Chiral transfer-dictated self-assembly of chiral block copolymers
Abstract
Chiral structures not only exist in nature widely, they also emerge in artificial systems, attracting myriad attentions due to their excellent mechanical, optical, electrical, and magnetic properties. Self-assembly of chiral block copolymers (BCPs*), where at least one block consists of chiral centers, represents a facile strategy to form helical/spiral/network structures with a controlled chirality. Usually, morphological chirality of BCP* assemblies was closely associated with molecular and conformational chirality of the chiral block. Generally, chiral assemblies arose from molecular chirality of BCPs*, transferring up in the assembly process and dictated the chirality at a higher hierarchical level. In contrast, notwithstanding similar assemblies could be observed from achiral BCPs under certain conditions, both left- and right-handed ones were usually observed simultaneously without a preference. Moreover, unique feature of BCPs* to access to controllable chiral assemblies affords an opportunity to prepare advanced functional materials. Herein, we dedicated a review on assembly of BCPs* into chiral assemblies in bulk/films, selective solvents, and confined spaces. The chiral transfer process in these assembly scenarios were discussed and highlighted as a key contributor to morphological chirality. Functionalities and representative applications of BCP* assemblies were also described, followed by present challenges and future prospects of BCP* self-assembly.
INTRODUCTION
Self-assembly refers to a plethora of spontaneous phenomena that structural units organize into ordered structures.[1] In nature, lipids, proteins, and carbohydrates assemble into biological membranes with recognition and transportation functionalities.[2] In artificial systems, self-assembly endows a well-defined structural order with enhanced spectroscopic, electrical, and mechanical performance.[3-6] Composed of different polymeric blocks with covalent bond junctions, block copolymers (BCPs) display interesting assembly behaviors and afford an essential bottom-up strategy to structurally ordered functional materials, calling for increasing research interests.[7, 8] The thermodynamic incompatibility between blocks played a significant role in the self-assembly of BCPs. In bulk or films, BCPs could self-assemble into spheres, cylinders, gyroids, lamellae, and other phases structures.[9] In selective solvents, spherical micelles, rod-like micelles, vesicles, and lamellar assemblies were commonly observed, whereas their further evolution under certain conditions gave rise to various superstructures, to name a few, loops, giant segmented worms, polymersomes, and large compound micelles.[10-17]
Beyond these classic assembly morphologies, chiral structures are fascinating and appealing, on considering of the ubiquitous existence of chirality in daily life, and the significance of chiral structures to optical, electromagnetic and chiral recognition properties.[8-22] At a molecular level, biological organisms showed a strong selectivity to proteinogenic sinistral α-amino acids. Also, the functionality and toxicity of a great number of medicines were strongly associated with their molecular chirality and chiral purity.[23-25] At a conformational level, the chiral conformation of peptide backbone (e.g., α-helix and β-sheet) resulted from the chiral configuration, encoded precise sequence and intramolecular interactions. At a morphological level, different peptides packed and folded into proteins, twisted fibrils at a nano-scale, and fibers at a micro-scale.[26, 27] In another exquisite and complicated manner, Towel gourd tendrils, Paphiopedilum dianthum petals, and many other plants grew in a chiral direction at a macro-scale, speculatively attributed to the hierarchical assembly of dextral sugar units, in the name of a chiral transfer from low to high hierarchy (Figure 1A).[28, 29]
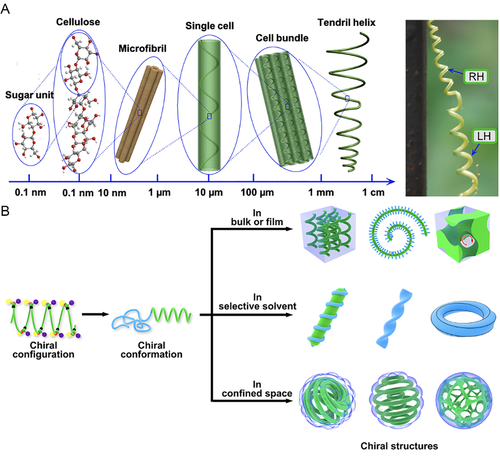
The self-assembly of synthetic chiral molecules features an analogous hierarchical evolution, shedding lights on the vast yet puzzling formation of chiral structures in nature.[31] Besides, it unlocks attractive functionalities for applications. For example, the chiral helical structure of assembled carbon nanotube ropes and electrospun polymeric fibers yielded enhanced deformability and elastic property, when compared to the conventional achiral counterparts.[32-34] However, the formation of synthetic chiral structures and an understanding on the corresponding mechanism and governing principles were usually challenged by the sophisticated assembly behaviors.
With the introduction of chiral centers, chiral BCPs* were capable of self-assembling into chiral structures with a controlled chirality (Figure 1B).[35, 36] Nolte et al. firstly reported the solution assembly of α-amino acid-based BCPs* into chiral helical (helix*) superstructures. [37] In a bulk or film state, Ho et al. claimed a unique helix* phase from polystyrene-block-poly(L-lactide) (PS-b-PLLA) and enantiomeric PS-block-poly(D-lactide) (PS-b-PDLA) BCPs*.[38] Both presentations unveiled unprecedented homochiral characteristics on the morphological level, implying a strong correlation between the morphological chirality and molecular chirality of BCPs*. Not only did extensive studies on BCPs* prove so and underline so, they also deepened the understanding on the assembly behavior of BCPs*. Other fascinating chiral assemblies were evidenced from BCPs*, including chiral networks, roll cake-like spirals, twisted ribbon-like fibers, and Moebius stripes.[36]
However, owing to the complicated assembly behaviors of BCPs*, the understanding on BCP* assembly is still elusive so far. For the self-assembly of BCPs* in bulk/films and selective solvents, there are divergent assertions on how the conformational chirality determined the morphological chirality. For the self-assembly of BCPs* under confinement, it is only until recently that this field received attentions.[39] More essentially and attractively, it is not clear so far how the spatial confinement affects the chiral transfer process and morphological chirality. In a comparison, notwithstanding similar chiral structures could be observed from the self-assembly of achiral BCPs, it is usually challenging to achieve a preferred chirality.[40-48] Due to these interesting phenomena and the remaining challenges in BCP* self-assembly, it is of great emergency to retrospect the development of BCP* self-assembly and pinpoint the recent progress, which may be helpful for the future research in this field.
In this paper, we highlighted the unique characteristics of BCPs* to generate innovative and fascinating assemblies with a controllable morphological chirality, and summarized the rationales correlating them to the molecular chirality of BCPs*, namely, the chiral transfer process. In the second section, the molecular and conformational chirality of BCPs* were basically introduced. In the third and fourth section, we respectively dedicated the self-assembly of BCPs* in bulk/films and in selective solvents. In the fifth section, the to-date progress on the emerging confined assembly of BCPs* was provided. In the sixth section, the functionalities and representative applications of BCP* assemblies were summarized, as well as the potentials they hold. Finally, challenges and future prospects in self-assembly of BCPs* were outlined.
