Comparison of short-term outcomes between robot-assisted and laparoscopic rectal surgery for rectal cancer: A propensity score-matched analysis using the Japanese Nationwide diagnosis procedure combination database
Abstract
Background
The use of robot-assisted surgery for rectal cancer is increasing, but its short-term results remain unclear. We compared the short-term outcomes of robot-assisted and laparoscopic surgery for rectal cancer using a nationwide inpatient database.
Methods
We analyzed patients registered in the Japanese Diagnosis Procedure Combination database who underwent robot-assisted or laparoscopic surgery for rectal cancer from April 2018 to March 2020. Postoperative complication rates, anesthesia time, length of hospital stay, and cost were compared using propensity score matching for low anterior resection (LAR), high anterior resection (HAR), and abdominoperineal resection (APR).
Results
Among 38 090 rectal cancer cases, 1992 LAR, 357 HAR, and 310 APR pairs were generated by propensity score matching and analyzed. Anesthesia time was longer for robot-assisted surgery compared with laparoscopic surgery (LAR: 388.6 vs. 452.8 min, p < 0.001; HAR: 300.9 vs. 393.5 min, p < 0.001; APR: 4478.5 vs. 533.5 min, p < 0.001). Robot-assisted surgery was associated with significantly shorter hospital stay for LAR (22.3 vs. 20.0 days, p < 0.001) and APR (29.2 vs. 25.9 days, p = 0.029). Total costs for LAR were significantly lower for robot-assisted surgery (2031511.6 vs. 1955216.6 JPY, p < 0.001). The complication rates for robot-assisted surgery tended to be fewer than laparoscopic surgery for all procedures, but the differences were not significant.
Conclusions
Although the anesthesia time was longer for robot-assisted surgery, the procedure resulted in shorter hospital stay for LAR and APR, and lower costs for LAR compared with laparoscopic surgery. Robot-assisted surgery can thus help to reduce costs and can be performed safely.
1 INTRODUCTION
More than 700 000 people worldwide are newly diagnosed with rectal cancer each year, and more than 300 000 people die from the disease, with particularly high incidence rates in East Asia, including Japan.1 Furthermore, the incidence of early-onset rectal cancer has been increasing in recent years.2 Continuing to improve surgical accuracy in the treatment of rectal cancer is an important issue. Surgery for rectal cancer has recently become minimally invasive. Since the world's first case of robot-assisted colorectal resection was reported in 2002,3 robot-assisted colorectal surgery has made remarkable progress and its use has spread throughout the world. In particular, the invention of the da Vinci® Surgical System (Intuitive Surgical) has revolutionized surgery. Its articulated robotic arm with a wide range of joint motions, three-dimensional imaging, stabilization, and motion scale functions have gained the support of many surgeons, and its market share is now expanding worldwide, with the da Vinci® Surgical System being the most widely used surgical robot in the world. According to the manufacturers, there were 7135 units globally up to the end of June 2022, representing a 13% increase over the previous year.
Robotic surgery for prostate cancer was included in the Health Insurance List in Japan in 2012, and although the indications were not expanded significantly in the following years, 12 procedures were added simultaneously in 2018, including esophageal, gastric, rectal, and uterine cancers. In the area of colorectal cancer, the indication was expanded to include colon cancer in 2022. The use of robotic surgery in Japan has thus exploded over the past decade, becoming widely used not only in a few highly specialized facilities but also in general hospitals.
Despite the speed of its development, large-scale evidence for the use of robot-assisted surgery for rectal cancer has been lacking for a long time. The robot versus laparoscopic resection for rectal cancer (ROLARR) trial (N = 466) reported by Jayne et al. in 2017 was one of the large randomized controlled trials, and found no significant difference between robot-assisted and laparoscopic surgery in terms of the conversion rate to laparotomy, as the primary endpoint.4 Several other RCTs found no significant differences in open-conversion rates or complications.5, 6 However, the robot versus laparoscopic surgery for middle and low rectal cancer (REAL) trial (N = 1240) conducted by Feng et al. in 2022 found that robotic surgery was associated with a significantly shorter postoperative hospital stay, lower open-conversion rate, and fewer intraoperative complications.7 But these RCTs primarily collect data from some high-volume centers and often do not include hospitals that are not proficient in robotic surgery. In the rapid spread of robot-assisted surgery, we considered it essential to have real-world data, including general hospitals that are not proficient in robot-assisted surgery.
In addition to RCTs, database-based studies of robot-assisted surgery for rectal and colon cancer have been reported from Taiwan (N = 113 180),8 the United States (N = 128 288),9 Norway (N = 1284)10 and Denmark (N = 2393).11 However, some of these studies had small numbers of cases10, 11 and some others had small percentages of robotic surgery (0.4–2.7%).8, 9 Matsuyama et al.12 analyzed data over a similar period to the present study, using the National Clinical Database (NCD), Japan's extensive surgical database. The NCD is a vast database dedicated to the surgical field and includes perioperative information.13 The database requires registration of all gastrointestinal surgeries, but it also requires exceptionally detailed records for some procedures. One of these procedures is LAR. Matsuyama analyzed data for 17 377 laparoscopic cases and 2843 robotic cases by propensity score (PS) matching. They showed significant decreases in open-conversion rate, blood loss, in-hospital mortality, and length of hospital stay, but increased operation time. However, the analysis was limited to patients undergoing LAR. However, there was a limitation in that only one procedure for rectal cancer was included.
The Diagnosis Procedure Combination (DPC) is another large inpatient database in Japan. Unlike NCD, DPC does not include detailed data on surgery, However, it includes records on resource use and medical costs, so we speculated it would be possible to evaluate rectal cancer from a different perspective than previously reported. The current study thus aimed to clarify the short-term outcomes and medical costs of robot-assisted rectal surgery compared with laparoscopic rectal surgery using data from the DPC.
