Effect of Intraoperative Posture on Accurate Diagnostic Rate of Intraoperative Nerve Monitoring During Esophagectomy
Funding: The authors received no specific funding for this work.
ABSTRACT
Background
The benefits of intraoperative nerve monitoring for identifying recurrent laryngeal nerves during esophageal cancer surgery have recently been reported. However, no standardized procedures have been established for the use of this system. This study aimed to identify factors affecting the diagnostic accuracy of intraoperative nerve monitoring for recurrent laryngeal nerve palsy and explore approaches to improve the precision and efficiency of intraoperative nerve monitoring in esophageal cancer surgery.
Methods
We analyzed 187 consecutive patients who underwent esophagectomy between 2011 and 2018, of whom 142 underwent intraoperative nerve monitoring. We evaluated factors affecting the diagnostic accuracy of intraoperative nerve monitoring for recurrent laryngeal nerve palsy.
Results
The overall incidence of postoperative recurrent laryngeal nerve palsy was 22% (32/142). Univariate analysis identified the left lateral decubitus position (vs. prone position) and not using video laryngoscope during intubation as risk factors for discrepancies between intraoperative nerve monitoring findings and postoperative recurrent laryngeal nerve palsy diagnosis. Multivariate analysis confirmed that the left lateral decubitus position (odds ratio: 4.24; 95% confidence interval: 1.09–13.4, p = 0.019) and not using video laryngoscope during intubation (odds ratio: 9.51; 95% confidence interval: 2.94–15.9, p = 0.001) were independent risk factors for recurrent laryngeal nerve palsy diagnostic discrepancies.
Conclusion
Adequate contact between the intubation tube and vocal cord muscles is crucial for effective intraoperative nerve monitoring during esophagectomy. Additionally, the intraoperative posture significantly affects diagnostic outcomes and should be carefully considered.
1 Introduction
Surgery remains a mainstay curative treatment for esophageal cancer, even in recent years; however, it is associated with significant postoperative morbidity [1, 2]. Various perioperative strategies, such as enhanced postoperative recovery programs [3, 4], minimally invasive surgical techniques [5, 6], and prehabilitation [7, 8], have contributed to reducing postoperative complication rates, shortening hospital stays, and lowering perioperative costs in recent years. Postoperative pneumonia is one of the most prevalent complications, which can occasionally be fatal. Notably, studies have shown that pulmonary complications following esophagectomy negatively affect postoperative survival [9-11]. Therefore, reducing the incidence of postoperative complications may improve short- and long-term outcomes in patients undergoing esophagectomy.
Recurrent laryngeal nerve (RLN) palsy is a common complication of esophageal cancer surgery. It can lead to motility disorders of the vocal cord muscles, causing occasional severe respiratory complications [12, 13]. As thoracic esophageal cancer most often metastasizes to the lymph nodes surrounding the RLN, safe and accurate dissection of these lymph nodes is essential to improve curative outcomes. Misidentification of the RLN is the leading cause of RLN palsy. However, the inability to detect intraoperative nerve injury visually makes it challenging for surgeons to prevent this complication.
Recent studies have highlighted the benefits of intraoperative nerve monitoring (IONM) for nerve identification during esophageal cancer surgery [14-17]. Previously, we reported the diagnostic accuracy and effectiveness of IONM in such procedures [18], demonstrating its role in reducing the risk of RLN palsy following esophagectomy [19]. In Japan, IONM during esophagectomy was included under medical insurance coverage in April 2020 and is now widely adopted by many institutions. Despite its advantages for both surgeons and patients, standardized procedures and guidelines for the proper use of IONM during esophagectomy have yet to be established. Therefore, this study aimed to identify the factors affecting the diagnostic accuracy of IONM for RLN palsy and to explore strategies for improving the accuracy and efficiency of the system in esophageal cancer surgery.
2 Materials and Methods
2.1 Patients and Study Design
This single-center, retrospective, observational cohort study was conducted at Jikei University School of Medicine (Tokyo, Japan) between January 2011 and December 2018. A total of 222 consecutive patients with esophageal cancer who underwent esophagectomy were enrolled. After excluding 35 patients who underwent salvage surgery, pharyngolaryngectomy, or palliative surgery, 187 patients were considered eligible for inclusion. Of these, 142 patients provided informed consent for the use of IONM during surgery. The study protocol was approved by the institutional review board of Jikei University (approval number: 32–482).