MOLECULAR AND CONFORMATIONAL CHIRALITY OF BCPs*
The utilization of chiral molecules as monomers represents a facile access to BCPs* with an intrinsic chiral configuration (Figure 2A).[36] Such molecular chirality could be characterized by circular dichroism (CD) spectroscopy, where the chiral entities displayed a Cotton effect.[49-51] The vibrational CD (VCD) spectroscopy affords a credible confirmation on the conformational chirality of BCPs*, as well as the intramolecular chiral interaction between the chiral and achiral blocks. The latter could be verified by induced CD (ICD) spectroscopy as well.[52] Additionally, the chiral signals at an excited state could be detected by circularly polarized light luminescence (CPL) spectroscopy.[53] Beyond the chiral configuration and conformation, BCPs* usually carry other characteristics, for example, semicrystalline, liquid crystalline, the tunability/inversion of conformational chirality.[36] For example, the ring-opening polymerization of L-lactide led to PLLA-based BCPs*, whereas enantiomeric D-lactide led to PDLA-based BCPs*. Owing to the regular configuration, the PLLA/PDLA block also showcased a semicrystalline feature.[54] Given the relatively well-established understanding on the synthesis, physical properties, good degradability, and biocompatibility of PLLA/PDLA, there has been a litany of studies on the self-assembly of PLLA-/PDLA-based BCPs*.[35, 38, 55-58]
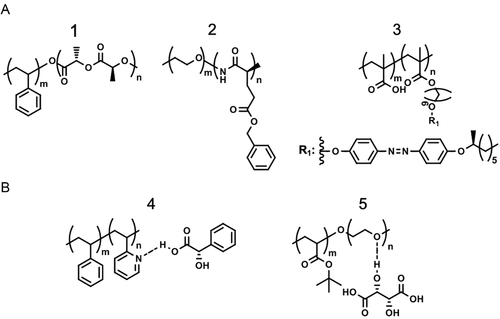
Besides, α-amino acids were also widely employed for the preparation of BCPs*.[37] For instance, PBLG-/PBDG-based BCPs* (PBLG: poly(γ-benzyl-L-glutamate), PBDG: poly(γ-benzyl-D-glutamate)) attracted a large number of studies.[59, 60] More than simply a conformational chirality of the PBLG/PBDG block, the overall molecular mass of BCP*, temperature, and solvent property imposed great impacts on the conformation of PBLG/PBDG.[61-63] For example, Lin and Wang et al. reported a conformational chirality inversion of PEG-b-PBLG upon the changes on temperature and solvent property.[63] Similar stimuli-responsive chiral-achiral conformation switch was found on azobenzene (Azo) pendant group-containing liquid crystalline BCPs*.[64] The conformational chirality of these BCPs* could be demolished by UV light irradiation and recovered by thermal annealing, owing to the reversible trans-cis isomerization of Azo mesogens.[65, 66]
As an alternative to prepare BCPs* with intrinsic chiral centers, selective incorporation of chiral small molecular dopants into achiral BCPs emerged as another strategy to prepare BCPs* (Figure 2B). So far, there have been two class of references: (1) S-/R-mandelic acid (S-/R-MA) was introduced to P2VP-based BCPs (P2VP: poly(2-vinyl pyridine)); and (2) L-/D-tartaric acid (L-/D-TA) was introduced to PEO-based BCPs (PEO: poly(ethylene oxide)) via hydrogen bonding.[67, 68] By learning from the combination of achiral homopolymers and chiral dopants, we suggested the arsenal of BCP/chiral dopant supramolecules could be expanded to PPA-based BCPs (PPA: poly(phenyl acetylene)) and other chiral primary amines and amino alcohols.[69-71]
For intrinsic BCPs*, there is usually a chiral consistency all the way along the monomer configuration, the configuration of chiral polymeric block, and the chain conformation of chiral polymeric block. However, confusing spectral results and assertions on the conformational chirality were found for the achiral BCP/chiral dopant supramolecules.[52, 72] Yu and Lu et al. suggested that the chiral dopants usually led to a reversed conformational chirality for the supramolecular systems, whereas Ho et al. found the opposite tendency.[58, 73, 74] Owing to this difference, the assembly behavior of BCPs* and the chiral transfer from chain conformation to assembly morphology might differ as the chemical structure of BCPs* and assembly scenario varied. Therefore, in the following sections, we respectively dedicated and discussed the self-assembly of BCPs* in bulk/films, selective solvents, and confined spaces.
SELF-ASSEMBLY OF BCPs* IN BULK OR FILMS
As the self-assembly of BCPs occurs in bulk or films, the thermodynamic incompatibility between different blocks enforces their segregation into individual domains, to achieve a minimized interface with the lowest enthalpy. However, this is shadowed by the constrain of the covalent junctions and the tendency of polymeric chains to form a random coil-like conformation. As a result, microphase-separated structures emerge in a balance of the enthalpic drive and entropic penalty.[44] For BCPs*, their self-assembly in bulk/films unlocked several fascinating phase structures, with a controlled chirality usually unattainable from the corresponding achiral BCPs.
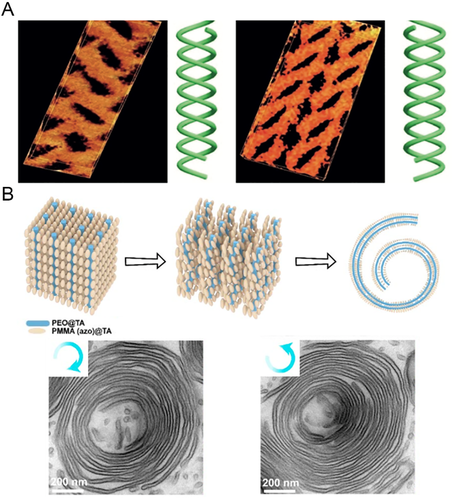
The most studied BCPs* in bulk/films belonged to PS-b-PLLA, where single helixes*, networks, core-shell cylinders, and concentric/roll cake-like spiral lamellae were obtained from a manipulation on the volume fraction of PLLA (fPLLA) and the overall molecular mass.[75, 76] The helix* phase from PS-b-PLLA/PS-b-PDLA BCPs* were systemically characterized by Ho et al.[35, 38, 55-58] In a combination of transmission electron microscopy images (TEM) and small-angle X-ray scattering (SAXS) results, it was evidenced that this intriguing helix* phase contained PLLA/PDLA spring-like single helixes* embedded in a PS matrix, with an interdigitated and hexagonal fashion (Figure 3A).[35] This unique feature was verified by 3D TEM tomography, as well as the morphological chirality. Left-handed homochiral helixes* were identified from PS-b-PLLA with a left-handed conformational chirality, while right-handed homochiral helixes* were formed by PS-b-PDLA BCPs* with a right-handed conformational chirality (Figure 3B).[77]
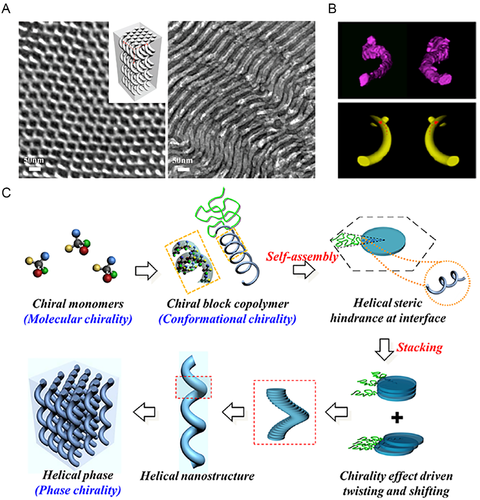
Moreover, it was found that the acquisition of helix* phase from PS-b-PLLA/PS-b-PDLA relied on an overall molecular mass over 20 kDa to ensure a sufficient segregation strength between the chiral PLLA/PDLA and achiral PS blocks, and the volume fraction of PLLA/PDLA (fPLLA/fPDLA) within 35%–39% to realize a columned phase geometry.[38-78] No less than these thermodynamics, assembly kinetics was another essential factor to form a helix* phase from PS-b-PLLA/PS-b-PDLA. When processed by solution casting, the BCP* films must be cast through a rapid solvent evaporation (several hours).[78, 79] Otherwise, order-order transitions from single helixes* to cylinders and double-gyroids took place, experimentally proving the PLLA/PDLA helixes* as a kinetically-trapped metastable structure.[38]
To explain the formation of helix* phase, Ho et al. proposed a chiral transfer from molecular to morphological (phase) level (Figure 3D).[77] The PLLA/PDLA block featured a chiral helical conformation due to the chiral configuration, while the PS block remained a random coil-like conformation. Hence, an intramolecular chiral interaction was formed between the two blocks. When the microphase separation occurred, the chiral conformation and intramolecular chiral interaction caused chiral steric hindrances, which enforced twists and shifts of the columned PLLA/PDLA domains and increased enthalpic penalty of system. To counterbalance this enthalpic penalty and minimize the Gibbs free energy of the system, the intermolecular chain packing of BCPs* was rearranged into a partially ordered state. Thereby, the PLLA/PDLA evolved into spring-like single helixes* in local, and at a higher level, all the helixes* bespoke the same chirality as the PLLA/PDLA conformation. Some of the key elements in this elucidation were extensively studied and manifested, that is, the intramolecular chiral interaction between PS and PLLA/PDLA, and the chiral steric hindrances.[55, 57, 80]
