Three timepoint perioperative CEA levels are a prognostic factor for recurrence after adjuvant chemotherapy in patients with Stage II and III colorectal cancer
Shodai Mizuno and Ryosuke Hara contributed equally to this work.
Abstract
Aim
To investigate the relationship between the three timepoint perioperative CEA (ttpCEA) calculated at three timepoints and recurrence during the perioperative period in Stage II and III colorectal cancer (CRC) patients.
Methods
We performed a multi-institutional retrospective analysis of patients with Stage II and III CRC who underwent surgery and adjuvant chemotherapy from 2010 to 2020. Patient data from three facilities were used as training data, and data from three other facilities were used as validation data. The primary endpoint was the time to recurrence (TTR).
Results
A total of 538 patients were included for the training data. To validate the feasibility of ttpCEA, 329 patients were included for the validation data. Training data patients were categorized as ttpCEA low (n = 365) and ttpCEA high (n = 173). The 5-y TTR was significantly greater in the ttpCEA-low subgroup than in the ttpCEA-high subgroup (84.3% vs. 69.6%, respectively; p < 0.001). Validation data patients were categorized as ttpCEA low (n = 221) and ttpCEA high (n = 108). The 5-y TTR was significantly greater in the ttpCEA-low subgroup than in the ttpCEA-high subgroup (82.9% vs. 68.7%, respectively; p = 0.003).
Conclusion
The ttpCEA calculated from perioperative CEA levels at different timepoints was a prognostic factor for recurrence in Stage II and III CRC patients who underwent adjuvant chemotherapy according to both the training and validation data.
1 INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer in the male and female population, just behind prostate cancer, breast cancer, and lung cancer, which are the most common cancers in both sexes.1 Regarding Stage II CRC, multiple guidelines recommend adjuvant chemotherapy for patients with high-risk factors. However, these guidelines differ slightly in defining high-risk factors, and there is no clear unified criterion.2-4 We recently published a prognostic stratification score for Stage II colon cancer patients based on clinicopathological factors to overcome this difficulty.5 However, although this novel scoring system may be helpful for identifying patients who would benefit from adjuvant chemotherapy, it is not useful for predicting recurrence after adjuvant chemotherapy. Adjuvant chemotherapy after curative resection is recommended for patients with Stage III CRC. Nevertheless, the 5-y overall survival rate for patients with Stage III CRC ranges from 12.9% to 73.7%, and it is controversial whether adjuvant chemotherapy should be administered to all patients within this wide range of survival outcomes.6, 7 If the prognosis of each patient can be predicted from clinical information that can be easily obtained for each patient, follow-up procedures can be tailored to the individual patient, leading to early detection of recurrence.
Carcinoembryonic antigen (CEA) is a standard serum marker for colorectal cancer and is routinely measured for the detection of CRC recurrence during follow-up.8-10 The North American guidelines suggest that during follow-up, the serum CEA level should be measured every 3–6 mo, and patients with a CEA level above an absolute threshold (5.0 ng/mL according to the American Society of Clinical Oncology guidelines) should undergo radiological imaging surveillance.11-13 However, the evidence underpinning these guidelines in routine practice is limited.14, 15 Moreover, patients with Stage II and III CRC have more recurrences than patients with Stage I CRC. Therefore, a more accurate predictive factor for recurrence among these patients is needed.
While some reports indicate that high preoperative CEA or elevated postoperative CEA is a prognostic factor, other reports suggest that preoperative CEA is not a prognostic factor.15-17 Furthermore, although there is a slight change in the CEA level during the follow-up period, the majority of patients remain within the normal range, and this change might not be useful in a clinical setting. Thus, we hypothesized that perioperative changes in CEA levels might be more useful for accurately predicting recurrence. The purpose of this study was to investigate the relationship between the risk score calculated by the serum CEA concentration during the perioperative period and recurrence after adjuvant chemotherapy in Stage II and III CRC patients.
