based on commercially available dyes, though economic, have low photostability, and laborious synthetic strategies are needed to bring about any structural alteration for desirable photophysical properties.[9] Furthermore, due to their narrow absorption range, requirement of high energy radiation source (Blue LED) is mandatory for achieving good catalytic outcome. Alternatively, with meticulous designing and less synthetic inputs, it is relatively convenient to prepare ‘metal-free’ efficient organophotosensitizers based on synthetic dyes.[10] Since most of the reported organophotosensitizers are designed for photodynamic therapy (PDT) as the target application, hence, much research efforts are focused on developing new strategies to improve their sensitization potential. Consequently, a variety of approaches such as photon upconversion, modulation of intersystem crossing (ISC), incorporation of heavy atoms have been developed to enhance ROS generation in designed ‘metal free’ materials.[11, 12] In case of PDT, the duration is quite short (several minutes); thus, photostability of the photosensitizer is not a matter of concern. On the other hand, in case of photosensitized organic transformations, photostability of photosensitizer is of utmost importance. Unfortunately, there is scarcity of systematic studies on understanding various structural parameters for developing photostable sensitizers. Very recently, development of a cross-linked conjugated polymer based on donor (triphenylamine)-acceptor (fumaronitrile) building block has been reported for photooxidation of aromatic aldehydes where polymerization enhanced the photostability of the material.[13] Although the as-prepared polymer showed ‘quenched’ excited state and poor solubility in common organic solvents, the polymerization-induced modulation of singlet-triplet energy gap resulted in efficient ISC and, hence, high catalytic activity.[14, 15] Interestingly, monomeric ‘lighted’ donor-acceptor building block of the cross-linked ‘quenched’ polymer shows promising applications in the preparation of optoelectronics devices due to its efficient reverse ISC (RISC).[16, 17] The subtle interplay between ‘lighted’ molecular and ‘quenched’ polymeric level for RISC/ISC encouraged us to develop ‘lighted’ organophotosensitising supramolecular assemblies using noncovalent interactions in aqueous media. We planned to undertake the study focusing on the impact of supramolecular polymerization on photostability, sensitizing and catalytic efficiencies of the as-prepared assemblies. Designing a photosensitizer around donor-acceptor (D-A) scaffold is a tested strategy to achieve absorption in the visible region and charge transfer state with efficient ISC. The D-A systems having intramolecular charge transfer (ICT) state are known to exhibit quenched excited state in polar protic media, and thus the development of materials having high luminescence efficiency in excited state/aqueous media is a challenge. It is important to focus on structure-function relationship to establish a rational design strategy to achieve luminescent materials having desired optoelectronic properties. Recently, a variety of approaches such as incorporation of electron donating and electron accepting moieties with depressed ICT properties,[18] development of π-conjugated materials[19] having multi-photon absorption[20] and thermally activated delayed fluorophor with multiple through space charge transfers have been explored to prepare emissive materials. The D-A systems which show through space intermolecular charge transfer are known to exhibit ‘lighted’ excited state. Through space charge transfer materials exhibit high photostability, low singlet-triplet energy gap and emit at longer wavelength with large Stokes shift[21, 22] (Scheme 1). D-A systems which show ICT are known to act as triplet sensitizers for the light amplification.[23] Due to their efficient energy transfer and carrier transportability, potential of through space energy transfer materials has been very well explored for the fabrication of optoelectronic devices.[24, 25] With an aim to develop through space intersystem charge transfer materials, we planned to develop a building block having potential to undergo self-assembly and strong ICT state. Recently, strong acceptor-weak acceptor systems have been reported, which exhibit strong ICT.[26] These studies inspired us to develop a strong acceptor-weak acceptor building blocks for the prepration of ‘lighted’ photosensitizing assemblies, and thus, we designed and synthesized FN-TPy derivative having fumaronitrile as the strong acceptor and terpyridyl unit as the weak acceptor. We chose fumaronitrile as the scaffold due to its known potential as strong acceptor. The presence of cyano groups is further expected to lower the HOMO-LUMO energy gap, while the terpyridyl unit is selected as weak acceptor due to its well-established contribution towards aggregation-induced emission enhancement activity (AIEE).[27-29] We envisage that the presence of multiple aromatic rings in the molecule may enhance aggregation potential of FN-TPy in aqueous media through intermolecular π-π stacking, which could lead to generation of through space charge transfer assemblies. As expected, FN-TPy exhibits interesting photophysical, aggregation and photosensitizing properties in mixed aqueous media.