The crystallization of PLLA/PDLA was demonstrated as another key factor to the self-assembly of PS-b-PLLA/PS-b-PDLA BCPs* in bulk/films. By taking advantage of real time in situ SAXS and wide-angle X-ray diffraction, Grason and Russell et al. indicated that the self-assembly of PS-b-PLLA/PS-b-PDLA entailed a sophisticated competition between incompatibility-driven microphase separation and the crystallization of PLLA/PDLA. The later was severely impacted by the solvent property (Table 1).[81] In a PS-selective solvent (e.g., toluene), the chiral transfer process and the formation of helix* phase was prohibited by a rapid precipitation of BCP* chains and PLLA/PDLA crystallization in the early stage of microphase separation. Consequently, a semicrystalline non-helix* phase was formed instead.[81] To obtain the helix* phase with a high order and suppress the destructive impact of PLLA/PDLA crystallization, the PS-b-PLLA/PS-b-PDLA films have to be cast in a good solvent for both blocks (e.g., dichloromethane, chloroform).[78] Trickily, in a solvent with a barely “moderate” selectivity to PS, such as 1,3-dioxolane, a semicrystalline helix* phase was formed, suggesting the helix* phase and PLLA/PDLA crystallization could coexist in a subtle manner.[78] By using thermal annealing treatment to an amorphous helix* phase, Ho et al. showed the PLLA/PDLA crystallization introduced a stress to the microphase separated helix* domains, “stretching” single helixes* into semicrystalline cylinders.[82]
| Phase structure | Solvents |
|---|---|
| Semicrystalline non-helixes* | Benzene, toluene, tetrahydrofuran, methyl ethyl ketone, ethyl acetate, carbon disulfide, and butyronitrile |
| Amorphous helixes* obtainable upon rapid assembly | Chlorinated solvents (dichloromethane, chloroform, 1,2-dichloroethane, 1,1,2-trichloroethane), dibromomethane, and dimethyl formamide |
| Semicrystalline helixes* | 1,3-Dioxolane and chlorobenzene |
| Amorphous non-helixes* | 1-Nitropropane |
Similarly, for PS-b-PLLA/PS-b-PDLA melts, in order to yield a helix* phase, they have to be quenched quickly to avoid the destructive impact of PLLA/PDLA crystallization.[35] By using this method, a core-shell cylindrical phase was formed from the melts of PS-b-PLLA with a minor PS block and major PLLA block (Figure 4A).[83] It was proposed that an inversed helix* phase was first formed, in which PS single helixes* were hexagonally packed in a PLLA matrix. Then, core-shell cylinders derived from a tight scrolling of the PS helixes*.[84] However, when the PS-b-PLLA with a similar fPLLA was cast from a solution state, it was hypothesized that the long PLLA block with a chiral helical conformation displayed a smectic liquid crystalline-resembling behavior, forming bilayer lamellar domains first. With a further scrolling and spiraling induced by the conformational chirality, concentric and roll cake-like spiral lamellae emerged (Figure 4B).[75]
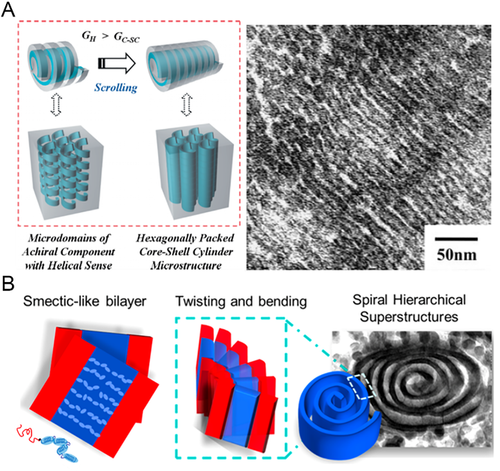
A special chiral alternating gyroid phase could be realized from a more complicated PIP-b-PS-b-PLLA and PIP-b-PS-b-PDLA triblock BCPs* (PIP: polyisoprene).[85] In this network phase, the PLLA/PDLA block formed single gyroids with the same chirality as its configuration and conformation, the PI block formed the single gyroids with the opposite chirality to PLLA/PDLA, and the PS block formed a matrix.
It was shown that the chemical structure and rigidity of the chiral block determined the equilibrium state of the assembled structures and other features, such as crystallization and liquid crystalline property. The self-assembly of PLLA-/PDLA-based BCPs* in bulk/films characterized in a kinetic dependence, and the helix* phase was a kinetically trapped metastable state for these BCPs*.[79] In a sharp contrast, single helixes* could be an equilibrium structure for poly(benzyl methacrylate)-block-poly(L-cyclohexylglycolide) (PBnMA-b-PLCG) and enantiomeric PBnMA-block-poly(D-cyclohexylglycolide) (PBnMA-b-PDCG) BCPs*. The morphological chirality of single helixes* from these BCPs* stayed consistent with the conformational chirality of PLCG/PDCG. [86] It was ascribed to that the PLCG/PDCG blocks possessed a bulkier aromatic pendant group than a methyl one in PLLA/PDLA, endowing the PBnMA-b-PLCG/PBnMA-b-PDCG BCPs* with a higher twisting power in the chiral transfer process and a higher thermodynamic stability to the helix* phase.[87] In addition, the replacement of PLLA/PDLA with PLCG/PDCG avoided the crystallization of the chiral block.
Single helixes* were also accomplished from the supramolecular systems of cylinder-forming PEO-based BCPs (e.g., PS-b-PEO and PtBA-b-PEO) and L-/D-TA, with a L-/D-TA composition of ∼25 wt%.[52] The supramolecular systems of poly(1,4-butadiene)-block-PEO (PBd-b-PEO) and L-/D-TA realized hexagonally-packed double helixes* (Figure 5A), with an L-/D-TA composition of 10–19 wt%, while irregular helixes were formed with 25 wt%.[88] The formation of these chiral phase structures strongly depended on the volume fraction of PEO/TA complex (fPEO/TA).[72] Controversially, these single and double helixes* exhibited a morphological chirality in a consistency with the PEO/TA conformational chirality, but both were opposite to the intrinsically molecular chirality of TA. Thus, the sinistral L-TA gave rise to right-handed conformation of PEO/TA and morphological chirality, while the dextral D-TA gave rise to left-handed conformation of PEO/TA and morphological chirality.[52, 72] This differed from the chiral transfer process of BCPs*, and such controversy has not been unfolded so far.
With high rarity, by introducing L-/D-TA to an achiral liquid crystalline BCP containing a PEO block and Azo pendant groups in the other block (Figure 5B), Lu et al. realized reversible spiral patterns consisting of PEO cylinders in a matrix of the liquid crystalline block.[89] In this demonstration, L-/D-TA interacted with the both blocks via hydrogen bonding, and the morphological chirality of spiral cylinders was closely associated with the intrinsic molecular chirality of L-/D-TA. Owing to the reversible trans-cis isomerization of Azo mesogens, the chirality of patterns was easily demolished under UV light irradiation and restored by thermal annealing. Besides the incompatibility-driven microphase separation, the molecular and conformational chirality of BCPs* impacted the crystallization and liquid crystalline behaviors of BCPs* as well, bestowing a chiral sense to the crystal growth and liquid crystal mesogens orientation.[90-92]
The bulk assembly of BCPs* was also investigated by self-consistent field theory (SCFT) simulations, which echoed the chiral transfer mechanism and the formation of helix* phase.[93, 94] Beyond, it was found the thermodynamically equilibrium states of BCPs* closely depended on segregation strength, BCP* composition (f), and chiral strength (q0), which was correlated with the pitch length of chiral conformation.[95] Moreover, an undulated lamellar phase was predicted, which has not been experimentally accomplished yet from the incompatibility-driven microphase separation of BCPs*.[96]
In brief, the molecular and conformational chirality played an essential and appealing role in the assembly behavior of intrinsic BCPs* and achiral BCP/chiral dopant supramolecules. A chiral transfer from the molecular level to the morphological (phase) level epitomized the incompatibility-driven microphase separation of BCPs* in bulk or films. However, controversies exist and raise a series of questions waiting for an explanation.