2 MATERIALS AND METHODS
2.1 Patients
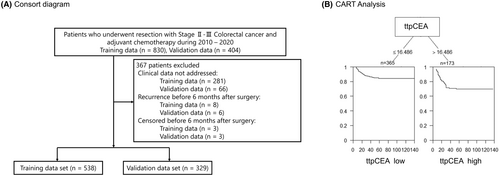
We conducted a retrospective, multi-institutional analysis of patients with pathological Stage II or III CRC who underwent primary tumor resection and received adjuvant chemotherapy from January 2010 to December 2020. Patient data from three different institutions, each with at least two colorectal surgeons, were selected and used as the training data. Data from the remaining three institutions, each with only one colorectal surgeon, were used for the validation data. The inclusion criteria were as follows: (1) adult patients (>18 y old); and (2) patients with histologically confirmed Stage II and III CRC. The exclusion criteria were as follows: (i) had recurrence before 6 mo after surgery; (ii) had less than 6 mo of follow-up; and (iii) had undergone neoadjuvant chemotherapy. A total of 538 patients were included in the training cohort, and 329 patients were included in the validation cohort (Figure 1A,B). This study was approved by the Ethics Committees of all the included institutions and was conducted in accordance with the Helsinki Declaration of 1996 (approval number: 20211148).
2.2 Study endpoints
The primary endpoint was time to recurrence (TTR), defined as the postoperative length of time during which the patient survived without any evidence of cancer recurrence as identified by imaging studies (computed tomography [CT], ultrasonography, magnetic resonance imaging, and positron emission tomography) or histological examination of biopsy specimens. Tumor recurrence was categorized as local recurrence (at the site of anastomosis or pelvis), peritoneal dissemination, or distant metastasis (liver, lung, or distant lymph nodes).
2.3 Calculation method of the risk calculator
The values of each coefficient were retrospectively measured at each timepoint based on the presence or absence of recurrence 3 y after surgery (Table S1).
2.4 Statistical analysis
Continuous variables are presented as the mean ± standard deviation (SD) and were analyzed using a t-test. Categorical variables were compared using the chi-squared test. The TTR rates were estimated by the Kaplan–Meier method, and the differences in survival between the two groups were assessed with the log-rank test. A Cox proportional hazards model was used to quantify the prognostic impact of individual covariates, and hazard ratios (HRs) and confidence intervals (CIs) were calculated. Covariates with p values <0.05 in the univariate Cox models were included in further multivariate Cox models.
Classification and Regression Tree (CART) analysis was used to determine the cutoff values of the ttpCEA for TTR.19 This cutoff value was used to predict patient prognosis by applying the statistical software R v. 4.2.1 (R Foundation Statistical Computing, Vienna, Austria). The statistical data were generated using the Stata 12 software program (StataCorp, College Station, TX, USA).
3 RESULTS
3.1 Patient characteristics: Training data
A total of 538 patients were enrolled in the training cohort (Figure 1A). Patients were assigned to two groups according to a ttpCEA cutoff value of 16.486 as calculated by survival analysis (Figure 1B). The patient characteristics of the two classified groups are detailed in Table 1, and Table S2 shows the demographic and oncological characteristics of all patients in the training and validation cohorts. Additionally, Table S3 shows the number of patients with high CEA values at 3 and 6 mo postoperatively. Of the 538 patients, 365 (67.8%) were in the ttpCEA-high subgroup, and 173 (32.2%) were in the ttpCEA low subgroup. Significant differences were observed in age, sex, tumor location, preoperative CEA level, postoperative CEA level, and recurrence.