The present study demonstrates the systematic morphological modulations of ICT blue light emitting FN-TPy molecules to emission-enhanced assemblies and finally generation of entropically favoured stable yellow emitting assembsslies. Various experimental observations suggest the cooperative assembly of FN-TPy molecules to generate entropically favoured intermolecular charge transfer assemblies in mixed aqueous media supported by intermolecular π-π interactions, hydrophobic effect etc. The FN-TPy molecules in assembled state exhibit excellent photostability and photosensitizing properties in comparison to that in dispersed state. To understand the validity of design, we also prepared building blocks TPy-FN-Br, TPy-FN-TPy and TPy-PCQ-TPy having heavy atom/additional weak acceptor groups. Increasing the number of additional ‘weak acceptor’ groups in derivative TPy-FN-TPy did not bring any significant change in the photosensitized efficiency of the system. On the other hand, on switching the acceptor unit from fumaronitrile to pentacenequinone in TPy-PCQ-TPy and upon introducing heavy atoms (bromine) ROS generation through type II pathway was significantly affected. Among the various systems examined, the FN-TPy through space assemblies in H2O:THF (8:2) enabled the photooxidation reactions of aldehydes and alcohols in aqueous media using air as an oxidant. In literature, a variety of metal-based, ‘metal free’ catalytic systems have been reported for the oxidations of aldehydes/alcohols, which require ‘oxygen environment’, heating at high temperature to furnish the oxidized product, and in most of these cases the oxidation is unregulated.[30-32] On the contrary, FN-TPy assemblies could catalyse the regulated oxidation of aldehyde and alcohol to furnish easy to purify target compounds under mild conditions using visible light radiations as source of energy (Scheme 2) (for details, see Supporting Information Tables S1 and S2).
2 RESULTS AND DISCUSSION
2.1 Synthesis and characterization of fumaronitrile-based derivative
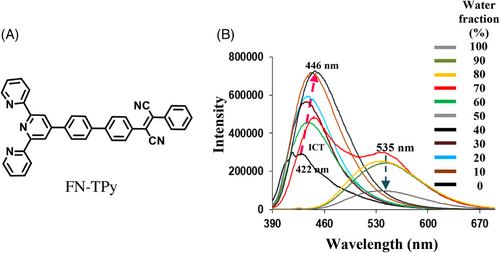
The Suzuki–Miyaura protocol was followed for the synthesis of FN-TPy by carrying out the reaction between 2,3-bis(4-bromophenyl)fumaronitrile[33] and terpyridinylphenyl boronic acid to furnish FN-TPy derivative in 53% yield (Figure 1A). The structure of the compound was corroborated by various spectroscopic and analytical studies (Supporting Information, Figure S1A-D).
2.2 Photophysical behaviour of compound FN-TPy
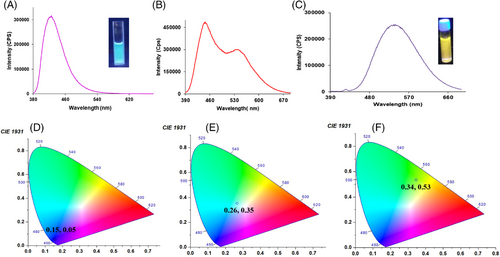
After the complete structural characterization of compound FN-TPy derivative, we examined its photophysical behaviour using absorption and emission spectroscopy. The Ultraviolet-visible (UV-vis) spectrum of FN-TPy in tertahydrofuran (THF) shows an intense absorption band at 290 nm corresponding to π- π* transitions of terpyridyl group[27] and low energy ICT band at 360 nm. The solvent dependent absorption studies of derivative FN-TPy show red shifting of the absorption band from 360 nm to 366 nm upon switching the solvent from nonpolar (hexane) to polar (MeOH) solvent (Supporting Information, Figure S5).[34, 35] The solvent dependent emission studies also show positive solvatochromism, and both of these studies support the presence of charge transfer state in compound FN-TPy. To examine the self-assembly behaviour, we carried out absorption and emission studies of FN-TPy in mixed aqueous media. On addition of water fractions in the THF solution of FN-TPy up to 60% (volume fraction), an enhancement in the absorption intensity was observed. Further, increase in water fraction up to 80% results in red shifting in the absorption band from 360 nm to 380 nm with the decrease in absorption intensity (Supporting Information, Figure S6). In the fluorescence spectrum, THF solution of FN-TPy exhibits an emission band at 422 nm (φ = 0.06) when excited at 370 nm, upon addition of water fractions up