Firstly, Ho et al. and Watkins et al. suggested the chiral and achiral blocks in BCPs* possess a higher segregation strength than the corresponding racemic BCPs, which could be ascribed to the intramolecular chiral interaction between two blocks.[38, 67] However, in another probe on polypeptoid-based BCPs*, it was stated that BCPs* exhibited a lower segregation strength (e.g., a lower order-disorder transition temperature).[97] Therefore, there is still lacked understanding on the impact of chiral configuration onto the conformation of BCPs* and the incompatibility between the achiral and chiral blocks.
Secondly, Ho et al. claimed that the achiral block still adopted a random coil-like conformation in the assembled chiral structures, indicated by its silence in VCD spectra.[58, 77] As for the supramolecules of PS-b-PEO/TA and PIP-b-PEO/TA, the PS and PIP displayed VCD signals and a chiral conformation, as well as the PEO/TA complex. Lu et al. hypothesized that the conformation chirality of PS/PIP block might be adopted from a cooperative motion of BCPs, even without a direct interaction to TAs.[52] Hence, it is puzzling why different conformations were demonstrated from the achiral blocks in the intrinsic BCPs* and the non-PEO blocks in supramolecules without a direct interaction with the chiral dopants.
Thirdly, the chiral transfer proposed by Ho et al. highlighted a strict chiral consistency along the monomer configuration, BCP* configuration, chiral block conformation, local structure, and phase structure. In the PEO-based BCP/TA supramolecules, although the helix* phases exhibited the same chirality as the conformational chirality of PEO/TA, both were opposite to the intrinsic molecular chirality of TA.[52, 72] In this case, we suggested it still required further investigation that how chirality developed in the assembly process (chiral transfer) and determined the morphological chirality.
SELF-ASSEMBLY OF BCPs* IN SELECTIVE SOLVENTS
In addition to the incompatibility between different blocks, the interactions between the blocks and solvent contribute to a more complicated assembly behavior of BCPs in selective solvents. The dogma is that, solvent selectivity induces the tight packing of solvophobic blocks into a core structure, and the solvophilic blocks are stretched from the coil-like conformation to a swollen state.[98] The equilibrium state of BCP assemblies is determined by interfacial area minimization between the core and solvent, the repulsion of corona chains, and the entropic penalty.[10-17] The self-assembly of BCPs* in selective solvents basically followed the above principles. However, it showcased other interesting assembly behaviors, due to the chiral conformation.
The first report on the solution assembly of BCPs* belonged to PS-block-poly(isocyano-L-alanine-L-alanine) (PS-b-PIAA) and PS-block-poly(isocyano-L-alanine-L-histidine) (PS-b-PIAH). [37] Through a delicate control on the ionic interactions and pH value of solution, helix* assemblies were observed (Figure 6A), which could be attributed to the helical conformation of PIAA/PIAH blocks, the steric interactions induced by the bulky pendant groups, and a stabilization effect induced by the intramolecular hydrogen bonding of amides. It was interesting that PS-b-PIAA with a right-handed helical conformation formed left-handed twists on the assemblies, whereas PS-b-PIAH with a left-handed helical conformation formed right-handed twists. The morphological chirality was opposite to the conformational chirality, alike the chiral inversion that occurred during the hierarchical assembly in nature.[99]
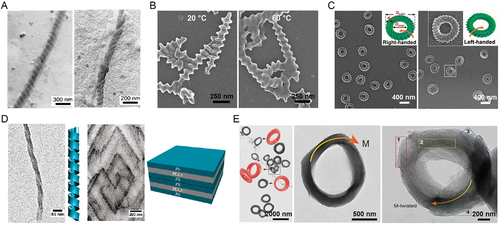
In combination with simulative studies, Cai and Lin et al. reported the co-assembly of PEG-b-PBLG/PEG-b-PBDG BCPs* with PBLG chiral homopolymers. Such co-assembly in selective solvents also accessed to assemblies with a helix* topology opposing to the molecular and conformational chirality of PBLG/PBDG block, emphasizing the pivotal role of molecular and conformation chirality in determining the morphological chirality.[103] In short, the assembly mechanism involved the deposition of amphiphilic PEG-b-PBLG/PEG-b-PBDG onto a template of PBLG.[100, 104] Extensive investigations demonstrated the morphological chirality of these assemblies was also effectively manipulated by temperature and solvent property (Figure 6B).[63] Replacing the rod-like templates by PBLG loops or carbon nanotubes, the PEG-b-PBLG/PEG-b-PBDG BCPs* still enabled a helix* topology (Figure 6C).[100, 104, 105] Also, the same group demonstrated the co-assembly of PEG-b-PBLG/PEG-b-PBDG BCPs* with PS homopolymers into spherical assemblies with striped topologies.[106, 107] It was found that chiral spiral patterns were obtainable from PEG-b-PBLG/PS and PEG-b-PBDG/PS, whereas an achiral meridian-like pattern was obtained from PEG-b-PBLG/PEG-b-PBDG/PS. These works highlighted the significance of the molecular and conformational chirality of BCPs* to the morphological chirality of assemblies, and enriched the formation of spiral assemblies.
Ho et al. also investigated the self-assembly of PS-b-PLLA BCPs* in selective solvents (Figure 6D), finding the molecular and conformational chirality of PLLA block played a key role in the formational of ribbon-like helix* superstructures. Astonishingly, the morphological chirality of these superstructures was in agreement with the molecular and conformational chirality of PLLA.[101] Equally important, the solution assembly of PS-b-PLLA was also demonstrated as a kinetically dependent behavior. A rapid assembly kinetics prevented PLLA crystallization and left solvophobic interaction-driven microphase separation as a dominating governor to the assembly process. In this case, the PLLA chiral conformation likely enforced a shift and twist power that resulted in amorphous helix* ribbons and tubes, resembling the formation of tilted chiral lipid bilayers.[108] In comparison, a slow assembly kinetics provided sufficient time for the PLLA crystallization. Thereby, lozenge-shaped single crystals were formed, announcing the complexity of the solution assembly PLLA-/PDLA-based BCP*, where the crystallization of PLLA/PDLA exerted considerable impacts on the chiral transfer process.
In an intriguing investigation on the self-assembly of PS-b-PDLA BCPs* in selective solvents, Zhu and Yang et al. reported the formation of Moebius stripe superstructures (Figure 6E), which relied on an elegant synergy of solvophobic interaction-driven microphase separation, stirring-induced shearing flow, chiral transfer, and PDLA crystallization.[102] They asserted that the assembly process entailed a chiral transfer from low to high hierarchy. At a molecular level, the PDLA block showed a dextral configuration, which gave rise to a right-handed β-strand conformation. As the assembly proceeded, the PDLA block adapted to a compacted conformation. At a morphological level, right-handed twisted ribbons of PDLA were locally discerned inside the Moebius stripes. Appealingly, at a higher level, the frequency of left-handed twisted topology was overwhelmingly higher than that of right-handed one and achiral one. It was possible that an internal right-handed strain was imposed by the conformational chirality and crystallization of PDLA. To counterbalance this internal strain, at least for a partial release, the Moebius stripes were twisted in a inversed direction, that is, a chiral inversion occurred in the chiral transfer process.[102] This hypothesis symbolized the self-assembly of BCPs* in selective solvents, where similar chiral transfer proposals were also manifested as a rationale to the origin of chiral assemblies.[64, 109]
The solution assembly of double BCPs* containing two chiral polymeric blocks displayed a higher complexity and mystery. Rudra et al. founded that tape-like helix* assemblies only arose from a double BCP* composed of two heterochiral peptide blocks.[110] In a comparison, the BCP* counterparts consisting of two homochiral peptide blocks only assembled into nanofibers. This finding highlighted the significance of an intramolecular chiral interaction between the two blocks for BCPs* to form chiral assemblies.