2 MATERIALS AND METHODS
2.1 Database
The DPC is a large-scale medical database that records information on hospitalized patients at acute care hospitals in Japan. Data include basic patient information such as age, sex, height, and weight; primary illness at admission; comorbidities at admission; comorbidities that developed during hospitalization; surgeries and procedures performed; all medical resources used; anesthesia time; and discharge outcome. The database records all disease names as ICD-10 codes. It is a nationwide administrative claims database that covers over 1700 hospitals and 7 million inpatients.14, 15 The database is linked to hospitalized patients' insurance information and records total medical costs.
Robot-assisted surgery for rectal cancer has been covered by national insurance from April 2018, and the current study thus covered the period from April 2018 to March 2020, i.e., the first 2 years after the introduction of health coverage.
2.2 Patients
Data from patients who underwent robot-assisted or laparoscopic rectal surgery for rectal cancer during the study period were extracted from the DPC database. Patients whose clinical stage was unknown or 0, and patients who underwent concurrent surgery for other malignancies were excluded.
The study was approved by the institutional review board of Tokyo Medical and Dental University (M8000-788).
2.3 Study variables
The primary outcome of this study was postoperative complications, and the secondary outcomes were in-hospital mortality, anesthesia time, length of hospital stay, and total medical costs. Postoperative complications were extracted using ICD-10 codes. The following complications were analyzed in this study: myocardial infarction (I21, I22), pulmonary embolus (I26), pneumonia (J10, J12, J13, J14, J15, J16, J18, J958), venous thrombosis (I80), peritonitis (K65, K659), surgical site infection (T814), cerebrovascular disease (I60, I61, I62, I63, I64, I65, I66), ileus (K56, K913), intestinal ischemia (K55), intra-abdominal bleeding (k66), urinary tract infection (N30, N34, N39, N10), dysuria (N31, N391, N99), septic shock (R572), and anastomotic leakage (T813). Anesthesia time was used as a proxy for operation time because the DPC database does not include operation time. We defined reoperation as surgery under general anesthesia after the first postoperative day.
2.4 Analysis
Short-term outcomes were compared between robot-assisted surgery and laparoscopic surgery separately for each of the three techniques: LAR, high anterior resection (HAR), and abdominoperineal resection (APR). One-to-one PS matching was used to adjust for confounding for LAR, HAR, and APR, respectively. The PS was estimated using a logistic regression model, including the following variables: sex, age, year, clinical T stage, clinical N stage, educational hospital or not, activities of daily living (ADL), dementia, smoking history, body mass index, diabetes (E10, E11, E12, E13, E14), hypertension (I10), chronic obstructive pulmonary disease (J43, J44), cardiac failure (I110, I130), ischemic heart disease (I20, I21, I22, I24, I25), chronic kidney disease stage 5 (N185), cerebrovascular event (I60, I61, I62, I63, I64, I65,I66 < I69), cirrhosis (K74), and emergency transport. Matching was performed by nearest-neighbor matching within a caliper of 0.001.
After PS matching, categorical outcome variables were compared with conditional logistic regression analyses and continuous outcome variables were compared with fixed effect linear regression analyses. A p-value < 0.05 was considered significant.
All statistical analyses were performed using Stata SE 16.0 (StataCorp).
3 RESULTS
A flow diagram of the patient selection process is shown in Figure 1. A total of 38 090 patients underwent scheduled robot-assisted surgery or laparoscopic surgery for rectal cancer in 951 hospitals between April 2018 and March 2020. Among them, we excluded 4064 patients whose clinical stage was unknown or 0 and 1571 patients who underwent concurrent surgery for other malignancies. After PS matching, we included 3984 patients (1992 in each group) for analysis for LAR, 714 patients (357 in each group) for HAR, and 620 patients (310 in each group) for APR.
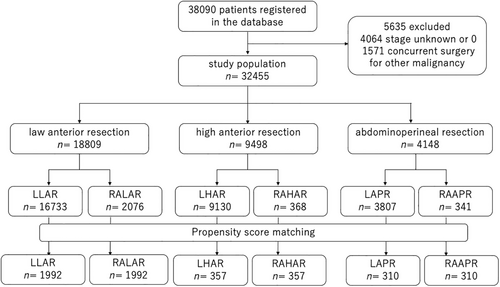
Tables 1–3 show the characteristics of the patients and hospitals among unmatched and PS-matched patients for each procedure.
| Characteristics of patients | Before propensity score-matching | After propensity score-matching | ||||
|---|---|---|---|---|---|---|