to 60%, the emission band is red shifted to 446 nm and its emission intensity is enhanced (φ = 0.21). The red shifting of the emission band is attributed to the formation of J-aggregates, and the increase in the emission intensity of FN-TPy in H2O:THF (6:4) solvent is attributed to the AIEE characteristics of the molecule. The AIEE behaviour was further confirmed by viscosity dependent emission studies of FN-TPy in DMSO-glycerol solvent mixture (Supporting Information, Figure S7). Interestingly, upon increasing the water fractions up to 70%, dual emission was observed due to the appearance of a red shifted (Δ = 89 nm) emission band at 535 nm (Figure 1B). These studies suggest coexistence of J-type assemblies and intermolecular charge transfer assemblies in the reaction mixture. When the water fraction was increased up to 80%, the band at 446 nm disappears completely, and the intensity of the band at 535 nm decreases (φ = 0.09). Further, to understand the dual emission, we performed an experiment using FN-TPy assemblies in H2O/THF (8:2) solvent mixture. On gradual addition of THF (1–150 μl) to the 80% aqueous solution of FN-TPy, the emission intensity of the band at 535 nm corresponding to through space charge transfer assemblies decreases and a new band corresponding to J-aggregates appears at 446 nm (Supporting Information, Figure S8). Thus, by controlling the H2O:THF ratio in solvent mixture, dual emission can be obtained due to coexistence of through space charge transfer assemblies and J-aggregates. The emission studies of FN-TPy in mixed aqueous media clearly indicate solvent dependent switching of emission from blue (Commission Internationale de I'Eclairage (CIE) coordinates at 0.15, 0.05, THF) to yellow (CIE coordinates 0.34, 0.53, [H2O:THF, 8:2]) in the presence of water as co-solvent (Figure 2). The appearance of emission band at 535 nm in H2O:THF (8:2) solvent mixture with a Stokes shift of 155 nm indicates the existence of through space intermolecular charge transfer assemblies of FN-TPy.[36] The temperature dependent emission studies show slight blue shifting (∼2–3 nm) of emission band at 535 nm with decrease in emission intensity (Supporting Information, Figure S9). The emission studies of the solution of FN-TPy (H2O:THF, 8:2) in the presence of trifluoroacetic acid show quenching of emission band at 535 nm, which confirms the generation of intermolecular charge transfer assemblies in mixed aqueous media (Supporting Information, Figure S10).[37]
The time-resolved fluorescence studies of FN-TPy in THF show the biexponential decay and lifetime up to 2.71 ns (85%) when measured at 422 nm. In H2O-THF (6:4) solution of FN-TPy, lifetime of 3.26 ns in major fraction (91.41%) and decay time of 0.40 ns in small fraction (8.59%) of the molecules are observed when measured at 446 nm. The increase in lifetime in H2O-THF (6:4) mixture suggests the formation of ordered aggregates, which supports the enhancement of emission intensity as observed in fluorescence spectrum of the solution. Further, in H2O-THF (7:3) solvent mixture, the lifetime of major fraction (85.97%) is further increased to 3.33 ns, and a decrease in decay time to 0.295 ns in minor fraction of molecules (14%) was observed when measured at 446 nm. On the other hand at 535 nm, shorter lifetime of 0.08 ns is observed in case of major portion (65.3%) of molecules. The decrease in the decay time of molecules of FN-TPy in H2O:THF (7:3) solvent mixture is attributed to the energy transfer between the blue emitting assemblies and the intermolecular charge transfer assemblies of FN-TPy. In H2O-THF (8:2) solvent mixture of FN-TPy, triexponential decay up to a timescale of 2.40 ns in major fraction of molecules (48%) and 9.64 ns (38%) and 0.28 ns (13%) in the minor fractions was observed when measured at 535 nm (Supporting Information, Figure S11 and Table S3). The increase in lifetime indicates the formation of ordered assemblies.