2.2 Surgical Posture and Procedure
The thoracoscopic approach through the right thoracic cavity with the patient in the prone position was the preferred choice. During the study period, the primary surgical approach shifted from thoracotomy to thoracoscopic surgery, and the intraoperative posture changed from left lateral decubitus to prone. Lymph node dissection along the right and left RLN was routinely performed according to the guidelines for thoracic squamous cell carcinoma [20]. Following the thoracic procedure, the patient was repositioned to the supine position for esophageal reconstruction using a gastric tube and cervical lymph node dissection. Cervical lymph node dissection was performed in all patients at clinical Stage II or higher. It was omitted in patients with clinical Stage I disease and risk factors, such as elderly patients. IONM was used to assess the viability of the RLN in both the thoracic and cervical regions before and after lymph node dissection.
2.3 IONM Procedure
The patient was first intubated by an anesthesiologist using a specialized intubation tube equipped with electrodes to detect bilateral vocal cord activity (NIM Response System 3.0; Tri-Vantage EMG tube; Medtronic, Tokyo, Japan). Starting in April 2014, video laryngoscopy was used to ensure proper contact between the electrodes and vocal cord muscles. When the stimulation probe contacted the RLN, electrical stimulation triggered contraction of the vocal cord muscles, which was visible on the electromyogram in the IONM system. Vocal cord activity was monitored using auditory signals.
Before lymph node dissection, the RLN was identified by observing vocal cord activity as a baseline response. After completing the dissection, the RLN was stimulated at the segment most proximal to the dissected area to verify its viability.
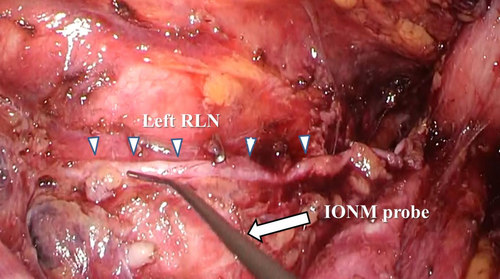
If the RLN is injured during lymph node dissection, the vocal cord muscles would fail to respond to electrical stimulation, indicating impaired function. Loss of response (LOR) was defined as the absence of action potentials during IONM stimulation. If LOR occurred, the stimulation point was gradually moved distally along the RLN to locate the site of injury. Figure 1 illustrates a representative image of intraoperative nerve stimulation along the left RLN in the thoracic cavity. Intraoperative neural electrical activity recorded by IONM was used to evaluate the accuracy of postoperative RLN palsy diagnosis.
2.4 Definition of Loss of Response (LOR)
In this study, the cutoff value for predicting postoperative RLN palsy was set as complete LOR, that is, 0% amplitude after lymph node dissection. The data from the preliminary study are presented in Table S1 and Figure S1. ROC curve analysis of the amplitude during IONM for postoperative RLN palsy showed that the AUC value was 0.857, and the optimal cutoff value was a 46.5% amplitude change. These data are not highly reproducible because the application of the intermittent IONM method was applied. The amplitude before lymph node dissection varied widely from a minimum of 114 μV to a maximum of 1160 μV. To perform intermittent IONM during thoracoscopic surgery, the probe must be placed in contact with the nerve through a surgical port with a limited range of motion. Furthermore, if the moist environment for nerve stimulation is not maintained, the amplitude becomes unstable. Setting the cutoff value to 0% amplitude increases the false-negative rate but enables the surgeon to more intuitively identify RLN palsy during surgery and facilitates localization of neurological damage.
2.5 Definition of Postoperative Complications
Complications were categorized according to the criteria outlined in the National Clinical Database, the Japanese nationwide surgical database system [21]. RLN palsy is defined as a functional disorder of the vocal muscles, presenting as postoperative hoarseness or aspiration, diagnosed by an otolaryngologist using laryngeal fiber examination within 1 week after surgery. A swallowing study was not conducted before initiating oral intake because of the inherent risk of aspiration associated with the test. The severity of complications was assessed using the Clavien-Dindo (CD) classification system [22], in which RLN palsy was defined as CD Grade I or higher.