| LLAR (N = 16 733) | RALAR (N = 2076) | p-Value | LLAR (N = 1992) | RALAR (N = 1992) | p-Value | |
| n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | |||
| Age (years) | 67.5 (11.2) | 64.3 (11.6) | <0.001 | 64.4 (11.5) | 64.7 (11.5) | 0.468 |
| Sex | 0.584 | 0.299 | ||||
| Male | 10 933 (65.3) | 1369 (65.9) | 1333 (66.9) | 1302 (65.4) | ||
| Female | 5800 (34.7) | 707 (34.1) | 659 (33.1) | 690 (34.6) | ||
| BMI (kg/m2) | 22.8 (3.5) | 22.7 (3.4) | 0.271 | 22.7 (3.4) | 22.7 (3.4) | 0.628 |
| Smoking history | <0.001 | 0.559 | ||||
| Never | 7589 (45.4) | 874 (42.1) | 824 (41.4) | 851 (45.7) | ||
| Past or current | 7722 (46.2) | 967 (46.6) | 957 (48.0) | 923 (46.3) | ||
| Missing | 1422 (8.5) | 235 (11.3) | 211 (10.6) | 218 (10.9) | ||
| ADL (ability to walk) | <0.001 | 0.478 | ||||
| Walks without aid | 15 779 (95.1) | 2007 (97.1) | 1939 (97.4) | 1925 (97.1) | ||
| Walks with aid | 374 (2.3) | 25 (1.2) | 17 (0.9) | 24 (1.2) | ||
| Moves with wheelchair | 155 (0.9) | 18 (0.9) | 15 (0.8) | 18 (0.9) | ||
| Requires total assistance | 186 (1.1) | 13 (0.6) | 13 (0.7) | 13 (0.7) | ||
| Missing | 91 (0.6) | 3 (0.2) | 6 (0.3) | 2 (0.1) | ||
| Preoperative comorbidities | ||||||
| Diabetes mellitus | 3243 (19.4) | 312 (15.0) | <0.001 | 282 (14.2) | 302 (15.2) | 0.370 |
| Hypertension | 4126 (24.7) | 286 (13.8) | <0.001 | 267 (13.4) | 281 (14.1) | 0.520 |
| COPD | 340 (2.0) | 35 (1.7) | 0.287 | 27 (1.4) | 35 (1.8) | 0.306 |
| Ischemic heart disease | 961 (5.7) | 81 (3.9) | 0.001 | 88 (4.4) | 80 (4.0) | 0.528 |
| Heart failure | 27 (0.2) | 2 (0.1) | 0.476 | 1 (0.1) | 2 (0.1) | 0.500* |
| CKD stage 5 | 254 (1.5) | 24 (1.2) | 0.197 | 19 (1.0) | 23 (1.2) | 0.535 |
| Cerebrovascular event | 610 (3.7) | 45 (2.2) | 0.001 | 47 (2.4) | 44 (2.2) | 0.750 |
| Dementia | 3066 (18.3) | 410 (19.8) | 0.114 | 436 (21.9) | 391 (19.6) | 0.079 |
| Cirrhosis | 26 (0.2) | 3 (0.1) | 0.905 | 0 | 3 (0.2) | 0.125* |
| Clinical T | <0.001 | 0.468 | ||||
| 1 | 2781 (16.6) | 443 (21.2) | 438 (22.0) | 408 (20.5) | ||
| 2 | 3260 (19.5) | 452 (27.8) | 416 (20.9) | 430 (21.6) | ||
| 3 | 7852 (46.9) | 915 (43.9) | 867 (43.5) | 892 (44.8) | ||
| 4 | 2650 (15.8) | 244 (11.8) | 257 (12.9) | 241 (12.1) | ||
| Missing | 190 (1.1) | 22 (1.1) | 14 (0.7) | 21 (1.1) | ||
| Clinical N | <0.001 | 0.575 | ||||
| 0 | 9620 (57.5) | 1302 (62.7) | 1231 (61.8) | 1240 (62.3) | ||
| 1 | 4640 (27.7) | 528 (25.4) | 511 (25.7) | 512 (25.7) | ||
| 2 | 2027 (12.1) | 176 (8.5) | 185 (9.3) | 175 (8.8) | ||
| 3 | 291 (1.7) | 55 (2.7) | 57 (2.9) | 50 (2.5) | ||
| Missing | 155 (0.9) | 15 (0.7) | 8 (0.4) | 15 (0.8) | ||
| Hospital type | <0.001 | 0.773 | ||||
| Educational hospital | 3560 (21.3) | 1196 (57.6) | 1138 (57.1) | 1129 (56.7) | ||
| Other hospital | 13 173 (78.7) | 880 (42.4) | 854 (42.9) | 863 (43.3) | ||
- Abbreviations: ADL, activity of daily living; BMI, body math index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; LLAR, laparoscopic low anterior resection; RALAR, robot-assisted low anterior resection.
- * Fisher's exact test.
| Characteristics of patients | Before propensity score-matching | After propensity score-matching | ||||
|---|---|---|---|---|---|---|
| LHAR (N = 9130) | RAHAR (N = 368) | p-Value | LHAR (N = 357) | RAHAR (N = 357) | p-Value | |
| n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | |||
| Age (years) | 69.0 (11.4) | 67.4 (11.6) | 0.008 | 68.1 (11.6) | 67.4 (11.6) | 0.441 |
| Sex | 0.073 | 0.696 | ||||
| Male | 5478 (60.0) | 238 (64.7) | 233 (65.3) | 228 (63.9) | ||
| Female | 3652 (40.0) | 130 (35.3) | 124 (34.7) | 129 (36.1) | ||
| BMI (kg/m2) | 22.6 (3.5) | 22.4 (3.3) | 0.158 | 22.7 (3.4) | 22.4 (3.3) | 0.170 |
| Smoking history | 0.023 | 0.540 | ||||
| Never | 4512 (49.4) | 165 (44.8) | 147 (41.2) | 161 (45.1) | ||
| Past or current | 3809 (41.7) | 156 (42.4) | 161 (45.1) | 153 (42.9) | ||
| Missing | 809 (8.9) | 47 (12.8) | 49 (13.7) | 43 (12.0) | ||
| ADL (ability to walk) | 0.469 | 0.347 | ||||
| Walks without aid | 8363 (92.9) | 349 (95.1) | 345 (96.9) | 340 (95.5) | ||
| Walks with aid | 306 (3.4) | 10 (2.7) | 4 (1.1) | 9 (3.5) | ||
| Moves with wheelchair | 124 (1.4) | 2 (0.5) | 2 (0.6) | 2 (0.6) | ||
| Requires total assistance | 165 (1.8) | 4 (1.1) | 5 (1.4) | 3 (0.8) | ||
| Missing | 49 (0.5) | 2 (0.5) | 0 | 2 (0.6) | ||
| Preoperative comorbidities | ||||||
| Diabetes mellitus | 1775 (80.6) | 63 (17.1) | 0.269 | 48 (13.5) | 58 (16.3) | 0.293 |
| Hypertension | 2246 (24.6) | 54 (14.7) | <0.001 | 52 (14.6) | 54 (15.1) | 0.833 |
| COPD | 160 (1.8) | 7 (1.9) | 0.830 | 5 (1.4) | 6 (1.7) | 0.500* |
| Ischemic heart disease | 550 (6.0) | 21 (5.7) | 0.802 | 20 (5.6) | 20 (5.6) | 1.000 |