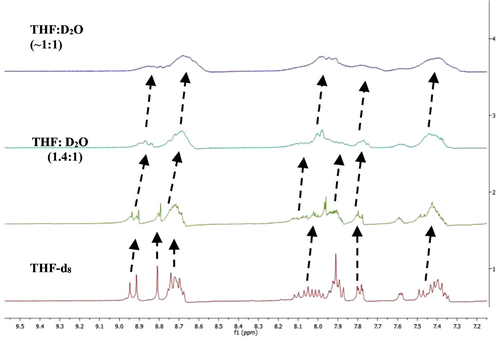
To understand the self-assembly behaviour of FN-TPy molecules, we carried out nuclear magnetic resonance (1H-NMR) studies of FN-TPy in THF-d8 (500 μl) and THF-d8-D2O (1:1; v/v) solvent mixtures. The significant upfield shift and broadening of all the aromatic protons was observed upon gradual addition of D2O (350-450 μl) to the THF-d8 solution of FN-TPy, which indicates increased intermolecular π-π stacking between FN-TPy molecules in mixed aqueous media (Figure 3). The powder X-ray diffraction (XRD) studies of FN-TPy show sharp peaks at 2θ of 20.39°, 24.37° and 25.72° with d-spacing value of 4.35 Å, 3.65 Å and 3.46 Å, which supports intermolecular π-π stacking of the FN-TPy molecules.[38] (Supporting Information, Figure S12).
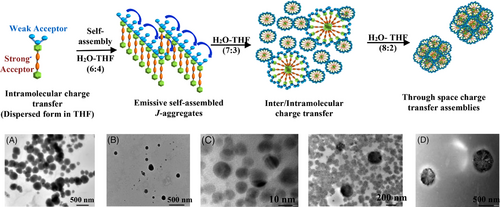
The transmission electron microscopy (TEM) and dynamic light scattering (DLS) studies of FN-TPy suggest the presence of spherical assemblies having size in the range of 320–340 nm in THF while dispersed spheres having size in the range of 220–260 nm in case of H2O-THF (6:4) solvent mixture. The TEM image of FN-TPy in H2O-THF (7:3) solvent mixture shows the presence of small as well as large size of spherical assemblies (Figure 4). The DLS studies support the presence of two different sets of particles with size distribution of ∼18 nm (12%–15%) and 150–180 nm (50%–60%) (Supporting Information, Figure S13A-D). On the other hand, dispersed spheres are observed in the TEM image of FN-TPy in H2O-THF (8:2) solvent mixture.
To get more information about the assembling process, we carried out the isothermal titration calorimetric studies of FN-TPy at 25 °C in THF and mixed aqueous media. The dissociation of FN-TPy aggregates induced by the introduction of THF to the highly concentrated solution of FN-TPy produces an endothermic heat flow (Supporting Information, Figure S14), which indicates that the disaggregation is entropically driven (ΔS = 4.478 KJ mol−1 deg−1) and enthalpically disfavoured, or aggregation is enthalpically favoured and entropically disfavoured in THF.[39] Interestingly, the disassembly of aggregates in H2O-THF (7:3) solvent was found to be enthalpically favoured, or aggregation is enthalpically disfavoured and entropically favoured (ΔS = 7.74 KJ mol−1 deg−1). Further disassembly of aggregates of FN-TPy in H2O-THF (8:2) was found to be more enthalpically favoured, or aggregation in H2O-THF (8:2) is strongly entropically favoured (ΔS = 171.5 KJ mol−1 deg−1). Contribution of positive entropy towards aggregation may be attributed to the release of ordered water molecules in bulk during the self-assembly of FN-TPy.
The solvent dependent emission and subsequent morphological changes are attributed to the modulations of packing arrangement of the molecules upon introducing water as the cosolvent. As evident from the above studies, initially, upon introduction of water fraction upto 60% to the THF solution of FN-TPy, emissive aggregates are formed due to restriction to motion attributed to AIE phenomenon and J-aggregation. Increase in water fractions up to 80% led to further aggregation of these emissive assemblies to form entropically favoured through space intermolecular charge transfer assemblies. In accordance with various spectroscopy studies, we believe that hydrophobic effect, favorable entropical changes and enhanced intermolecular π-π stacking upon self-assembly are the key contributing factors towards the generation of these assemblies.
The cyclic voltammetry studies of FN-TPy in ACN using Ag/AgCl as reference electrode show the ground state reduction potential of −0.85 V for the compound (Supporting Information, Figure S15). The HUMO–LUMO gap (∆Egap) determined from the absorption studies comes out to be 3.2 eV. These studies support the potential of FN-TPy to activate aerial oxygen.