| Low ttpCEA n = 365 | High ttpCEA n = 173 | p Value | |
|---|---|---|---|
| Age | 64.6 ± 10.9 | 67.5 ± 10.7 | 0.004 |
| Sex: Female | 183 (50%) | 71 (41%) | 0.048 |
| Location | |||
| Right | 121 (33%) | 75 (43%) | 0.028 |
| Left | 160 (44%) | 72 (42%) | |
| Rectum | 84 (23%) | 26 (15%) | |
| Operation time (min) | 281.4 ± 108.4 | 283.7 ± 111.6 | 0.818 |
| Blood loss (mL) | 174.1 ± 543.8 | 163.8 ± 352.0 | 0.820 |
| Complication+ | 110 (30%) | 54 (31%) | 0.800 |
| Tumor histology: por, sig, muc | 27 (7%) | 11 (6%) | 0.660 |
| Tumor depth | |||
| T1, 2 | 54 (15%) | 19 (11%) | 0.090 |
| T3 | 244 (67%) | 109 (63%) | |
| T4 | 67 (18%) | 45 (26%) | |
| Lymphatic invasion+ | 251 (69%) | 129 (75%) | 0.168 |
| Venous invasion+ | 279 (76%) | 138 (80%) | 0.387 |
| N1 | 189 (52%) | 88 (51%) | 0.808 |
| N2 | 67 (18%) | 29 (17%) | |
| II | 109 (30%) | 56 (32%) | 0.556 |
| III | 256 (70%) | 117 (68%) | |
| Preoperative CEA >5 | 86 (24%) | 95 (55%) | <0.001 |
| Chemotherapy regimen | |||
| Oral 5-FU | 294 (81%) | 135 (78%) | 0.498 |
| Oxaliplatin | 71 (19%) | 38 (22%) | |
| Postoperative CEA >5 | 2 (0.5%) | 81 (47%) | <0.001 |
| Follow-up month | 51.9 ± 24.6 | 49.9 ± 26.1 | 0.381 |
| Recurrence+ | 50 (14%) | 46 (27%) | <0.001 |
| Death+ | 21 (6%) | 19 (11%) | 0.031 |
| Facility | |||
| A | 164 (45%) | 77 (45%) | 0.465 |
| B | 126 (35%) | 53 (31%) | |
| C | 75 (21%) | 43 (25%) | |
- Note: The values are presented as the mean ± standard deviation or number of patients (%).
- Abbreviations: CEA, carcinoembryonic antigen; muc, mucinous adenocarcinoma; por, poorly differentiated adenocarcinoma; sig, signet ring cell adenocarcinoma.
3.2 Time to recurrence: Training data
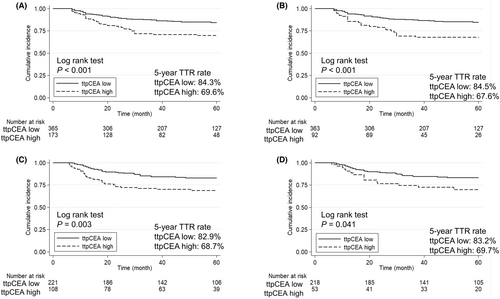
The 5-y TTR was significantly greater in the ttpCEA-low subgroup than in the ttpCEA-high subgroup (84.3% vs. 69.6%, respectively; p < 0.001; Figure 2A). Univariate analysis revealed that tumor localization, tumor depth, lymph node metastasis, lymphatic invasion, and high ttpCEA significantly increased the risk of recurrence. Moreover, multivariate analysis revealed that high ttpCEA was an independent prognostic factor for TTR [HR 2.38, 95% CI 1.58–3.59; p < 0.001; Table 2). According to the subgroup analysis of patients with normal CEA at both 3 and 6 mo postoperatively, the 5-y TTR was significantly greater in the ttpCEA-low subgroup compared to the ttpCEA-high subgroup (84.5% vs. 67.6%, respectively; p < 0.001; Figure 2B). Univariate analysis revealed that tumor localization, tumor depth, lymph node metastasis, and high ttpCEA significantly increased the risk of recurrence. Moreover, multivariate analysis revealed that high ttpCEA was an independent prognostic factor for TTR ( HR 2.35, 95% CI 1.45–3.80, p < 0.001; Table 3).