Recently, some new assembly scenarios in selective solvents have been developed, which could be employed to assemble BCPs* into chiral assemblies. As an alternative to the laborious process of synthesis and solution assembly, polymerization-induced self-assembly (PISA) method accomplished the hierarchical assembly of BCPs* in selective solvents simultaneously as the polymerization of solvophobic monomers proceeded.[64] As the length of chiral solvophobic block increased, chiral assemblies emerged. Zhang et al. recently demonstrated that the formation of helix* fibers via the PISA of BCPs* with Azo mesogens and chiral centers in the pendent groups, where the fibers possessed the same chirality as the molecular and conformation chirality.[64] However, in another study, helix* ribbons of PPA-derived BCPs* (PPA: poly(phenyl acetylene)) displayed a morphological chirality contrary to the molecular and conformational chirality.[109] The reason behind this difference has not been clear so far.
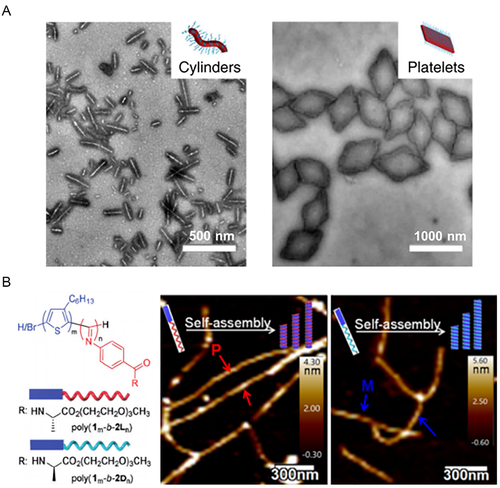
As mentioned above, the crystallization of the chiral semicrystalline block might interrupt the chiral transfer and prohibit the formation of chiral assemblies, since it preferred the BCPs* to from low curvature assemblies.[102, 111] The crystallization-driven self-assembly (CDSA) of PLLA-/PDLA-based BCPs* with a chiral semicrystalline PLLA/PDLA block yielded 1D cylinders and 2D lozenge-shaped platelets (Figure 7A), when crystallization overwhelmed solvophobic interaction as the dominating driving force of assembly.[112-122] In another example, the CDSA of a triple BCP* consisting of three heterochiral peptide blocks formed 2D square-shape platelets.[123] Unprecedentedly, Wu et al. reported the CDSA of poly(3-hexylthiophene)-block-poly(phenyl isocyanide) (P3HT-b-PPI) BCPs* with chiral pendant groups attached to the solvophilic PPI block.[124] It was found that such chiral PPI block led to asymmetric crystallization of the P3HT block in a helical direction. As a result, right-handed helix* fibers were discerned from sinistral P3HT-b-PPI, while left-handed ones were observed from dextral P3HT-b-PPI (Figure 7B). In this case, the molecular and conformational chirality of BCPs* might break the translational symmetry of crystallization and impose a chiral sense to the assemblies.
In brief, the self-assembly of BCPs* in selective solvents bespoke a much higher complexity than the self-assembly in bulk/films. Nevertheless, astonishing chiral superstructures were still accessible from BCPs*, and the morphological chirality was still strongly correlated with the molecular and conformational chirality of BCPs*. In some investigations, the morphological chirality was the same as the molecular and conformational chirality.[64, 102, 125] In more studies, a chiral inversion occurred in the assembly process.[100, 102, 104, 109] It seemed that the morphological chirality favored to be opposite to that of the configuration and conformation in the solution assembly of BCPs*.[126, 127] In addition, in most of these studies, the conformational chirality of BCPs* was consistent to the molecular chirality. In this case, it was puzzling hence worth mentioning that, Wu et al. found a right-handed helical conformation of the sinistral PPI blocks and a left-handed one of the dextral PPI block in both dissolution state and semicrystalline assemblies.[124] Nevertheless, these existing ambiguities highlighted the mysteries of BCP* self-assembly, which might contribute to decoding the chiral transfer of hierarchical assembly in biological organisms.
Consequently, we suggest extensive works were required to elucidate the chiral transfer in the hierarchical assembly of BCPs*, especially on how the chirality at a low hierarchy determined the chirality at a high hierarchy, and how/why the chiral inversion occurred. In addition, the erasing/inversion of morphological chirality by manipulating the stimuli-responsive conformational chirality of BCPs* not only proved the strong relationships between conformational and morphological chirality, but it also represented a novel methodology on morphological control.[128]
SELF-ASSEMBLY OF BCPs* UNDER CONFINEMENT
Spatial confinement imposes considerable impacts on the microphase separation of BCPs, including interfacial interactions between polymers and boundary, symmetry breaking, structural frustration, bending, and curvature.[129-131] As a result, the confined assembly of achiral BCPs leads to unpredicted periodically ordered nanostructures and physical properties, unattainable from self-assembly in bulk/films and selective solvents.[132-136]
Under confinement, the interactions between BCPs and boundary are intensified.[131, 137] With equivalent interfacial selectivity, each block could cover the surface of assemblies. A strong interfacial selectivity to one block attracts it to the surface of assemblies, hence determines the orientation of microphase separated structures.[137, 138] If the confined space is soft and deformable, the interfacial selectivity also affects the overall shape of assemblies.[139-142]
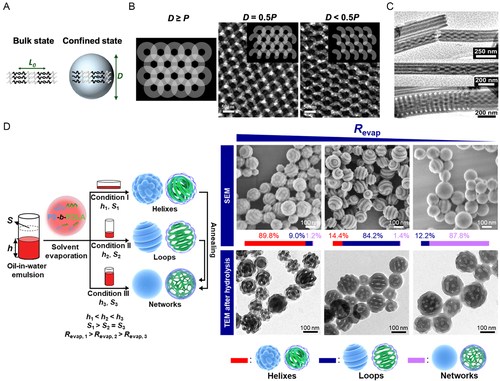
When the dimension of confined space (D) is commensurable with the periodicity of BCP microphase separation (L0, Figure 8A), the periodicity and symmetry of assemblies under confinement is similar to that in a bulk state. When D is incommensurable with L0, the symmetry and periodicity are sabotaged, and frustration is imposed on BCP chains by stretching or compressing BCP chains, which is entropically unfavorable. [130] Thus, the BCP chains tend to further rearrangement into a morphology that could relieve this frustration.[131, 138] These impacts are considerable under a high confining strength (D ≈ L0) and moderate when 3 ≤ D/L0 ≤ 30.[143]
The geometry of confined space is another key factor to the confined assembly, since it introduces bending and curvature to microphase separated structures and shapes the overall shape of assemblies.[131] Under 1D confinement, the microphase separation of BCPs usually takes place in an ultra-thin film.[145, 146] Under 2D confinement, the microphase separation occurs inside a cylindrical or prismatic space (e.g., electrospun fibers, cylindrical pores).[147-149] As for 3D confinement, the microphase separation occurs in a spherical, spheroidal, or polyhedral space (e.g., evaporative emulsion droplets, spherical pores).[139, 150, 151]
As a synergistic outcome of above impacts, chiral structures were formed from the 2D and 3D confined assembly of achiral BCPs, including single/double/multiple helixes and spirals.[148, 152-170] Although these examples differed from each other, demanding different requirements to form the chiral structures, a high incommensurability between D and L0 was usually mandatory, that is, a non-integral D/L0 value.[40, 41, 148, 153-155, 171-174] This high incommensurability ensured the orientation of columned microphase separated domains of BCPs according to the geometry of confined space. However, these chiral structures usually lacked a controlled morphological chirality, and both left-handed and right-handed structures were equally identified.[169, 171, 175, 176] The reason can be ascribed to the fact that there was no free energy difference between these two chiral structures, unless a chiral potential field was deliberately applied.[171, 174, 177-180]
The exploration on confined assembly of BCPs* and understanding on it lag far behind than the ones on the self-assembly of BCPs* in bulk/films and selective solutions, given the sophistication of BCP* assembly behavior and confining effects. Although there has not been a systematic investigation on the 1D confined assembly of BCPs* yet, it is sensible to expect that a low D value would impact the integrity and thread of the helix* domains. Ho et al. discovered that the presentation of helix* phase showed a high sensitivity to the film thickness along helical central axes (Figure 8B).[38] When this dimension (D) exceeded helical pitch length (P), one helical periodicity was exhibited in fullness, giving a honeycomb-like projection to a surface normal to the helical central axes. However, if D was lower than P, the single helixes* became curved cylinders, showing a semicircular or crescent-like projection.