2.6 Statistical Analysis
All statistical analyses were conducted using SPSS version 22.0.0.0 (IBM SPSS Statistics, Armonk, NY, USA). Univariate and multivariate logistic regression analyses were performed to identify factors associated with the diagnostic accuracy of IONM in predicting postoperative RLN palsy. Statistical significance was set at p < 0.05.
3 Results
3.1 Patients' Characteristics and Postoperative Outcomes
The clinicopathological characteristics of the patients are summarized in Table 1. The median age of the participants was 66.5 years, and there were 118 men (83%) and 24 women.
| Variables | n = 142 |
|---|---|
| Age median (years) | 66.5 |
| Sex | |
| Male | 118 (83) |
| Female | 24 (17) |
| BMI median | 21.5 |
| Comorbidity | |
| Circulatory disease | 13 (9) |
| COPD | 8 (6) |
| Diabetes mellitus | 11 (8) |
| Liver disease | 5 (4) |
| Preoperative treatment | |
| No | 52 (37) |
| Chemotherapy | 86 (61) |
| Chemoradiotherapy | 4 (3) |
| Location of cancer | |
| Upper | 16 (11) |
| Middle | 76 (54) |
| Lower | 50 (35) |
| Histological type | |
| SCC | 133 (93) |
| Adenocarcinoma | 5 (4) |
| Others | 4 (3) |
| cT stage | |
| 1 | 46 (32) |
| 2 | 19 (13) |
| 3 | 75 (54) |
| 4 | 2 (1) |
| cN stage | |
| 0 | 65 (46) |
| 1 | 45 (32) |
| 2 | 21 (15) |
| 3 | 11 (7) |
| cM stage | |
| 0 | 134 (94) |
| 1 (supraclavicular lym) | 8 (6) |
| cStage | |
| I | 36 (25) |
| II | 44 (31) |
| III | 53 (37) |
| IV | 9 (7) |
| Metastatic lymph node | |
| Around RLN | 28 (20) |
| Cervical lymph node dissection | 131 (92) |
- Note: Data are presented as median or n (%). Tumor staging was based on the 11th edition of the TNM classification of thoracic esophageal cancer, published by the Japan Esophageal Society.
- Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; RLN, recurrent laryngeal nerve; SCC, squamous cell carcinoma.
The postoperative outcomes are summarized in Table 2. RLN palsy was observed in 32 (22%) patients, including three cases of bilateral palsy. Mild postoperative pneumonia, classified as Grade II or higher according to CD classification, occurred in 28 (20%) patients. Moderate postoperative pneumonia, classified as Grade III or higher, was reported in 12 patients (8%).
| Variables | Monitoring |
|---|---|
| n = 142 | |
| Morbidity | |
| Surgical | |
| Anastomotic leakage | 10 (7) |
| Anastomotic stenosis | 30 (21) |
| Recurrent laryngeal nerve palsy | 32 (22) |
| Nonsurgical | |
| Pneumonia CD > 2 | 28 (20) |
| Pneumonia CD > 3 | 12 (8) |
| Hospital stay (d) | 31.6 |
| 30-day mortality | 0 (0) |
- Note: Data are presented as median or n (%).
- Abbreviation: CD, Clavien-Dindo classification.
3.2 Details of Left and Right RLN Palsy
The relationship between IONM findings and the postoperative diagnosis of RLN palsy is shown in Table 3. IONM-LOR “yes” was defined as the disappearance of electromyographic responses during IONM, indicating postoperative RLN palsy. Five cases were positive and 132 were negative for the right RLN, yielding 137 (96%) correctly diagnosed cases. For the left RLN, 20 cases were positive, and 109 were negative, resulting in 129 (91%) correctly diagnosed cases. The overall diagnostic accuracy was 94%.
| (A) Right RLN (n = 142) | (B) Left RLN (n = 142) | ||||||
|---|---|---|---|---|---|---|---|
| IONM-LOR | IONM-LOR | ||||||
| Yes | No | Yes | No | ||||
| Postoperative RLN palsy | Yes | 5 | 2 | Postoperative RLN palsy | Yes | 20 | 7 |
| No | 3 | 132 | No | 6 | 109 | ||
- Abbreviations: LOR, loss of response; RLN, recurrent laryngeal nerve.