| Heart failure | 24 (0.3) | 0 | 0.325 | 0 | 0 | |
| CKD stage 5 | 165 (1.8) | 12 (3.3) | 0.043 | 8 (2.2) | 10 (2.8) | 0.633 |
| Cerebrovascular event | 394 (4.3) | 12 (3.3) | 0.327 | 12 (3.4) | 12 (3.4) | 1.000 |
| Dementia | 1679 (18.4) | 82 (22.3) | 0.060 | 68 (19.1) | 78 (21.9) | 0.353 |
| Cirrhosis | 16 (0.2) | 0 | 0.422 | 0 | 0 | |
| Clinical T | 0.112 | 0.342 | ||||
| 1 | 1595 (17.5) | 73 (19.8) | 76 (21.3) | 71 (19.9) | ||
| 2 | 1474 (16.1) | 75 (20.3) | 67 (18.9) | 72 (20.2) | ||
| 3 | 4204 (45.9) | 155 (42.0) | 144 (40.3) | 150 (42.0) | ||
| 4 | 1731 (18.9) | 61 (15.5) | 58 (16.3) | 60 (16.8) | ||
| Missing | 126 (1.4) | 4 (1.1) | 12 (3.4) | 4 (1.1) | ||
| Clinical N | 0.086 | 0.896 | ||||
| 0 | 5379 (58.9) | 244 (66.3) | 237 (66.4) | 236 (66.1) | ||
| 1 | 2636 (28.9) | 89 (24.2) | 91 (25.5) | 88 (24.7) | ||
| 2 | 906 (9.9) | 29 (7.9) | 21 (5.9) | 27 (7.6) | ||
| 3 | 98 (1.1) | 3 (0.8) | 4 (1.1) | 3 (0.8) | ||
| Missing | 111 (1.2) | 3 (0.8) | 4 (1.1) | 3 (0.8) | ||
| Hospital-level factors | ||||||
| Hospital type | <0.001 | 0.454 | ||||
| Educational hospital | 1767 (19.4) | 174 (47.3) | 176 (49.3) | 166 (46.5) | ||
| Other hospital | 7363 (80.7) | 194 (52.7) | 181 (50.7) | 191 (53.5) | ||
- Abbreviations: ADL, activity of daily living; BMI, body math index; CKD, chronic kidney disease.; COPD, chronic obstructive pulmonary disease; LHAR, laparoscopic high anterior resection; RAHAR, robot-assisted high anterior resection.
- * Fisher's exact test.
| Characteristics of patients | Before propensity score-matching | After propensity score-matching | ||||
|---|---|---|---|---|---|---|
| LAPR (N = 3807) | RAAPR (N = 341) | LAPR (N = 310) | (RAAPR N = 310) | |||
| n (%) or mean (SD) | n (%) or mean (SD) | p-Value | n (%) or mean (SD) | n (%) or mean (SD) | p-Value | |
| Age (years) | 70.3 (11.1) | 68.3 (12.6) | 0.001 | 69.1 (11.3) | 68.5 (12.2) | 0.552 |
| Sex | 0.437 | 0.618 | ||||
| Male | 2447 (64.3) | 212 (62.2) | 188 (60.7) | 194 (62.6) | ||
| Female | 1360 (35.7) | 129 (37.8) | 122 (39.4) | 116 (37.4) | ||
| BMI (kg/m2) | 22.2 (3.4) | 22.2 (3.3) | 0.984 | 22.2 (3.4) | 22.3 (3.3) | 0.855 |
| Smoking history | 0.665 | 0.968 | ||||
| Never | 1771 (46.5) | 150 (44.0) | 140 (45.2) | 137 (44.2) | ||
| Past or current | 1658 (43.6) | 155 (45.5) | 138 (44.5) | 141 (45.5) | ||
| Missing | 378 (9.9) | 36 (10.6) | 32 (10.3) | 32 (10.3) | ||
| ADL (ability to walk) | 0.286 | 0.555 | ||||
| Walks without aid | 3398 (91.1) | 320 (93.8) | 287 (92.6) | 290 (93.6) | ||
| Walks with aid | 158 (4.2) | 12 (3.5) | 9 (2.9) | 12 (3.9) | ||
| Moves with wheelchair | 74 (2.0) | 5 (1.5) | 9 (2.9) | 5 (1.6) | ||
| Requires total assistance | 60 (1.6) | 4 (1.2) | 5 (1.6) | 3 (1.0) | ||
| Missing | 39 (1.1) | 0 | 0 | 0 | ||
| Preoperative comorbidities | ||||||
| Diabetes mellitus | 699 (18.4) | 49 (14.4) | 0.066 | 49 (15.8) | 46 (14.8) | 0.738 |
| Hypertension | 953 (25.0) | 57 (16.7) | 0.001 | 54 (17.4) | 55 (17.7) | 0.916 |
| COPD | 84 (2.2) | 4 (1.2) | 0.205 | 2 (0.7) | 3 (1.0) | 0.500* |
| Ischemic heart disease | 208 (5.5) | 14 (4.1) | 0.286 | 14 (4.5) | 14 (4.5) | 1.000 |
| Heart failure | 5 (0.1) | 0 | 0.651* | 0 | 0 | |
| CKD stage 5 | 63 (1.7) | 2 (0.6) | 0.128 | 1 (0.3) | 2 (0.7) | 0.500* |
| Cerebrovascular event | 166 (4.4) | 9 (2.6) | 0.130 | 6 (1.9) | 7 (2.3) | 0.779 |
| Dementia | 668 (17.6) | 77 (22.6) | 0.020 | 69 (22.3) | 65 (21.0) | 0.696 |
| Cirrhosis | 12 (0.3) | 2 (0.6) | 0.408 | 2 (0.7) | 2 (0.7) | 0.688* |
| Clinical T | 0.012 | 0.703 | ||||
| 1 | 294 (7.7) | 34 (10.0) | 31 (10.0) | 30 (9.7) | ||
| 2 | 776 (20.4) | 61 (18.0) | 56 (18.1) | 56 (18.1) | ||
| 3 | 1961 (51.5) | 153 (44.9) | 130 (41.9) | 143 (46.1) | ||
| 4 | 722 (19.0) | 87 (25.5) | 84 (27.1) | 76 (24.5) | ||
| Missing | 54 (1.4) | 6 (1.8) | 9 (2.9) | 5 (1.6) | ||
| Clinical N | 0.750 | 0.588 | ||||
| 0 | 2016 (53.0) | 180 (52.8) | 158 (50.7) | 169 (54.5) | ||
| 1 | 1023 (26.9) | 84 (24.6) | 78 (25.2) | 76 (24.5) | ||
| 2 | 486 (12.8) | 50 (14.7) | 53 (17.1) | 40 (12.9) | ||
| 3 | 232 (6.1) | 21 (6.2) | 16 (5.2) | 20 (6.5) | ||
| Missing | 50 (1.3) | 6 (1.8) | 6 (1.9 | 5 (1.6) | ||
| Hospital-level factors | ||||||
| Hospital type | ||||||
| Educational hospital | 939 (24.7) | 223 (65.4) | <0.001 | 195 (62.9) | 196 (63.2) | 0.934 |
| Other hospital | 1868 (75.3) | 188 (34.6) | 115 (37.1) | 114 (36.8) | ||
- Abbreviations: ADL, activity of daily living; BMI, body math index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; LAPR, laparoscopic abdominoperineal resection; RAAPR, robot-assisted abdominoperineal resection.