| Factors | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age | 1.00 | 0.98–1.01 | 0.709 | |||
| Sex: Female | 0.95 | 0.64–1.42 | 0.818 | |||
| Location | ||||||
| Right | Ref | Ref | ||||
| Left | 1.37 | 0.84–2.22 | 0.207 | 1.58 | 0.97–2.57 | 0.067 |
| Rectum | 1.98 | 1.17–3.36 | 0.011 | 3.21 | 1.84–5.60 | <0.001 |
| Tumor differentiation: por, sig, muc | 1.02 | 0.47–2.21 | 0.951 | |||
| Tumor depth | ||||||
| T1-2 | Ref | Ref | ||||
| T3 | 3.26 | 1.18–9.00 | 0.023 | 4.33 | 1.56–12.05 | 0.005 |
| T4 | 7.92 | 2.82–22.23 | <0.001 | 13.00 | 4.50–37.55 | <0.001 |
| Lymph node metastasis | ||||||
| N0 | Ref | Ref | ||||
| N1 | 2.34 | 1.32–4.14 | 0.003 | 3.03 | 1.69–5.42 | <0.001 |
| N2 | 3.30 | 1.74–6.27 | <0.001 | 3.44 | 1.72–6.89 | <0.001 |
| Lymphatic invasion+ | 1.88 | 1.13–3.14 | 0.016 | 1.65 | 0.98–2.80 | 0.061 |
| Venous invasion+ | 1.13 | 0.69–1.85 | 0.623 | |||
| Complication+ | 0.83 | 0.53–1.29 | 0.410 | |||
| Chemotherapy regimen: Oxaliplatin | 1.70 | 1.08–2.67 | 0.022 | 1.00 | 0.60–1.67 | 0.997 |
| Preoperative CEA: >5 | 1.45 | 0.96–2.18 | 0.074 | |||
| Postoperative CEA >5 | 1.61 | 0.98–2.63 | 0.059 | |||
| ttpCEA: High | 2.16 | 1.45–3.22 | <0.001 | 2.38 | 1.58–3.59 | <0.001 |
- Abbreviations: CEA, carcinoembryonic antigen; CI, confidence interval; HR, hazard ratio; muc, mucinous adenocarcinoma; por, poorly differentiated adenocarcinoma; por, poorly differentiated; sig, signet ring cell adenocarcinoma; sig, signet ring cell carcinoma.
| Factors | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age | 1.00 | 0.98–1.02 | 0.640 | |||
| Sex: Female | 1.05 | 0.67–1.65 | 0.830 | |||
| Location | ||||||
| Right | Ref | Ref | ||||
| Left | 1.64 | 0.92–2.93 | 0.096 | 1.73 | 0.96–3.09 | 0.067 |
| Rectum | 2.48 | 1.34–4.60 | 0.004 | 3.70 | 1.94–7.04 | <0.001 |
| Tumor differentiation: por, sig, muc | 1.05 | 0.46–2.43 | 0.902 | |||
| Tumor depth | ||||||
| T1-2 | Ref | Ref | ||||
| T3 | 3.66 | 1.13–11.82 | 0.030 | 5.27 | 1.62–17.08 | 0.006 |
| T4 | 9.31 | 2.84–30.47 | <0.001 | 16.52 | 4.91–55.54 | <0.001 |
| Lymph node metastasis | ||||||
| N0 | Ref | Ref | ||||
| N1 | 2.36 | 1.22–4.56 | 0.011 | 3.51 | 1.80–6.87 | <0.001 |
| N2 | 3.36 | 1.61–7.01 | 0.001 | 3.83 | 1.83–8.03 | <0.001 |
| Lymphatic invasion+ | 1.53 | 0.89–2.62 | 0.123 | |||
| Venous invasion+ | 1.17 | 0.67–2.02 | 0.586 | |||
| Complication+ | 0.81 | 0.49–1.34 | 0.408 | |||
| ttpCEA: High | 2.40 | 1.50–3.84 | <0.001 | 2.35 | 1.45–3.80 | <0.001 |
- Abbreviations: CEA, carcinoembryonic antigen; CI, confidence interval; HR, hazard ratio; muc, mucinous adenocarcinoma; muc, mucinous adenocarcinoma; por, poorly differentiated; sig, signet ring cell adenocarcinoma; sig, signet ring cell carcinoma.
Furthermore, ttpCEA consistently showed the highest time-dependent area under the curve (AUC) compared to preoperative CEA and CEA levels at 3 and 6 mo postoperatively (Figure S1). Differences in recurrence sites are shown in Table S4, where the number of patients with peritoneal dissemination was significantly higher in the ttpCEA-high group. Kaplan–Meier curves by recurrence site are presented in Figure S2. Although the ttpCEA-high group showed no significant difference in recurrence rates for liver metastases and local recurrences compared to the ttpCEA-low group, it demonstrated significantly higher rates of lung metastases and peritoneal dissemination recurrences.