Thurn-Albrecht and Steinhart et al. probed the 2D confined assembly of a lamellae-forming PS-b-PLLA inside rigid cylindrical pores, where the confining strength was moderate (D/L0 = 4.8 and 7.3).[39] This investigation highlighted the considerable impacts of 2D confined cylindrical geometry, interfacial selectivity, and the crystallization of PLLA on the microphase separated structure and the formation of intertwined multiple helixes (Figure 8C). The interfacial selectivity to PLLA lured it to the surface of cylindrical walls and orientated the lamellae into a concentric tubular fashion resembling the stem of scallion. Intertwined multiple helixes arose from an isothermal crystallization of PLLA above the glass transition temperature (Tg) of PS, where the PS and amorphous PLLA segments afforded a soft confined space. It was proposed that the PS-b-PLLA chains suffered from elastic frustrations imposed by the 2D cylindrical confinement, which strongly absorbed outmost PLLA and PS lamellar domains. Then, this frustration was passed through and approached the inner lamellar domains, leading to their breaking into intertwisted helixes. Finally, such microphase-separated structure was preserved by PLLA crystallization. However, the chirality of these helical structures has not been addressed.
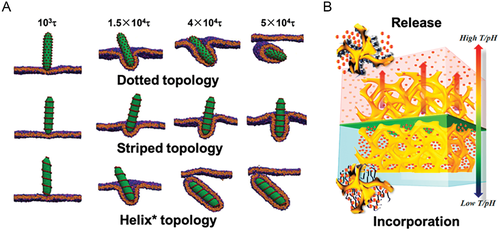
Wang et al. and Gröschel et al. respectively probed the 3D confined assembly of linear and bottlebrush BCPs* containing chiral PLLA blocks.[181, 182] These two contributions manifested that the interfacial selectivity played an important role in the 3D confined assembly of BCPs*, but they did not spot a light on the morphological chirality. Xu and Zhu et al. launched a systematic examination on the 3D confined assembly of helix*-forming PS-b-PDLA BCPs* in evaporative emulsion droplets (Figure 8D), demonstrating the essentialness of solvent evaporation rate (Revap).[30] This work accomplished a kinetic control (by fine-tuning the Revap) on the 3D confined assembly of BCPs* into colloidal assemblies with both controllable topologies and internal microphase separated structures. Rough spheres with an internal helical structure arose from combined impacts of rapid assembly kinetics (i.e., a high Revap), chiral transfer, and 3D confined geometry. Smooth spheres with an internal network structure were obtained from a slow assembly kinetic and demonstrated as an equilibrium state, echoing the kinetically dependent phase evolution of PS-b-PLLA in bulk.[39] Moreover, they found that a moderate confining strength (2.00 ≤ D/L0 ≤ 8.60) granted most particles (89.9%) with a coil-like feature of the internal helical structure, exhibiting a higher complexity with an increasing D/L0 value. Intriguingly, when the thread of these PDLA domains was relatively simple within a range of 2.25 ≤ D/L0 ≤ 4.49, right-handed PDLA helixes* were identified. Such morphological chirality coincided with the molecular and conformation chirality of PDLA. Successive studies from the same group underlined the kinetically dependence of PS-b-PLLA/PS-b-PDLA BCPs* during the 3D confined assembly in evaporative emulsion droplets and demonstrated the formation of chiral particles relied on a rapid assembly kinetics. Furthermore, it evidenced the homochiral transfer from the PLLA/PDLA configuration to the PLLA/PDLA internal helixes*.[183] Not only did these contributions paved a pathway to a controlled confined assembly of BCPs*, it also inspired further investigations on how confinement would impact the morphological chirality of BCP* assemblies.
The solidification of chiral homo-polypeptides and statistical co-polypeptides in evaporative emulsion droplets realized spherical assemblies with a chiral spiral topology, exhibiting a chirality opposite to the molecular and conformational chirality of polypeptides.[184] Although the pertinent mechanism did not involve the incompatibility-driven microphase separation of BCPs*, but nevertheless this work demonstrated the significant role of 3D confined geometry and provoked a debate on the relationship between the morphological and molecular/conformational chirality under confinement.
FUNCTIONALITIES AND APPLICATIONS OF BCP* ASSEMBLIES
Templated synthesis
BCP* assemblies could be utilized as a template to synthesize other materials with the same chiral structures (Figure 9). By taking advantage of chemical/enzymic hydrolysis or UV irradiation, it is feasible to selectively remove partial domains of degradable BCP* assemblies, such as those consisting of PLLA/PDLA (Figure 9A). This selective removal generates channels with preserved structures and functional groups on the walls.[56, 185-187] These ordered channels are applicable as hard templates to be filled with other components through sol-gel reaction, atomic layer deposition, polymerization, electrochemical deposition, electroless plating, and so on. Therefore, a large variety of new materials could be prepared, including metals (Ni, Au, and Ag,), metal oxides (TiO2, ZnO), silicon dioxide, polymers (epoxy), and others.[56, 188-198] Moreover, the co-assembly of BCPs* and inorganic nanoparticles realized chiral spatial arrangement of nanoparticles (Figure 9B).[88, 199-204] A fine-tuning of the dimension and loading of nanoparticles were required to accommodate the nanoparticles inside BCPs* assemblies commensurately.[202-204] As an alternative to these two approaches, it is feasible to implement the co-assembly of BCPs* and precursors into designed assemblies (Figure 9C), then in situ generated chiral inorganic materials, for example, Ni and Ti networks.[205-208]
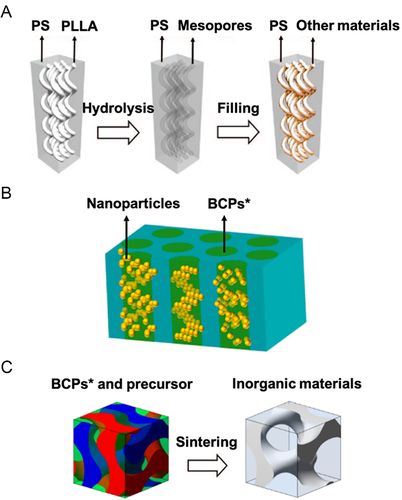
These three approaches yield hybrid materials with a precise morphological chirality, where the removal of the remaining BCP* block gives rise to porous materials, which are able to be further re-filled or incorporated into well-design devices.[209, 210] Despite the complexity of material process and device preparation, the chiral structures from BCPs* and their derivates possess several unique functionalities and appeal to the practical applications, for example, enantioselective membrane separation, catalysts, solar cells, and supercapacitors.[190, 196, 211-215]
Optical and luminescent functionalities
Both helix* and chiral network structures attract application interests because of their optical and luminescent functions. It is found that these functions are tightly associated intrinsic material properties (e.g., metallic or dielectric materials), the morphologies (e.g., helixes, spirals, or networks), the dimension of structural unit, and the spatial arrangement of structural units (the packing order and domain spacing).[216-219] Based upon a manipulation on these factors, it is feasible to tune the optical functions. [217, 220-222]
Chiral helical/spiral/network structures were applied in photonic devices and liquid crystal displays.[209, 217, 219, 223] It is found that plasmon oscillations took place in metallic helix* structures, with a circular polarization dichroic band determined by the plasmon frequency, while photonic stop bands could be induced by dielectric helixes*.[224] Consequently, these helix* structures are applicable as circular polarizers, only blocking the circular polarization light with the same chirality as the morphological chirality of helixes*.[225-228] Moreover, the helix* structures could display a surface-enhanced Raman scattering behavior as well.[229]
Some of the gyroid, primitive, and diamond networks displayed controllable structural colors, making them great choices to prepare photonic materials.[87, 209, 217-219] Specifically, the gyroid networks characterized in a homogeneous feature (e.g., uniform nanoporous geometry, high porosity, high-specific surface area value, and structural symmetry).[188, 189] Gu et al. prepared a chiral beam splitter using photonic chiral networks, which allowed left-handed circularly polarized light to transmit through more, but reflected right-handed circularly polarized light more (Figure 10A).[230] In addition, the inorganic gyroid metamaterials derived from BCP* networks carried other optical functions, for instance, a controllable refractive index and a surface plasmon resonance in near-infrared region.[188, 231]