3.3 Analysis of Discrepancies Between IONM Findings and Postoperative RLN Palsy Diagnosis
Logistic regression analysis was performed to identify the risk factors contributing to discrepancies between IONM findings and the postoperative diagnosis of RLN palsy. Univariate analysis identified the left lateral decubitus position (vs. the prone position) and not using video laryngoscopy during intubation as risk factors for these discrepancies (Table 4). Multivariate analysis further demonstrated that the left lateral decubitus position (odds ratio [OR]: 4.24; 95% confidence interval [CI]: 1.09–13.4, p = 0.019) and not using video laryngoscopy (OR: 9.51; 95% CI: 2.94–15.9, p = 0.001) were independent risk factors for discrepancies in RLN palsy diagnosis.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | (95% CI) | p | OR | (95% CI) | p | |
| Age > 66.5 years | 1.63 | (0.60–4.42) | 0.335 | |||
| Male | 4.14 | (0.52–3.26) | 0.177 | 4.65 | (0.68–6.60) | 0.102 |
| Tumor location | ||||||
| Middle (vs. lower) | 2.04 | (0.51–8.18) | 0.311 | |||
| Upper (vs. lower) | 2.83 | (0.73–10.9) | 0.130 | 2.38 | (0.81–6.75) | 0.321 |
| cT ≧ 3 | 1.94 | (0.60–6.22) | 0.263 | |||
| cN≧1 | 0.92 | (0.35–2.44) | 0.881 | |||
| Node-positive around RLN | 0.35 | (0.12–1.01) | 0.051 | 0.37 | (0.22–5.91) | 0.120 |
| Preoperative treatment | ||||||
| Chemotherapy (vs. none) | 1.58 | (0.53–4.70) | 0.407 | |||
| Thoracoscopic (vs. thoracotomy) | 0.91 | (0.47–1.76) | 0.783 | |||
| Left lateral decubitus (vs. prone) | 3.65 | (1.01–13.2) | 0.048 | 4.24 | (1.09–13.4) | 0.019 |
| Not using of video laryngoscope | 2.97 | (1.09–8.09) | 0.033 | 9.51 | (2.94–15.9) | 0.001 |
| Operation time > 565 min | 0.87 | (0.32–2.31) | 0.780 | |||
| Blood loss > 481 g | 2.07 | (0.74–5.78) | 0.163 | 3.42 | (0.81–6.55) | 0.157 |
| Cervical lymph node dissection | 0.93 | (0.76–5.20) | 0.664 | |||
- Abbreviations: CI, confidence interval; OR, odds ratio.
4 Discussion
This study identified the left lateral decubitus position and not using video laryngoscopy during intubation as independent risk factors for discrepancies between IONM findings and postoperative RLN palsy diagnosis. Although the appropriate use of IONM in esophageal cancer surgery has not yet been established, this study is the first to assess the effects of surgical posture and video laryngoscopy on IONM outcomes.
Why do discrepancies occur between IONM findings and postoperative paralysis diagnoses? The primary reason is thought to be inadequate contact between the intubation tube electrodes and vocal cord muscles during surgery. The IONM system relies on the electromyographic responses from the electrodes of the intubation tube, which are in contact with the vocal cord muscles. Proper electrode placement on both sides during video laryngoscopy and intubation is critical and requires careful observation by an anesthesiologist. Additionally, studies have shown that the lateral position alters the upper airway anatomy differently on the left and right sides [23, 24]. In clinical practice, it is well documented that patients with sleep apnea experience reduced dyspnea when sleeping in the lateral position [25, 26]. This is attributed to left–right differences in the upper airway anatomy, which can cause misalignment between the intubation tube and vocal cord muscles. Such a misalignment causes the accumulation of secretions in the airway and compromises electrode contact. As a result, the left lateral decubitus position is less suitable for IONM during esophagectomy than the prone position because of anatomical shifts and airway obstruction caused by secretions. This issue is specific to esophageal cancer surgery, highlighting the need for a comprehensive understanding of these challenges when using IONM. To ensure optimal IONM performance, surgeons must collaborate with anesthesiologists during surgery to maintain proper contact between the intubation tube electrodes and the vocal cord muscles throughout the procedure.