3.3 Patient characteristics and time to recurrence: Validation data
A total of 329 patients were included for validation data (Figure 1A). The patient characteristics of the patients included in the validation cohort are detailed in Table 4, and Table S2 shows the demographic and oncological characteristics of all patients in the training and validation cohorts. Additionally, the number of patients with elevated CEA values at 3 and 6 mo postoperatively is provided in Table S5. Of the 329 patients, 221 (67.2%) were classified as ttpCEA-low, and 108 (32.8%) were classified as ttpCEA-high. Significant differences were observed in preoperative CEA levels, postoperative CEA levels, recurrence rate, and mortality rate. The 5-y TTR was significantly greater in the ttpCEA-low subgroup than in the ttpCEA-high subgroup (82.9% vs. 68.7%, respectively; p = 0.003; Figure 2C). According to the subgroup analysis of patients with normal CEA levels at both 3 and 6 mo postoperatively, the 5-y TTR was significantly longer in the ttpCEA-low subgroup compared to the ttpCEA-high subgroup (83.2% vs. 69.7%, respectively; p = 0.041; Figure 2D). The differences in recurrence sites are detailed in Table S6, where the number of patients with liver metastases and peritoneal dissemination was significantly higher in the ttpCEA-high group. Kaplan–Meier curves by organ of recurrence are shown in Supplemental Figure 3. While there was no significant difference in recurrence rates for lung metastases and local recurrences between the ttpCEA-high and ttpCEA-low groups, the ttpCEA-high group exhibited significantly higher rates of liver metastases and peritoneal dissemination recurrences. Compared to the training data, although there was a trend observed in both liver and lung metastases, the results for these recurrence sites were not consistent. On the other hand, ttpCEA was consistently effective in predicting peritoneal dissemination across both datasets, suggesting that ttpCEA may be particularly useful in forecasting peritoneal dissemination recurrence.
| Low ttpCEA n = 221 | High ttpCEA n = 108 | p Value | |
|---|---|---|---|
| Age | 65.0 ± 10.8 | 66.8 ± 9.1 | 0.150 |
| Sex: Female | 96 (43%) | 58 (54%) | 0.080 |
| Location | |||
| Right | 75 (34%) | 46 (42%) | 0.188 |
| Left | 82 (37%) | 30 (28%) | |
| Rectum | 64 (29%) | 32 (30%) | |
| Operation time (min) | 260.0 ± 107.0 | 258.6 ± 104.4 | 0.909 |
| Blood loss (mL) | 179.3 ± 389.2 | 232.3 ± 453.2 | 0.273 |
| Complication+ | 38 (17%) | 20 (19%) | 0.767 |
| Tumor histology: por, sig, muc | 14 (6%) | 13 (12%) | 0.077 |
| Tumor depth | |||
| T1, 2 | 29 (13%) | 9 (8%) | 0.152 |
| T3 | 147 (67%) | 68 (63%) | |
| T4 | 45 (20%) | 31 (29%) | |
| Lymphatic invasion+ | 161 (73%) | 87 (81%) | 0.128 |
| Venous invasion+ | 186 (84%) | 93 (86%) | 0.644 |
| Lymph node metastasis | |||
| N1 | 126 (57%) | 52 (48%) | 0.317 |
| N2 | 36 (16%) | 21 (19%) | |
| pStage | |||
| II | 59 (27%) | 35 (32%) | 0.282 |
| III | 162 (73%) | 73 (68%) | |
| Preoperative CEA >5 | 63 (29%) | 69 (64%) | <0.001 |
| Postoperative CEA >5 | 3 (1%) | 55 (51%) | <0.001 |
| Follow-up month | 63.9 ± 31.3 | 61.1 ± 28.0 | 0.428 |
| Recurrence+ | 36 (16%) | 32 (30%) | 0.005 |
| Death+ | 15 (7%) | 23 (21%) | <0.001 |
| Facility | |||
| A | 83 (38%) | 38 (35%) | 0.896 |
| B | 77 (35%) | 38 (35%) | |
| C | 61 (28%) | 32 (30%) | |
- Note: The values are presented as the mean ± standard deviation or number of patients (%).
- Abbreviations: CEA, carcinoembryonic antigen; muc, mucinous adenocarcinoma; por, poorly differentiated adenocarcinoma; sig, signet ring cell adenocarcinoma.