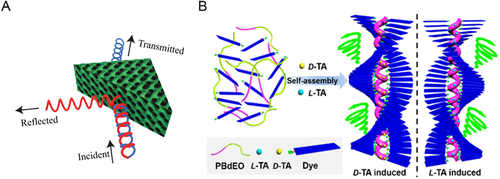
In addition, the structural anisotropy of chiral helical/network structures gifted a birefringent phenomenon under polarized light microscopy, holding a promise in optoelectronics industry.[233] It was found that the aggregation and chiral transfer of chiral small molecules, chiral homopolymers and chiral peptides endowed a chiral optical resonance of pertinent functional groups in the system, such as induced CD (ICD) and circularly CPL emission. [234-236] For BCPs*, Ho et al. manifested that the chiral transfer-dictated self-assembly of PS-b-PLLA into the bulk helix* phase caused the ICD of achiral perylene moiety, which served as the chemical junction of PS and PLLA blocks. Such ICD was due to the intramolecular chiral interaction between PS and PLLA blocks. [77, 80] Moreover, the ICD signals of the perylene moiety were memorized after the removal of PLLA block, presenting a promising chiral memory effect.[237] In another demonstration of Lu et al., a trinary supramolecular system assembled into the bulk helix* phase (Figure 10B), where both L-/D-TA and achiral fluorescent molecules were hydrogen-bonded to the PEO block of PBd-b-PEO. These intermolecular interactions accessed to the bulk helix* phase and the CPL emission of achiral dyes.[232] Besides, the helix* structures hold a good prospect to prepare light-emitting diodes with various colors and a high efficiency. [224, 238]
Beyond the chiral helical/spiral/network phase, it has been manifested that chiral liquid crystalline phases from small molecules displayed chiral optical and luminescent functionalities.[239, 240] These characters might be carried by liquid crystalline BCPs* as well.[89, 92]
Mechanical characters and applications
Helix* structures resemble macroscopic springs with elasticity. The helix* structures coil under a compression or elongate under a stress. Then, they return to the original shape when the compression/stress is released.[212] The pertinent mechanisms have been investigated by theoretical models, and such coiling-recoiling reversible phenomenon has been evidenced on inorganic helixes* at nano-/micro-scale.[28, 32, 213, 214, 241] Further, a shape memory/recovery capability was demonstrated on inorganic ZnO helixes*, prompting these materials as a robust candidate for soft robots.[242]
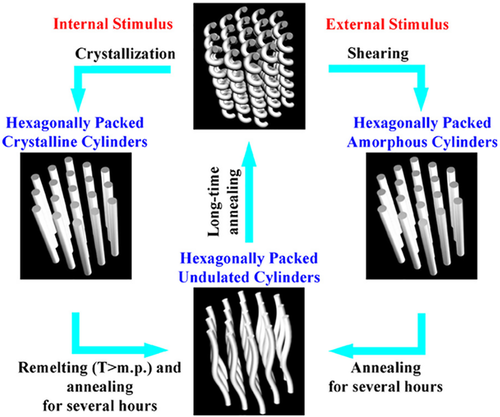
As for BCPs*, the PS-b-PLLA helix* phase showcased a spring-like reversible behavior (Figure 11), similar to the inorganic helixes*.[54] When an amorphous helix* phase was thermally annealed above the Tg of both PS and PLLA blocks, the crystallization of PLLA imposed a stress that stretched the amorphous single helixes* into semicrystalline cylinders. If this cylindrical phase was placed under melting and thermal annealing, it recovered to the helix* phase eventually. Alternatively, if an external shearing was applied to the melts of an amorphous helix* phase, the later was stretched into an amorphous cylindrical phase. Under a long-time thermal annealing, this cylindrical phase recovered into the amorphous helix* phase. Therefore, the spring-like reversible character of the helix* structure opened up a new avenue to nano-energic storage devices.[243, 244] By combining such spring-like behavior and stimuli-responsive capability, the BCP* helix* structures hold a solid promise in sensors, robots (e.g., actuators), and artificial organisms (e.g., artificial muscles).[245-248] The morphological chirality of BCP* helixes* could be a significant factor to the stimuli-responsibility.[248]
Network structures exhibit superior mechanical properties over conventional spherical, cylindrical, and lamellar phase structures.[249] The network structures showed high tensile and compression strengths, uphold ultra-high deformation, and recover both microscopically and macroscopically upon unloading and annealing. It was also manifested that these excellent features were correlated with a different deformation mechanism from the counterparts of other bulk phases, due to the unique interconnected morphological feature of network structures.[249, 250] Furthermore, it has been demonstrated that the mesoporous inorganic networks (e.g., TiO2, Al2O3@ZnO) derived from the bulk PS-b-PLLA double gyroid phase persisted the robust mechanical performances.[189, 192] Therefore, the BCP* network structures and their derives hold great promises as structural materials.
Bio-mimic characteristics and applications
BCPs* and their assembly process, particularly the chiral transfer process, render a reasonable illumination to decode the vast yet mysterious existence of chirality in nature. At a molecular level, polypeptides and polypeptoids were synthesized to mimic natural peptides. At a conformational level, the chiral blocks in BCPs* exhibited a rod-like chiral conformation, similar to peptide conformation in a degree.[26, 251] At a morphological level, the BCP* chains with a chiral conformation segregated and gave rise to a reversed morphological chirality in some cases, resembling the chiral inversion occurred all the way along that proteins folded into fibrils, and fibrils assembled into bundles and fibers thereafter.[36, 103] Such emulation is vitally important to the comprehension on living systems. For instance, one of challenges to treat amyloid-related diseases is to understand how proteins irreversibly assembled into highly stable amyloid fibrils.[252] Moreover, Lin, Cai, and Jiang et al. launched a series of research on BCP* rod assemblies with a helix* topology, demonstrating their superior cellular internalization efficiency over the assemblies with achiral surface patterns (Figure 12A).[26, 253, 254] These findings contributed to the understanding on the cellular internalization of virus and drug uptake mechanism. Further, at a micro-scale, the swimming bacterial flagella were mimicked by the stimuli-triggered motion of individual helix* assemblies.[247] At a macro-scale, Towel gourd tendrils and Paphiopedilum dianthum petals exhibited a chiral growth behavior, mimicked by the motion of BCP*/chiral homopolymer elastomers.[28, 29, 246]
Similar to the helix* structures, network structures widely exist in nature as well, providing inspirations for the application of synthetic BCP* network structures and their derivates. For example, Ho et al. demonstrated porous epoxy networks templated by the PS-b-PLLA double gyroid phase as a coated layer to enhance the absorbability for both lysozyme and bovine serum albumin.[193] The same group also used the PS-b-PLLA double gyroid phase as a template to prepare SiO2 networks coated with poly(2-(dimethyl amino)ethyl methacrylate) brushes, which have responses to thermal and pH stimuli. As a results, such hybrid network materials possessed a smart and controllable environment-selective release capability (Figure 12B).[187] Additionally, a high similarity to bone structure and competitive mechanical properties afforded the networks of biodegradable BCPs* or bio-ceramics a prospect of bio-mimic bone scaffolds.[255, 256]
SUMMARY AND PERSPECTIVE
In summary, we have reviewed the assembly behaviors of BCPs* in bulk/films, selective solvents, and confined spaces. Although the assembly process was intricate as it was governed by multiple factors, the BCPs* usually showcased a unique feature to form controllable chiral assemblies. These phenomena were elucidated as a chiral transfer process: the chiral configuration of BCPs* realized the conformational chirality and this conformational chirality transferred up in the assembly process, bestowing the morphological chirality. Although similar chiral assemblies were observed from achiral BCPs, both left- and right-handed ones were equally formed, lacking a controlled chirality. Therefore, the controlled morphological chirality bestowed BCP* assemblies and the corresponding derivates with many functionalities, offering a bright prospect for applications.