Further, we examined the photostability of the solution of FN-TPy in THF and various H2O/THF solvent fractions by continuous exposure to visible light radiations. No significant change in the absorption behaviour was observed after continuous irradiation of the solution of FN-TPy in H2O/THF (8:2) solvent fractions for 48 h, which indicates the high photostablity of assemblies of FN-TPy in mixed aqueous media (Figure 5C) (for details see Supporting Information, Figures S16 and S17). The THF solution of FN-TPy shows lowest photostability. An increase in photostability was observed upon introducing the water as cosolvent in the THF solution, which supports the contribution of supramolecular aggregation towards enhancing the photostability of the assembled architecture.

Encouraged by the high photostability of the ‘lighted’ FN-TPy assemblies, we examined their photosensitizing properties. To start with, we examined the potential of assemblies of FN-TPy in H2O/THF (8:2) solvent mixture to transport electron under visible light irradiation using methyl viologen (MV2+) as the electron acceptor and triethanolamine as the sacrificial donor. Within 5 min, the color of the solution changed from colorless to dark blue and the absorption spectrum of solution of FN-TPy in the presence of sacrificial donor and methyl viologen showed the appearance of two new bands at 395 and 603 nm corresponding to the reduced cationic species of (MV⋅+) methyl viologen (Supporting Information, Figure S18). Next, we examined the potential of assemblies to generate ROS (singlet oxygen and superoxide radical anion) using 9,10-anthracenediyl-bis(methylene) dimalonic acid (ABDA) and N,N,N′,N′- tetramethyl-p-phenylenediamine, respectively. These studies indicate the generation of singlet oxygen via quenching of absorbance intensity of ABDA (up to 79%, within 15 min) and superoxide radical generation within 3 min in case of FN-TPy in H2O/THF (8:2) solvent mixture (Figure 5A,B) (for details see Supporting Information, Figures S19 and S20). The solution of FN-TPy in H2O/THF (7:3) solvent mixture took 30 min for electron transportation, 5 min to generate superoxide radical and 55% quenching absorbance of ABDA in 30 min. On the other hand, the assemblies of FN-TPy in THF and H2O/THF (6:4) exhibit no potential to transport electron from acceptor to donor molecules (Supporting Information, Figures S21). No evidence was found for the generation of singlet oxygen and superoxide radical in case of THF solution of FN-TPy. Although assemblies of FN-TPy in H2O/THF (6:4) solvent mixture did not show any potential for electron transportation, but these assemblies could activate aerial oxygen to generate superoxide radical in 15 min and show 39% ABDA degradation in 5 h. These results clearly show the systematic enhancement in the electron transportation ability and generation of ROS via type I and type II reactions in case of FN-TPy assemblies upon increasing water portions in the THF solution. These studies also confirm the enhancement of photosensitizing ability of FN-TPy molecules upon supramolecular aggregation.
To understand the effect of additional acceptor groups on the photophysical properties, we also prepared TPy-FN-TPy and TPy-PCQ-TPy building blocks by following Suzuki–Miyaura protocol (Figure 6). The reduction potential of TPy-FN-TPy was found to be in the same range (−0.85 V) as that of FN-TPy derivative, whereas TPy-PCQ-TPy derivative shows reduction potential of −1.01 V (Supporting Information, Figure S22). The TPy-FN-TPy and TPy-PCQ-TPy derivatives also show the formation of through bond charge transfer assemblies in the mixed aqueous media (H2O/THF (8:2)). The TEM images of TPy-FN-TPy and TPy-PCQ-TPy derivatives in their respective H2O/THF (8:2) solvent mixture show the presence of spherical assemblies (Supporting Information, Figures S24-S27). The through space charge transfer assemblies of TPy-FN-TPy and TPy-PCQ-TPy exhibit high photostability in their respective H2O/THF (8:2) solvent mixture. Despite the presence of additional weak acceptor in case of TPy-FN-TPy assemblies, almost similar photosensitizing properties were observed as in case of FN-TPy assemblies. Thus, the presence of additional weak acceptor was not of much advantage. On the other hand, TPy-PCQ-TPy assemblies in H2O/THF (8:2) solvent mixture show high efficiency for electron transport ability (2.5 min) as well as for superoxide radical generation (2 min), but the singlet oxygen generation was found to be slow (67% in 3 h) (Supporting Information, Figures S29 and S30). We believe that TPy-PCQ-TPy assemblies due to the presence of highly conjugated system could well stabilize the external electron, thus, ROS generation via Type I pathway was more efficient; however, due to the presence of moderate acceptor unit of moderate strength (pentacenequinone, PCQ), HOMO-LUMO gap is not sufficiently low to generate singlet oxygen efficiently.