4 DISCUSSION
In this study we investigated the correlation between the ttpCEA level calculated at various perioperative timepoints and oncological outcomes in Stage II and III CRC patients who underwent adjuvant chemotherapy. For these patients, a high ttpCEA level was identified as an independent poor prognostic factor for TTR in both the training and validation datasets, even when the postoperative CEA level was normal. Similar findings were observed in the validation cohort, indicating that the ttpCEA level may be a universally effective predictive factor.
We were able to compare and validate the data in two different datasets. As shown in Table S2, the patient backgrounds in the training and validation cohorts were significantly different for several factors. As mentioned in the Methods section, training data were extracted from institutions with more than two colorectal surgeons. It is possible that the patients in the training dataset underwent extensive surgery compared with those in the validation dataset, resulting in differences in several factors. However, despite the differences in many factors between the two groups, a high ttpCEA level was an independent prognostic factor for poor TTR in both the training and validation cohorts. Notably, similar results were obtained for the ttpCEA levels in two datasets from different hospitals with different numbers of colorectal surgeons, although some reports have suggested that patient prognosis varies with hospital size or number of surgeons.20, 21 Taken together, the results of this study suggest that the ttpCEA level may be useful for identifying patients at high risk for recurrence in patients in various kinds of hospitals.
CEA measurement is recognized as an easy noninvasive test, and the results of the present study suggested that it is potentially useful for predicting the recurrence of Stage II/III CRC. Guidelines from the Japanese Society for Cancer of the Colon and Rectum (JSCCR) recommend regular blood tests, including the measurement of CEA and CA 19–9, every 3 mo after surgery.22 Similarly, guidelines from the American Society of Clinical Oncology (ASCO)23 and the European Society for Medical Oncology (ESMO)3 suggest CEA measurement every 3 mo for 3 y. However, a significant percentage (63%–73%) of patients with advanced cancer still have normal CEA levels.24 In contrast, the ttpCEA level can be easily calculated from the CEA level at three different timepoints. ttpCEA not only is superior for identifying patients with poor prognosis, but also overcomes the limitation of postoperative CEA values by identifying patients with poor prognosis, even if their postoperative CEA values are normal. In fact, when comparing the time-dependent AUC of ttpCEA to that of preoperative and postoperative CEA values (at 3 and 6 mo), ttpCEA consistently shows higher predictive accuracy, indicating it is a better predictor of recurrence. Considering all the facts above, the advantage of our new biomarker is its compatibility with the standard treatment protocols outlined in these guidelines, which allows for comprehensive data collection of CEA levels. It is important to emphasize that there is no need to deviate from current clinical practices as detailed in the existing guidelines, and ttpCEA can seamlessly integrate into routine clinical use.
We used three timepoints instead of two because we hypothesized that the effects of adjuvant chemotherapy would influence the results. Furthermore, the majority of patients relapse within 6 mo to 1 y after surgery (training data: 43% of all recurrences, validation data: 37% of all recurrences). Therefore, it is crucial to predict recurrence after postoperative treatment, as waiting until beyond 6 mo may be too late for effective prediction. Thus, we believe it is essential to be able to predict recurrence at 6 mo postoperatively. Moreover, the novelty of this study lies in our inclusion of multiple timepoints, which can be affected by postoperative treatment, to create a new predictive score. For these reasons, we believe our method is suitable and feasible for clinical practice.
In postoperative surveillance, a chest and abdomen CT scan is recommended every 6 mo after surgery for detection of recurrence.22 However, there is a risk of carcinogenesis from exposure to radiation from the CT scan.25, 26 It is important to balance the risk of exposure to radiation from CT scans with the benefit of detecting recurrence with such scans.25 The recommendation of CT scans every 6 mo was calculated based on the patient's status. Considering the results of this study, it is possible that the ttpCEA level might be useful for identifying patients who need frequent CT scans. For example, the frequency of CT scans can be reduced to once a year for patients with low ttpCEA levels due to the low risk of recurrence. In addition, compared with CT scans, CEA measurements are less expensive,27 and reducing the frequency of CT scans might also be useful from a cost perspective for patients whose ttpCEA concentrations are low.