In spite of great progress has been made in regard to the comprehension on BCP* self-assembly and the exploration for applications, significant challenges still remain. At the molecular level, most of hitherto reported BCPs* contained a chiral and an achiral block, showing a rod-coil linear architecture. The chiral blocks usually were composed of L-/D-lactide and α-amino acids, with a limited choice. In this case, the portfolio of BCPs* await a continuous expansion, with more chiral blocks to be synthesized to increase the diversity of BCPs* and more complex BCP* architecture to be designed. At the conformational level, it has been manifested that the chiral block of BCPs* exhibited a chiral helical/folded conformation rather than the classic coil-like conformation of the achiral blocks. However, there is still discrimination on whether the conformational chirality of the chiral block displays the same chirality as its configuration or not, whether the achiral block eventually would adopt a chiral conformation along with the chiral block or not, etc. Also, it is still elusive how the conformational chirality would affect the incompatibility between the achiral and chiral blocks, whether the former would enhance the latter or weaken the latter. No doubt an answer to these unsettled disagreements would deepen the comprehension on BCPs*. When the BCP* self-assembly took place in bulk/films or selective solvents, there has not been an unanimously accepted elucidation on how the conformation chirality dictated the assembly process in detail and determined morphological chirality of assemblies, although it has been noticed that the morphological chirality showed a tight association with the conformational chirality. Sometimes, the chirality stayed consistence from low to high hierarchy; but in other cases, the chiral inversion occurred. Additionally, it was found that the reported assembly mechanisms differed from each other. This not only emphasized the complexity of BCP* assembly, it also suggested that a consensus on the origin of chiral assemblies from the BCPs* is still vacant so far. To address these challenges, a methodology access to an in situ observation of the chiral transfer process on every hierarchical level and more simulative investigations on the self-assembly of BCPs* are highly beneficial and hence urged.
By contrast, the chiral transfer process in natural systems precisely dictated the formation of chiral structures, and a chiral inversion usually occurred during the hierarchical assembly, for example, gene expression, DNA processing.[23-26] In this case, it is still challenging to experimentally achieve a similar precise control on the chiral transfer process and the morphological chirality in synthetic BCP* systems. Furthermore, the understanding on the similarity and dissimilarity between the assembly behaviors in natural system and the counterparts in synthetic BCP* systems is limited to date. Such knowledge would not only deepen the comprehension on BCPs*, but also would deepen the comprehension on nature.
The emerging confined assembly of BCPs* presents a new horizon of the BCP* self-assembly, bridging two parallel lines, one is the unconfined assembly of BCPs* in bulk/films or selective solvents, forming controllable chiral assemblies, and the other is the confined assembly of achiral BCPs into chiral assemblies with a poor chirality control. Nevertheless, this research field is still in its infancy, and it is hitherto elusive how confining effects affect the BCP* self-assembly, particularly how they affects the chiral transfer process and the morphological chirality of BCP* assemblies. So far, it has been comprehended that the BCP* helixes exhibited a spring-like feature under 1D/2D confinement and a coil-like one under 3D confinement, similar to those from the achiral BCP assemblies under confinement. Hence, it is intriguing and essential to establish a controllable manipulation on the confined assembly of BCPs* and to understand the mechanisms behind. Specifically, it is vital to diversify the scenarios of the confined assembly of BCPs* and to examine the impacts of solvent selectivity, temperature, confining strength, confined space geometry, interfacial selectivity, and so on. A knowledge on the confined assembly of BCPs* would no doubt prompt the interpretation on BCP* self-assembly. It is also beneficial to the understanding and mimicking on DNA and RNA in natural systems, and the design of drug delivery systems.[21, 26, 254] In nature, chiral biomolecules such as DNA, RNA, and proteins showed a packed conformation under confinement (e.g., those inside a virus), considerably different from their conformation under unconfined conditions.[23, 257] Also, the confining effects imposed significant impacts to their physical properties and functionalities.[258, 259] For example, a tobacco mosaic virus contained a single RNA chain, associated with 2130 identical proteins. The formation of these proteins involved a series folding of the peptide chains, which displayed a disk-like feature with a dimension correlated with two turns of the RNA helix.[260]
In regard to exploring the functionalities and application of chiral structures generated by BCP* assembly, it is a challenge to prepare bulk materials with a well-preserved local structure (i.e., the chiral structures), a high long-range uniformity, and a high cost-efficiency. When BCP* colloidal assemblies from the solution assembly or confined assembly came to practical applications, it is challenging to achieve a massive preparation of such assemblies with a uniform dimension, shape and internal structure, and to effectively utilize these assembles as a template to synthesize other materials with the same assembly morphology.
Moreover, despite a series of unique physical properties even performance advantages the chiral structures showcased, it is a challenge to enrich these key features and extend them into an application level. We suggest that a comprehensive understanding on the functionalities of the chiral structures generated by the BCP* self-assembly requires a deep integration of multiple disciplines and cooperation of various research communities. This would enable a profound understanding and the maximum utilization of BCP* self-assembly at every level, from the molecular to the macro-scale level.[23] For example, inorganic nanoparticles were widely applied in advanced electrical devices, such as memristors. To date, these nanoparticles were usually spin-casted in the devices with a low order. It was found that the introduction of chiral small molecules considerably prompted the electrical performance of memristors, without an apparent change on structural order.[261, 262] This was because that the configuration of chiral small molecules allowed an efficient long-range electron transfer based on a chiral-induced spin selectivity effect, and this electrical behavior would apparently affect biorecognition processes.[263] At the morphological level, the chiral structures exhibited great potentials for application in electrical devices. The co-assembly of achiral BCPs and inorganic nanoparticles yielded various ordered arrangement of inorganic nanoparticles with a competitive electrical performance, but a controlled morphological chirality was lacked.[3, 202] In this case, a new memristor based on the co-assembly of BCPs* and inorganic nanoparticles into controllable chiral assemblies might combine the advantages of chirality at both molecular and morphological levels and exhibit a boosted memory performance.
ACKNOWLEDGMENT
We gratefully acknowledge financial supports from the National Natural Science Foundation of China (grant number: 51903098) and the Fundamental Research Funds for the Central Universities (grant number: 2019kfyXJJS077).
CONFLICT OF INTEREST
The authors declare no competing financial interest.
Biographies

Hao Li received his B.E. degree in 2016 from Donghua University, China. In 2017, he obtained his M.S. degree in Polymer Science from The University of Akron, U.S.A., under the supervision of Prof. Matthew Becker. Since 2018, he joined Prof. Jintao Zhu's group as a PhD student in Huazhong University of Science and Technology (HUST), China. His current research interests include the self-assembly of block copolymers.

Prof. Bijin Xiong received his bachelor's degree from Wuhan University of Technology, China, and completed his PhD degree at Institut National des Sciences Appliquées de Lyon, France. Then, he did his postdoctoral research in Changchun Institute of Applied Chemistry, Chinese Academy of Sciences. In 2018, he was appointed as an associate professor of chemistry in HUST. His research interests focus on the characterization and structure-property relationship of polymeric materials.

Prof. Jiangping Xu received his BS degree from University of Science and Technology of China and completed his PhD degree in Changchun Institute of Applied Chemistry, Chinese Academy of Sciences. Then, he did his postdoctoral research in HUST and University of Toronto, Canada. In 2018, he was appointed as a professor of chemistry in HUST. His research interests focus on the self-assembly of block copolymers and nanoparticles.

Prof. Jintao Zhu received his PhD in Polymer Chemistry and Physics from Changchun Institute of Applied Chemistry, Chinese Academy of Sciences. He then worked as a postdoctoral research fellow in the University of Alberta, Canada, and University of Massachusetts Amherst, USA. Since 2009, he has been appointed as a professor at the School of Chemistry and Chemical Engineering, HUST. Porf. Zhu is serving as an editor of Macromolecular Research. His current interests involve the self-assembled polymers and nanoparticles for responsive nanophotonics, healing materials, memory devices, and immunotherapy. Prof. Zhu is a recipient of Fellow of the Royal Society of Chemistry (2020), China National Funds for Distinguished Young Scholar (2015) and Chinese Chemical Society Youth Awards (2013).