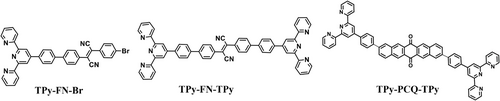
To understand the effect of heavy atoms, we also prepared TPy-FN-Br and examined its photophysical and photosensitizing properties. Although TPy-FN-Br exhibited formation of through space assemblies in aqueous media, the generation of ROS via Type-II mechanism was delayed in this case (Supporting Information, Figures S23 and S28).
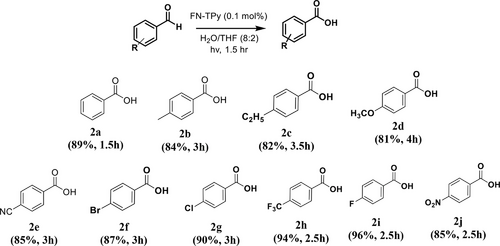
Excellent photosensitizing properties of FN-TPy assemblies in H2O/THF (8:2) solvent mixture prompted us to examine their activity in ‘metal-free’ photooxidation of benzaldehydes as the test reaction. To begin with, we carried out the model reaction by using benzaldehyde (2a) and assemblies of FN-TPy in H2O/THF (8:2) (0.1 mol%, 0.2 mg FN-TPy in 200 μl H2O/THF (8:2)) as photocatalyst (Scheme 2). After 15 min, the conversion of benzaldehyde to benzoic acid initiated as indicated by appearance of crystals of acid around the flask, and after 1.5 h the transformation was complete as indicated by thin layer chromatography (TLC) in 89% yield. These studies support the regulated oxidation of benzaldehyde under ‘green’ conditions using FN-TPy assemblies as catalyst in comparison to other literature reports (Scheme 2 and Table S1, Supporting Information).40-42
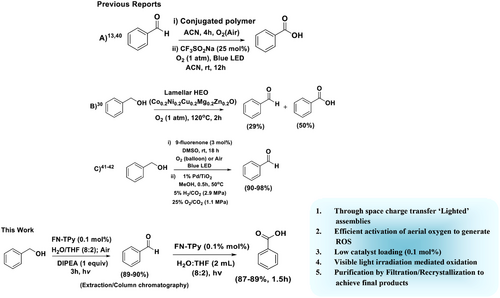
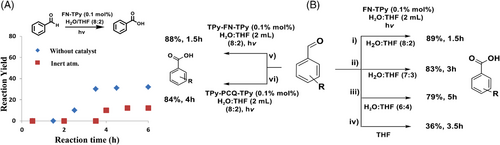
For the optimization of reaction conditions, we carried out the model reaction of benzaldehyde with less loading of catalyst (0.05 mol%), which results in slight reduction in the product yield. Further, to check the role of catalyst, we performed the model reaction in the absence of any catalyst, which results in the formation of the target product in lower yield (32%) in 3 h (Scheme 3A). When same reaction was carried out under inert atmosphere, as expected, no oxidation of benzaldehyde was observed. Furthermore, upon switching the solvent of the reaction to H2O/DMSO/EtOH/THF, the desired product was obtained in good yield except in the case of THF. The switching of H2O:THF ratio in the catalytic system from H2O/THF (8:2) to (6:4) led to slight decrease in the product yield (Scheme 3B (ii-iv)). The use of TPy-FN-TPy assemblies as catalyst in the model reaction furnished the desired product in comparable yield, while in the presence of TPy-PCQ-TPy assemblies as catalyst, relatively lower yield of the desired product (84%) was observed, and reaction took longer reaction time (4 h) for completion (Scheme 3B (v-vi)). Similarly, TPy-FN-Br catalysed oxidation reaction was found to be slow (82%, 4 h) which may be attributed to the delayed generation of singlet oxygen. Thus, various control experiments for the optimization of reaction parameters suggest the formation of desired product in excellent yield in the presence of FN-TPY and TPy-FN-TPy assemblies as catalysts (0.1 mol%) under mild reaction conditions. The high photocatalytic efficiency of FN-TPY and TPy-FN-TPy assemblies in H2O: THF (8:2) solvent mixture may be attributed to their relatively fast electron transport and more efficient singlet oxygen generation. Despite the ‘lighted’ excited state, FN-TPy in dispersed state exhibits almost negligible catalytic efficiency (with reference to the 32% yield of the product in the absence of any catalyst, Scheme 3A) due to its low photostability, poor electron transport ability and low efficiency for singlet oxygen generation. On account of comparable catalytic efficiency of FN-TPy and TPy-FN-TPy assemblies, for the next studies, we chose assemblies of FN-TPy as sensitizing catalyst.