Recently, circulating tumor DNA (ctDNA) has demonstrated promising results for monitoring several types of cancers, such as lung cancer and esophageal squamous cell carcinoma.28, 29 In terms of CRC, ctDNA analysis can potentially change the postoperative management of CRC patients by enabling risk stratification, adjuvant chemotherapy monitoring, and early relapse detection.30-32 However, ctDNA measurements are still in the clinical research phase and are unavailable to all patients because of cost or infrastructure issues. Therefore, the use of the ttpCEA level might be a useful and cost-effective method for identifying patients at high risk of recurrence. Furthermore, the use of the ttpCEA could be effective in identifying patients who need ctDNA evaluation for further treatment.
There are several limitations in this study. First, this was a retrospective study that potentially included several biases, such as selection bias. Second, patients who experienced recurrence before 6 mo after surgery were not included in this study; therefore, the ttpCEA level may not be predictive of early recurrence, i.e., within 6 mo after surgery. Third, we did not control for tobacco use, a known factor that raises CEA levels, as this factor is difficult to ascertain from patients. Similarly, we did not control for other factors that can lead to false-positive CEA elevation, such as gastritis, peptic ulcer disease, diverticulitis, liver disease, chronic obstructive pulmonary disease, diabetes, or acute or chronic inflammatory conditions. Finally, since this is a multi-institutional study, there may be inconsistencies in the methods of measuring CEA. To address these limitations, a prospective study should be conducted. We believe that the results of the present study should be validated in future prospective studies that include a larger number of patients and consider new treatment strategies.
In conclusion, the ttpCEA concentration calculated from perioperative levels at different timepoints was a prognostic factor for recurrence in Stage II and III CRC patients who underwent adjuvant chemotherapy even when the postoperative CEA concentration was normal.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, and/or acquisition of data, and/or analysis and interpretation of data; authors participated in drafting the article or revising it critically for important intellectual content; and authors gave final approval of the version to be published. Especially, Shodai Mizuno and Ryosuke Hara contributed to data analysis and drafted the article. Shodai Mizuno, Ryosuke Hara, Kohei Shigeta, Jumpei Nakadai, Hideo Baba, Hiroto Kikuchi, Yoko Adachi, Takehiro Shimada, Hirofumi Suzumura, and Kiyoaki Sugiura conducted data collection. Shimpei Matsui, Ryo Seishima, Koji Okabayashi, and Yuko Kitagawa provided a critical review of the article. Shodai Mizuno, Ryosuke Hara, and Kyoko Sakamoto performed the statistical analysis and review. Kohei Shigeta provided the final approval of the version for publication.
ACKNOWLEDGMENTS
The English in this document has been checked by a professional editor who is a native speaker of English.
FUNDING INFORMATION
There was no source of support for this work.
CONFLICT OF INTEREST STATEMENT
Dr. Kitagawa reports grants and personal fees from CHUGAI PHARMACEUTICAL CO., LTD., grants and personal fees from TAIHO PHARMACEUTICAL CO., LTD, grants from Yakult Honsha Co. Ltd., grants and personal fees from AsahiKASEI Co., Ltd., grants from Otsuka Pharmaceutical Co., Ltd., grants from Takeda Pharmaceutical Co., Ltd., grants from ONO PHARMACEUTICAL CO., LTD., grants from TSUMURA & CO., grants from Kyouwa Hakkou Kirin Co., Ltd., grants from DAINIPPON SUMITOMO PHARMA Co., Ltd., grants from EA Pharma Co., Ltd., grants from Astellas Pharma Inc., grants from Toyama Chemical Co., Ltd., grants from MEDICON INC., grants from KAKEN PHARMACEUTICAL CO. LTD., grants from Eisai Co., Ltd., grants and personal fees from Otsuka Pharmaceutical Factory Inc., grants from TEIJIN PHARMA LIMITED., grants from NIHON PHARMACEUTICAL CO., LTD., grants and personal fees from Nippon Covidien Inc., personal fees from SHIONOGI & CO., LTD., outside the submitted work.
Yuko Kitagawa is a Chief Editor of the Annals of Gastroenterological Surgery.
ETHICS STATEMENT
Approval of the research protocol: The protocol for this research project has been approved by a suitably constituted Ethics Committee of the institution and it conforms to the provisions of the Declaration of Helsinki. Committee of Keio University Hospital, Approval No. 20211148.
Informed Consent: N/A.
Registry and the Registration No. of the study/Trial: 20 211 148.
Animal Studies: N/A.