- * Fisher's exact test.
Table 1 shows the characteristics of patients who underwent LAR. Before PS matching, patients who underwent robot-assisted surgery were more likely to be younger than those who underwent laparoscopic surgery (64.3 vs. 67.5 years, p < 0.001) and to have better ADL (walks without aid, 97.1% vs. 95.1%, p < 0.001), and less likely to have diabetes (312 [15.0%] vs. 3243 [19.4%], p < 0.001) or hypertension (286 [13.8%] vs. 4126 [24.7%], p < 0.001). Table 2 shows the characteristics of patients who underwent HAR. For HAR patients, fewer patients receiving robot-assisted surgery had hypertension (54 [14.7%] vs. 2246 [24.6%], p < 0.001), and more patients receiving robot-assisted surgery had stage5 CKD (12 [3.3%] vs. 165 [1.8%], p = 0.043). Table 3 shows the characteristics of patients who underwent APR. For APR, fewer patients had hypertension (57 [16.7%] vs. 953 [25.0%], p = 0.001) in robot-assisted group. Patients with dementia were more likely to be in the robot-assisted group (77 [22.6%] vs. 668 [17.6%], p = 0.02). Robot-assisted LAR and APR were more often performed in educational hospitals than in other types of hospitals (LAR: 57.6% vs. 42.4%, p < 0.001, APR: 65.4% vs. 34.6%, p < 0.001), but HAR was more likely to be performed in non-educational hospitals (47.3% vs. 52.7%, p < 0.001). Balance of covariates was achieved because the standardized biases were <10% for all variables.
Table 4 shows the short-term outcomes of patients undergoing robot-assisted surgery and laparoscopic surgery for LAR. Although not significant, the complication rate tended to be lower for robot-assisted surgery than for laparoscopic surgery (12.7% vs. 14.7% [p = 0.060]). No significant differences were found in the frequency of reoperations, and inpatient mortality. Anesthesia time was significantly longer (452.8 min vs. 388.6 min, p < 0.001) and hospital stay was significantly shorter (20.0 days vs. 22.3 days, p < 0.001) for robot-assisted surgery compared with laparoscopic surgery, and total costs were significantly lower for robot assisted surgery (1955216.6 JPY vs. 2031511.6 JPY, p < 0.001).
| Before propensity score matching | After propensity score matching | ||||||
|---|---|---|---|---|---|---|---|
| LLAR (N = 16 733) | RALAR (N = 2076) | p-Value | LLAR (N = 1992) | RALAR (N = 1992) | OR (95% CI) or β (95% CI) | p-Value | |
| n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | ||||
| Postoperative complications | |||||||
| Ileus | 908 (5.4) | 92 (4.4) | 0.057 | 95 (4.8) | 89 (4.4) | 0.93 (0.69, 1.26) | 0.651 |
| Anastomotic leakage | 752 (4.5) | 64 (3.1) | 0.003 | 72 (3.6) | 61 (3.1) | 0.84 (0.60, 1.19) | 0.333 |
| Peritonitis | 438 (2.6) | 49 (2.4) | 0.463 | 60 (3.0) | 45 (2.3) | 0.75 (0.50, 1.10) | 0.141 |
| Intestinal Ischemia | 29 (0.2) | 3 (0.1) | 0.764 | 6 (0.3) | 3 (0.2) | 0.50 (0.13, 2.00) | 0.327 |
| Surgical site infection | 307 (1.8) | 44 (2.1) | 0.366 | 39 (2.0) | 43 (2.2) | 1.11 (0.71, 1.73) | 0.651 |
| Intraabdominal bleeding | 21 (0.1) | 1 (0.1) | 0.331 | 1 (0.1) | 1 (0.1) | 1.00 (0.06, 15.99) | 1.000 |
| Septic shock | 2 (0.0) | 0 | 0.791 | 0 | 0 | ||
| Urinary tract infection | 149 (0.9) | 18 (0.9) | 0.915 | 24 (1.2) | 16 (0.8) | 0.67 (0.35, 1.25) | 0.209 |
| Deep venous thrombosis | 106 (0.6) | 2 (0.1) | 0.002 | 6 (0.3) | 2 (0.1) | 0.33 (0.07, 1.65) | 0.178 |
| Pneumonia | 131 (0.8) | 10 (0.5) | 0.133 | 12 (0.6) | 10 (0.5) | 0.83 (0.36, 1.93) | 0.670 |
| Pulmonary embolism | 22 (0.1) | 2 (0.1) | 0.672 | 3 (0.2) | 2 (0.1) | 0.67 (0.11, 3.99) | 0.657 |
| Cardiac infarction | 10 (0.1) | 1 (0.1) | 0.653 | 2 (0.1) | 1 (0.1) | 0.50 (0.05, 5.51) | 0.571 |
| Cerebrovascular event | 40 (0.2) | 4 (0.2) | 0.680 | 2 (0.1) | 4 (0.2) | 2.00 (0.37, 10.92) | 0.423 |
| Dysuria | 206 (1.2) | 25 (1.2) | 0.917 | 31 (1.6) | 22 (1.1) | 0.71 (0.41, 1.23) | 0.219 |
| Any complications | 1607 (15.6) | 266 (12.8) | 0.001 | 293 (14.7) | 252 (12.7) | 0.84 (0.70, 1.01) | 0.060 |
| Reoperation | 1125 (6.7) | 115 (5.5) | 0.040 | 129 (6.5) | 107 (5.4) | 0.88 (0.67, 1.14) | 0.318 |
| In-hospital mortality | 53 (0.3) | 0 | 0.010 | 1 (0.1) | 0 | ||
| Anesthesia time (minutes) | 375.7 (121.1) | 453.6 (141.9) | <0.001 | 388.6 (126.9) | 452.8 (141.7) | 64.37 (55.95, 72.78) | <0.001 |
| Hospital stay (days) | 23 3 (17.6) | 20.0 (12.1) | <0.001 | 22.3 (16.2) | 20.0 (12.0) | −2.27 (−3.14, −1.40) | <0.001 |
| Cost (JPY) | 2063612.6 (737026.7) | 1955093.7 (578832.8) | <0.001 | 2031511.6 (651981.5) | 1955216.6 (581438.3) | −76295.06 (−114094.80, −38495.32) | <0.001 |
- Abbreviations: LLAR, laparoscopic low anterior resection; RALAR, robot-assisted low anterior resection.