The substrate scope with respect to various substituted aldehydes (bearing electron withdrawing/donating groups) was examined, and the respective benzoic acids were furnished in excellent yields under optimized conditions in all the cases. Slightly higher yield of the target product was obtained in case of benzaldehydes having electron withdrawing groups (-CN/Br/Cl/F/NO2/CF3) as electropositive nature of these substrates facilitates the photooxidation reaction under the optimized reaction conditions (Scheme 4).
To understand the mechanistic pathway of oxidation of benzaldehyde, we performed emission studies to examine the interactions between catalytic system and reactant (benzaldehyde). Upon addition of 80 equiv. of benzaldehyde, the band at 535 nm is quenched, and emission band at 440 nm is revived. The Stern–Volmer constant was found to be 3.8 × 105 M−1 (Supporting Information, Figure S31). The aerial exposure of the FN-TPy solution shows partial quenching (up to 58%) in 30 min (Supporting Information, Figure S32). These studies support the possibility of electron transfer between FN-TPy assemblies and benzaldehyde/oxygen. Further, the model reactions in the presence of 1,4-benzoquinone (superoxide scavenger) proceed well to provide the desired product in 70% yield. In the presence of tert-butanol (hydroxyl radical scavenger), the product was obtained in lower yield (30%). When the model reaction was performed in the presence of sodium azide and DABCO (singlet oxygen scavenger), yield of the product was drastically lowered. Further, no product was formed in the presence of TEMPO (Supporting Information, Scheme S1).
Based on these studies, we believe that the reaction proceeds through radical mechanism, and singlet oxygen is the main oxidant under the reaction conditions. The in situ generation of H2O2 during the reaction was confirmed by the absorption studies of the reaction mixture in the presence of horseradish peroxidase (Supporting Information, Figure S33).[43]
Subsequently, on the basis of all the studies, we propose that photoexcited (FN-TPy*) assemblies of FN-TPy activate the molecular oxygen to generate ROS such as (.OH, O2.−) to generate cation radical species (FN-TPy.+), which in turn accepts electron from benzaldehyde to produce radical intermediate B. Intermediate B is transformed to intermediate C, which finally furnishes the desired product (Supporting Information, Scheme S2).
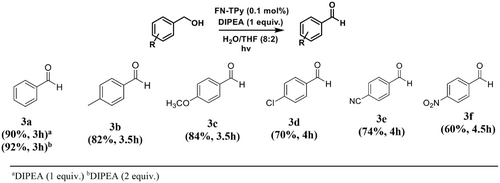
Encouraged by the facile photooxidation of benzaldehyde, we examined the photooxidation reaction of benzyl alcohols using FN-TPy assemblies under optimized conditions. For this, we carried out the model reaction of benzyl alcohol in the presence of 0.1 mol% of FN-TPy and H2O:THF (8:2) as solvent mixture under visible light irradiation. After 3–4 h of the reaction, no transformation was observed, monitored from TLC. Then we repeated the same experiment in the presence of DIPEA (1 equiv.) as a base under the optimized conditions. After 3 h, benzaldehyde was obtained in 90% yield, and the oxidation process was well controlled as the formation of benzoic acid was not detected under the reaction conditions. In comparison to previous literature reports (Scheme 2 and Supporting Information, Table S1), FN-TPy catalysed oxidation of benzyl alcohol is facile, ‘green’ and regulated using visible light radiation as the source of light and no ‘oxygen environment’ is needed. Next, we evaluated the substrate scope for this reaction by using substituted (electron releasing/electron withdrawing groups) benzyl alcohols. Benzyl alcohols bearing electron withdrawing groups furnished the target compound in lower yield and took longer reaction time for the oxidation (Scheme 5).