IONM is widely used in surgical specialties such as neurology and otorhinolaryngology, with its most common and well-documented application in recent years being thyroid surgery [27, 28]. However, unlike thyroid surgery, which is performed in a stable supine position, esophagectomy is often performed in the prone or left lateral decubitus position. Optimizing the use of IONM in less favorable environments requires secretion suctioning and precise adjustment of the tube position, which are essential. Recently, mediastinoscopic surgery, which allows esophagectomy to be performed in the supine position, has been reported [29, 30]. Mediastinoscopic esophagectomy is gaining attention for being minimally invasive, as it eliminates the need for intrathoracic procedures. Regarding surgical posture, this approach appears to be highly compatible with IONM for esophageal cancer surgery. Further research is necessary to validate its effectiveness in this context.
The overall diagnostic accuracy of IONM for postoperative RLN palsy was 94%, highlighting its value as a navigational tool for safely performing lymph node dissection along the RLN. IONM is recommended during esophagectomy for two reasons. First, IONM allows for the immediate and accurate identification of the RLN, reducing the risk of RLN injury during lymph node dissection [19]. Second, IONM can help identify the specific surgical step that causes RLN injury during lymph node dissection by cross-referencing IONM data with operation video reviews. This is particularly crucial because the nerve electrophysiological activity cannot be assessed using a surgeon's visual observation alone. In clinical practice, IONM has the potential to benefit patients by identifying those who can safely resume postoperative oral intake earlier and eliminating unnecessary swallowing rehabilitation. In addition, it serves as an effective teaching tool for trainees and promotes safe lymph node dissection.
This study had some limitations that should be acknowledged. First, because of its retrospective observational design, the analysis may have been influenced by confounding factors. To address this issue, prospective studies are required to minimize the impact of such confounders. Second, RLN palsy was defined as CD Grade 1 or higher in this study, which may have resulted in the inclusion of asymptomatic patients. Refining the criteria for defining complications could improve the accuracy of future analyses. Third, the cutoff value of the amplitude was set to 0% in this study, which reduced the accuracy of the diagnosis of RLN palsy using IONM. Diagnostic accuracy is expected to increase if the continuous IONM method is introduced and enhanced amplitude value analysis.
5 Conclusion
Adequate contact between the intubation tube and vocal cord muscles is crucial for effective IONM use during intubation. Additionally, intraoperative posture significantly affects diagnostic outcomes and should be carefully considered.
Author Contributions
Masami Yuda: conceptualization, methodology, data curation, investigation, formal analysis, writing – original draft, writing – review and editing. Keita Takahashi: conceptualization, data curation, investigation. Yoshitaka Ishikawa: methodology, data curation. Takanori Kurogochi: data curation, methodology. Akira Matsumoto: data curation, methodology. Naoko Fukushima: data curation, methodology. Takahiro Masuda: methodology, data curation. Naoto Takahashi: conceptualization, methodology, data curation. Fumiaki Yano: conceptualization, methodology, supervision, writing – original draft. Ken Eto: conceptualization, methodology, supervision, writing – original draft.
Acknowledgments
We would like to thank Drs. Yuichiro Tanishima and Katsunori Nishikawa for data acquisition and advice in preparing this manuscript. We would like to thank Editage (www.editage.jp) for the English language editing.
Ethics Statement
The protocol for this research project was approved by the Ethics Committee of Jikei University and conformed to the provisions of the Declaration of Helsinki. The study protocol was approved by the institutional review board of Jikei University (approval number: 32-482).
Consent
Informed consent was obtained from all subjects.
Conflicts of Interest
The authors declare no conflicts of interest.