Table 5 shows the short-term outcomes following robot-assisted surgery and laparoscopic surgery for HAR. No significant differences in the frequency of all complications, reoperations, and inpatient mortality were observed between the two procedures. Anesthesia time was significantly longer (393.5 min vs. 300.9 min, p < 0.001) for robot-assisted surgery compared with laparoscopic surgery. Hospital stay (17.4 days vs. 17.2 days, p = 0.787) and total costs (1777216.3 JPY vs. 1738661.9 JPY, p = 0.341) were not significantly different between the two techniques, but total costs were slightly higher for robot-assisted surgery than for laparoscopic surgery.
| Before propensity score matching | After propensity score matching | ||||||
|---|---|---|---|---|---|---|---|
| LHAR (N = 9130) | RAHAR (N = 368) | p-Value | LHAR (N = 357) | RAHAR (N = 357) | OR (95% CI) or β (95% CI) | p-Value | |
| n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | ||||
| Postoperative complications | |||||||
| Ileus | 318 (3.5) | 11 (3.0) | 0.611 | 17 (4.8) | 10 (2.8) | 0.56 (0.25, 1.27) | 0.167 |
| Anastomotic leakage | 205 (2.3) | 3 (0.8) | 0.066 | 8 (2.2) | 3 (0.8) | 0.38 (0.10, 1.41) | 0.147 |
| Peritonitis | 112 (1.2) | 6 (1.6) | 0.493 | 6 (1.7) | 5 (1.4) | 0.83 (0.25, 2.73) | 0.763 |
| Intestinal Ischemia | 16 (0.2) | 2 (0.5) | 0.111 | 1 (0.3) | 1 (0.3) | 1.50 (0.42, 5.32) | 0.530 |
| Surgical site infection | 118 (1.3) | 7 (1.9) | 0.314 | 4 (1.1) | 6 (1.7) | 2.50 (0.49, 12.89) | 0.273 |
| Intraabdominal bleeding | 17 (0.2) | 2 (0.5) | 0.133 | 1 (0.3) | 2 (0.6) | 2.00 (0.18, 22.06) | 0.571 |
| Septic shock | 1 (0.0) | 0 | 0 | 0 | |||
| Urinary tract infection | 52 (0.6) | 1 (0.3) | 0.452 | 3 (0.8) | 1 (0.3) | 0.33 (0.03, 3.20) | 0.341 |
| Deep venous thrombosis | 43 (0.5) | 2 (0.5) | 0.843 | 2 (0.6) | 2 (0.6) | 1.00 (0.14, 7.10) | 1.000 |
| Pneumonia | 59 (0.7) | 2 (0.5) | 0.809 | 0 | 2 (0.6) | ||
| Pulmonary embolism | 12 (0.1) | 0 | 0.486 | 0 | 0 | ||
| Cardiac infarction | 7 (0.1) | 0 | 0 | 0 | |||
| Cerebrovascular event | 33 (0.4) | 1 (0.3) | 0.778 | 0 | 1 (0.3) | ||
| Dysuria | 64 (0.7) | 2 (0.5) | 0.721 | 3 (0.8) | 2 (0.6) | 0.67 (0.11, 3.99) | 0.657 |
| Any complications | 911 (10.0) | 39 (10.6) | 0.698 | 42 (11.8) | 35 (9.8) | 0.82 (0.51, 1.31) | 0.407 |
| Reoperation | 267 (2.9) | 12 (3.3) | 0.708 | 6 (1.7) | 10 (2.8) | 1.67 (0.61, 4.59) | 0.323 |
| In-hospital mortality | 28 (0.3) | 1 (0.3) | 0.905 | 0 | 0 | ||
| Anesthesia time (minutes) | 304.3 (109.4) | 395.9 (120.0) | <0.001 | 300.9 (84.5) | 393.5 (118.7) | 92.34 (77.42107.27) | <0.001 |
| Hospital stay (days) | 19.3 (17.8) | 17.6 (10.0) | 0.076 | 17.2 (11.0) | 17.4 (9.7) | 0.21 (−1.32, 1.74) | 0.787 |
| Cost (JPY) | 1813539.2 (718755.2) | 1798502.5 (552102.7) | 0.692 | 1738661.9 (551372.4) | 1777216.3 (513187.2) | 38554.39 (−40944.73118053.50) | 0.341 |
- Abbreviations: LHAR, laparoscopic high anterior resection; RAHAR, robot-assisted high anterior resection.