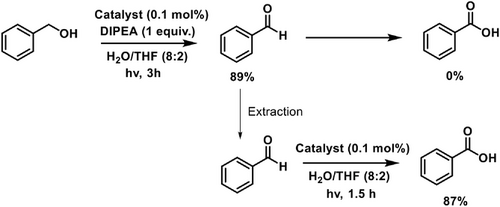
We also examined the possibility of stepwise photooxidation reaction of benzyl alcohol to generate benzoic acid. The oxidation of benzaldehyde proceeds smoothly, and formation of benzoic acid was not observed even after 8–10 h. This may be attributed to the inhibition of the oxidation of benzaldehyde to benzoic acid in the presence of trace amount of benzyl alcohol. To get rid of traces of benzyl alcohol in the repeated reaction, we isolated the benzaldehyde (extraction with organic solvent) after its formation (TLC) and set up its reaction again for further oxidation under the optimized conditions. The desired acid was obtained in 87% yield (Scheme 6). These studies support the potential of FN-TPy assemblies for regulated oxidation of alcohols and aldehydes.
To understand the mechanism of oxidation of benzyl alcohol, first we carried out the emission studies of FN-TPy in the presence DIPEA (80 equiv.), 85% decrease in emission intensity of assemblies of FN-TPy at 535 nm while only 30% quenching of emission band at 535 nm was observed in case of benzyl alcohol (Supporting Information, Figures S34 and S35). These studies are in accordance with the results obtained in the model reactions, and the presence of base is must to facilitate the oxidation of alcohols.
Next, we carried out the model reaction in the presence of 1,4-benzoquinone (superoxide scavenger), the reaction did not furnish the target product while in the presence of tert-butanol as hydroxyl radical scavenger, the product was observed in lesser yield (21%). When the model reaction was performed in the presence of sodium azide/DABCO as singlet oxygen scavenger, trace amount of the product was observed (Supporting Information, Scheme S3).
On the basis of all the experimental results and literature reports, we may conclude that there are two possible mechanistic routes, which involve the participation of FN-TPy.+ radical cation (pathway A) and FN-TPy.− radical anion (pathway B) (Supporting Information, Scheme S4 A).[44, 45] To check the relative participation of these reaction intermediates in the reaction, we performed the density functional theory (DFT) studies, which suggest relatively high stabilization of FN-TPy.− radical anionic species (ΔEM-A = -1.71 eV) in comparison to that of FN-TPy.+ radical cationic species (ΔEM-C = 7.07 eV) under the optimized conditions (Supporting Information, Figure S4B).
On the basis of these observations, we believe that the photoexcited FN-TPy* generates FN-TPy.− radical anion species in the presence of DIPEA (X), which consequently activates the molecular oxygen to generate ROS (such as 1O2, O2.−, .OH). Subsequently, benzyl alcohol is activated to generate intermediate P (radical cation), which in the presence of the singlet oxygen radical and superoxide radical anions furnishes the desired benzaldehyde derivative through the intermediate Q (Scheme 7).

3 CONCLUSION
To conclude, solvent dependent self-assembly of FN-TPy from J-aggregates to entropically favoured through bond charge transfer assemblies has been demonstrated in mixed aqueous media. As-prepared through bond charge transfer assemblies of FN-TPy exhibit strong absorption in the visible region and show sufficient potential to activate aerial oxygen to generate ROS such as superoxide ions and singlet oxygen. The work presented in this manuscript demonstrates the high photosensitizing activity of FN-TPy assemblies for regulated oxidation of benzyl alcohols to benzaldehyde and benzaldehyde to benzoic acid under visible light irradiation and aerial conditions without needing additional additive or pure oxygen environment.
ACKNOWLEDGEMENTS
Vandana Bhalla is thankful to SERB, New Delhi (ref. no. CRG/2018/001274), SERB Power fellowship (ref no. SPF/2021/000019) and CSIR, New Delhi (ref. no. 02(0358/19/EMR-II) for financial support.
ETHICS STATEMENT
There are no ethical issues in this work.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Dr. Vandana Bhalla and Prof. Manoj Kumar has planned, designed and supervised the whole investigation reported in this manuscript. Gurpreet Kaur, Harpreet Kaur and Mandeep Kaur contributed equally to this work.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are openly available at https://doi.org/10.1002/agt2.171, reference number [AGT2171].