Table 6 shows the short-term outcomes of patients undergoing robot-assisted surgery and laparoscopic surgery for APR. For this procedure, anastomotic leakage was not included as a complication. There were no significant differences in the frequency of complications, reoperations, and inpatient mortality. Anesthesia time was significantly longer (533.5 min vs. 478.5 min, p < 0.001) and hospital stay was shorter (25.9 days vs. 29.2 days, p = 0.029) in patients receiving robot-assisted surgery. Total costs tended to be lower for robot-assisted surgery (2136465.1 JPY vs. 2215140.0 JPY, p = 0.188), but the difference was not significant.
| Before propensity score matching | After propensity score matching | ||||||
|---|---|---|---|---|---|---|---|
| LAPR (N = 3807) | RAAPR (N = 341) | p-Value | LAPR (N = 310) | RAAPR (N = 310) | OR (95% CI) or β (95% CI) | p-Value | |
| n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | n (%) or mean (SD) | ||||
| Postoperative complications | |||||||
| Ileus | 222 (5.8) | 14 (4.1) | 0.187 | 19 (6.1) | 12 (3.9) | 0.63 (0.31, 1.30) | 0.213 |
| Peritonitis | 58 (1.5) | 8 (2.4) | 0.245 | 5 (1.6) | 7 (2.3) | 1.40 (0.44, 4.41) | 0.566 |
| Intestinal ischemia | 18 (0.5) | 0 | 0.203 | 1 (0.3) | 0 | ||
| Surgical site infection | 129 (3.4) | 8 (2.4) | 0.302 | 5 (1.6) | 7 (2.3) | 1.40 (0.44, 4.41) | 0.566 |
| Intraabdominal bleeding | 3 (0.1) | 0 | 0.773* | 0 | 0 | ||
| Septic shock | 1 (0.0) | 0 | 0.918* | 0 | 0 | ||
| Urinary tract infection | 80 (2.1) | 5 (1.5) | 0.428 | 2 (0.7) | 4 (1.3) | 2.00 (0.37, 10.92) | 0.423 |
| Deep venous thrombosis | 17 (0.5) | 1 (0.3) | 0.680 | 2 (0.7) | 1 (0.3) | 0.50 (0.05, 5.51) | 0.571 |
| Pneumonia | 42 (1.1) | 1 (0.3) | 0.157 | 5 (1.6) | 1 (0.3) | 0.20 (0.02, 1.71) | 0.142 |
| Pulmonary embolism | 6 (0.2) | 0 | 0.597* | 1 (0.3) | 0 | ||
| Cardiac infarction | 4 (0.1) | 0 | 0.709* | 0 | 0 | ||
| Cerebrovascular event | 18 (0.5) | 0 | 0.203 | 2 (0.7) | 0 | ||
| Dysuria | 77 (2.0) | 6 (1.8) | 0.740 | 3 (1.0) | 5 (1.6) | 1.67 (0.40, 6.97) | 0.484 |
| Any complications | 629 (16.5) | 41 (12.0) | 0.031 | 45 (14.5) | 35 (11.3) | 0.76 (0.47, 1.21) | 0.240 |
| Reoperation | 155 (4.1) | 12 (3.5) | 0.619 | 7 (2.3) | 12 (3.9) | 1.71 (0.67, 4.35) | 0.257 |
| In-hospital mortality | 25 (0.7) | 0 | 0.133 | 2 (0.7) | 0 | ||
| Anesthesia time (minutes) | 462.8 (146.5) | 532.2 (164.6) | <0.001 | 478.5 (154.1) | 533.5 (167.7) | 55.74 (29.54, 81.95) | <0.001 |
| Hospital stay (days) | 30.6 (19.7) | 26.0 (20.1) | <0.001 | 29.2 (16.4) | 25.9 (20.1) | −3.25 (−6.16, −0.34) | 0.029 |
| Cost (JPY) | 2245275.6 (823242.4) | 2150283.6 (870307.9) | 0.042 | 2215140.0 (640781.6) | 2136465.1 (814109.5) | −78674.82 (−196010.90, 38661.26) | 0.188 |
- Abbreviations: LAPR, laparoscopic abdominoperineal resection; RAAPR, robot-assisted abdominoperineal resection.
- * Fisher's exact test.
4 DISCUSSION
An important feature of the DPC database is that it is linked to medical insurance claims, and the total medical costs are thus recorded for each patient. This study was therefore uniquely able to compare the costs of robot-assisted and laparoscopic surgery in Japan. Notably, the study also included patients undergoing all surgical procedures for rectal cancer, compared with a previous study12 focusing only on LAR.
The present study showed that robot-assisted surgery was associated with longer anesthesia time for all procedures, and shorter hospitalization for LAR and APR, and lower costs for LAR, compared with laparoscopic surgery. Anesthesia time was longer for robot-assisted surgery for all three procedures, consistent with previous studies.4, 6, 7, 12 This is possibly because of the inclusion of a docking procedure that is not required for laparoscopic surgery. However, the time from admission to docking is expected to decrease as the team's surgical experience increases,16 and anesthesia time may thus decrease and become similar to that of laparoscopic surgery as the number of surgeries increases. Furthermore, this study covered the first 2 years since robot-assisted rectal cancer surgery became covered by insurance in Japan, when many facilities introduced robot-assisted surgery simultaneously. In Japan, the first 10 cases were self-funded and were not registered in the DPC database, and only the 11th and subsequent cases were registered at each institution. Previous studies have reported the learning curve for robot-assisted surgery levels of between 15 and 45 cases,17-19 and many hospitals were thus presumably in the middle of the learning curve. In addition, a comparison of the da Vinci Si and Xi systems reported shorter operation time and postoperative hospital stay in the Xi group.20 This may be due to simplification of the docking and setup procedures and the increased range of motion, which eliminates the need for re-docking. Further technological developments and platform advancements will continue to improve surgical outcomes.
This study found no significant differences in rates of all complications, in accord with several previous RCTs.4-6 The REAL trial showed a low rate of Clavien–Dindo II or higher complications.7 Furthermore, the rate of reoperation in the current study, defined as surgery under general anesthesia after the first postoperative day, was approximately 6% for LAR, which was similar to a previous study in Japan.12
For patients undergoing APR, rates of overall complications (35 [11.3%] vs. 45 [14.5%], p = 0.240) tended to be lower in the robot-assisted group. A single-center study by Kasai et al.21 showed that robot-assisted surgery tended to have a lower complication rate for APR, and shortened hospital stay by 3 days. The current results for hospital stay (25.9 days vs. 29.2 days, p = 0.029) confirmed the previous report.
In our study, robot-assisted surgery resulted in a significantly shorter hospital stay for LAR (20.0 days vs. 22.3 days, p < 0.001) and APR (25.9 days vs. 29.2 days, p = 0.029), consistent with previous studies. The shorter hospital stay may be associated with the open-conversion rate, time to first flatus, and overall complication rate.7, 22 Previous reports7, 12 also showed that the time to first flatus was significantly shorter following robot-assisted surgery, and the shorter recovery time for intestinal peristalsis may contribute to a shorter hospital stay. In the present study, the rate of all complications tended to be lower in patients undergoing LAR and APR, which may also have affected the length of hospital stay.
In this study, the total cost of robot-assisted surgery was significantly lower than that of laparoscopic surgery for LAR (1955216.6 JPY vs. 2031511.6 JPY, p < 0.001), and tended to be lower for APR (2136465.1 JPY vs. 2215140.0 JPY, p = 0.188) and higher for HAR (1777216.3 JPY vs. 1738661.9 JPY, p = 0.341), though these differences were not significant. LAR and APR tended to have lower overall complication rates (LAR; 252 [12.7%] vs. 293 [14.7%], p = 0.060, APR; 35 [11.3%] vs. 45 [14.5%], p = 0.240) though these results were not significant. LAR and APR showed shorter hospital stays in the robot-assisted group than in the laparoscopic group (LAR; 20.0 days vs. 22.3 days, p < 0.001, APR; 25.9 days vs. 29.2 days, p = 0.029). In contrast, although the difference was not significant, robot-assisted surgery for HAR was associated with an extended hospital stay (17.4 days vs. 17.2 days, p = 0.787), which may have led to cost differences.
Few studies have examined the costs of robot-assisted rectal cancer surgery,8, 9, 23, 24 but all of these found that the cost of robot-assisted surgery tended to be higher. However, Quijano et al.25 showed that the total costs were similar, and although the surgical cost was higher for robot-assisted compared with laparoscopic surgery, robotic surgery was more cost effective. Feng et al.7 found that the preoperative costs of robot-assisted and laparoscopic surgery were similar. One study suggested that the use of robots in multiple departments may decrease maintenance costs, thereby decreasing surgical costs, and further reducing the overall cost of robot-assisted surgery.26 Increasing the number of diseases for which robotic-assisted surgery is indicated may further lower costs.
Although none of the differences were significant, robot-assisted surgery tended to show a longer hospital stay than laparoscopic surgery, possibly due to the lower percentage of educational hospitals used for robot-assisted surgery (46.5% vs. 53.5%). Huang et al.27 reported on the postoperative results of robot-assisted surgery for rectal cancer at three high-volume centers in Taiwan. The complication rate was 14.4%, which was lower than previously reported. Yamaguchi et al.28 also reported a low postoperative complication rate at a high-volume center in Japan. These results suggest that surgical outcomes of robot-assisted surgery are good at high-volume centers. Carrying out robot-assisted surgery in non-educational hospitals with fewer cases may potentially increase the overall complication rates and hospital stays, resulting in higher costs, while carrying out the procedure in well-experienced high-volume centers may reduce total costs.
This study had several limitations. First, DPC data does not include objective data such as laboratory values, and disease names are added at the discretion of the attending physician. The criteria may therefore be ambiguous. However, Yamana et al.29 reported that the validity of diagnostic names in DPC was 78.9% for sensitivity and 93.2% for specificity, with some cases of underestimation in which the necessary diagnoses were not registered, but few cases of inaccurate diagnoses. There may thus be a certain degree of reliability in assessing complication rates using DPC data. Second, the DPC does not include several important pieces of information. For example, information needed to evaluate surgical outcomes, such as operation time, blood loss, distance from the anal verge, and the type of stapler used, is lacking. Furthermore, it was impossible to extract Hartmann's procedure from the rectal resections in this study. In addition, the presence of covering stoma in anus-preserving surgery was unknown due to uncertainties in tying up information on stomas. Third, the breakdown of costs is unknown because only the total amount billed at the piece rate is available. The cost obtained by DPC is the total estimated cost, not the amount paid by the patient. However, since this reflects the cost of medical care in Japan's social security system, we believe it deserves recognition. Fourth, although the DPC can capture hospitalizations for the same disease within a fiscal year, it cannot track data for outpatient care and long-term prognosis.
5 CONCLUSIONS
This study showed that robot-assisted surgery for rectal cancer could increase operation times for all techniques, but reduce hospital days and decrease costs for LAR, compared with laparoscopic surgery. The current study also suggests that complication rates for LAR and APR may be lower for robot-assisted surgery, although the differences were not significant. Robot-assisted surgery may potentially contribute to earlier postoperative recovery and lower costs when carried out in well-trained centers.
AUTHOR CONTRIBUTIONS
Study design: MM, MK, and TF. Data collection: MM and KF. Statistical analysis and interpretation of results: MM, MK, NI, YK, and TF. Drafting of the manuscript: MM. Critical revision of the manuscript for important intellectual content: MM, MT, NI, YK, and TF. Final approval of manuscript: all authors. Accountable for all aspects of the work: all authors.
ACKNOWLEDGMENTS
We thank Susan Furness, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
CONFLICT OF INTEREST STATEMENT
YK received lecture fee from Intuitive Surgical, Johnson&Johnson, and Medtronic. Other authors declare no conflict of interest for this article.
ETHICS STATEMENTS
Approval of the research protocol: All procedures were conducted in accordance with the ethical standards of the respective committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. The study was approved by Medical Research Institute Tokyo Medical and Dental University (M2000-788).
Informed Consent: The requirement for informed consent was waived because the customized database contained de-identified and anonymized data.
Registry and the Registration No. of the study/trial: The study was approved by Medical Research Institute Tokyo Medical and Dental University (M2000-788).
Animal Studies: N/A.




