International Retrospective Cohort Study of Conversion Therapy for Stage IV Gastric Cancer 1 (CONVO-GC-1)
[Correction added on 07 April 2023, after first online publication: The copyright line has been corrected.]
Abstract
Aim
Much attention has been paid to conversion therapy for stage IV gastric cancer, however, its operative comorbidities and survival benefit have not yet been clarified. CONVO-GC-1, an international retrospective cohort study, was designed to investigate the role of conversion surgery in Japan, Korea, and China.
Methods
The rate of operative complications was the primary endpoint and the overall survival (OS), according to the four-category criteria previously published (Gastric Cancer:19; 2016), was analyzed as the secondary endpoint.
Results
A total of 1206 patients underwent surgery after chemotherapy with curative intent. Operative complications were observed in 290 (24.0%) patients in all grades, including pancreatic fistula and surgical site infection. The median survival time (MST) of all resected patients was 36.7 mo (M) and those of R0, R1, and R2 resection were 56.6 M, 25.8 M, and 21.7 M, respectively. Moreover, the MST of R0 patients were 47.8 M, 116.7 M, 44.8 M in categories 1, 2, and 3, respectively, and not reached in category 4. Interestingly, the MST of P1 patients was as favorable as that of P0CY1 patients if R0 resection was achieved. The MST of patients with liver metastasis was also favorable regardless of the number of lesions, and the MST of patients with para-aortic lymph node (LN) No 16a1/b2 metastasis was not inferior to that of patients with para-aortic LN No 16a2/b1 metastasis.
Conclusion
Conversion therapy for stage IV gastric cancer is safe and could be a new therapeutic strategy to improve the survival of patients, especially those with R0 resection.
1 INTRODUCTION
Gastric cancer is the fifth most common cancer and third leading cause of cancer-related deaths worldwide. The Asian population accounts for ~75% of both incidence and mortality in patients with gastric cancer worldwide.1 Advanced gastric cancer with metastasis to another organ is categorized as M1 and classified as stage IV in both the UICC TNM 7th and 8th editions,2, 3 and the Japanese classification of gastric carcinoma 3rd English edition (Japanese classification).4 The initial treatment option for patients with stage IV gastric cancer is palliative systemic chemotherapy5, 6; a combination of fluoropyrimidine and platinum agents is commonly selected in East Asian countries.7-9 However, median overall survival (OS), demonstrated in several recent phase III clinical trials of first-line chemotherapy for gastric cancer patients with M1 disease was 12.5–13.8 mo,7, 9-11 which was unsatisfactory.
To improve the survival of patients, multidisciplinary treatment has recently received much attention. The REGATTA trial,12 a phase III study to determine the improvement in the survival of gastrectomy + chemotherapy vs chemotherapy alone, on stage IV gastric cancer with a single incurable factor (either hepatic metastasis, peritoneal metastasis, or para-aortic lymph node metastasis), has shown that palliative surgery followed by chemotherapy for stage IV gastric cancer did not improve the OS, and chemotherapy should be performed before primary tumor resection, except for tumor bleeding or obstruction. Therefore, conversion therapy could be the next-step strategy for stage IV gastric cancer13-22; however, its benefit has not yet been proven. Conversion therapy for gastric cancer is defined as a surgical treatment aimed at R0 resection after chemotherapy for tumors that are technically and/or oncologically unresectable or marginally resectable. Moreover, we have proposed classification categories for stage IV gastric cancer13 according to the biology of tumor burden. Using the new classification, we defined technically resectable metastasis as category 1. This category should not be regarded as conversion therapy, but as neoadjuvant chemotherapy (NAC). Categories 2, 3, and 4 were defined as patients with marginally resectable or unresectable metastasis, macroscopic peritoneal dissemination, and peritoneal disease and other organ metastasis, respectively. This classification system also defined “patients who can undergo surgery without induction chemotherapy” and “patients who can undergo surgery after chemotherapy.”
Several studies have reported the survival benefit of conversion therapy14-18, 20, 21 and attempted to answer various clinical questions such as safety of the operation, indication of the patients, chemotherapy regimens, timing of surgery, and treatment after resections. However, these reports invariably suffered from small sample size and inconsistency in patient selection. To obtain more convincing answers through analyses of big real-world data, a multi-institutional, large-scale international retrospective cohort study on conversion therapy for stage IV gastric cancer (CONVO-GC-1) was conducted in Japan, Korea, and China. In the present analysis, the safety profile and survival of patients who underwent surgery with curative intent after NAC or induction chemotherapy according to the category classification are demonstrated.
2 PATIENTS AND METHODS
2.1 Study design
This study was designed as a retrospective international cohort study to clarify the short- and long-term outcomes of conversion therapy for stage IV gastric cancer in Asian countries. Fifty-five institutions (43, five, and seven institutions from Japan, Korea, and China, respectively) participated. The primary endpoint was the postoperative complication rate in conversion therapy and the secondary endpoint was OS. Moreover, stage IV disease was stratified according to the new category classification13 as follows: category 1 included tumors that were technically resectable, such as solitary liver metastasis (up to 5 cm), para-aortic lymph node (LN) metastasis localized in station No. 16a2/b14 (para-aortic LN No 16a2/b1), or peritoneal lavage cytology positive disease without macroscopic peritoneal dissemination. These tumors were technically resectable at the initial diagnosis and could also be resected irrespective of the response to chemotherapy, except in the case of new lesions, which are not technically resectable. Chemotherapy given to this category should be regarded as NAC rather than induction chemotherapy. Category 2 included tumors with marginally resectable or unresectable metastasis such as solitary liver metastasis larger than 5 cm, multiple liver metastasis, or distant lymph node metastasis other than para-aortic LN No 16a2/b1, but without apparent peritoneal disease. Category 3 included tumors with peritoneal dissemination without any other distant metastasis. Category 4 included tumors with peritoneal dissemination accompanied by other distant metastasis. Since the aim of conversion therapy is to accomplish R0 resection, most eligible patients were expected to be responders from category 2, while patients with indication for surgery were estimated to be rare in category 3 and almost extinct in category 4.
2.2 Patients and data recruitment
Patient eligibility criteria were as follows: (a) histologically proven primary gastric cancer and clinically diagnosed stage IV patients based on the UICC classification 7th edition2 and Japanese classification of gastric carcinoma 3rd English edition (Japanese classification)4 in pretreatment evaluation; (b) patients who underwent systemic chemotherapy as initial treatment for stage IV gastric cancer, and consequently, underwent surgery composed of gastrectomy with or without resection of the metastatic lesion for curative intent; and (c) patients whose surgery was performed between January 1, 2001 and December 31, 2014. Patients with peritoneal disseminated disease (P+) (P1: metastasis of the tumors to the adjacent peritoneum, P2: metastasis of a few tumors to distant peritoneum, P3: metastasis of numerous tumors to the distant peritoneum) and/or positive lavage cytology, which were diagnosed with diagnostic staging laparoscopy or exploratory laparotomy and underwent surgery without preoperative systemic chemotherapy were also registered in the study. The chemotherapy regimens administered before and after surgery, indications, procedure, and extent of surgery were decided in each institution. Clinical and pathological evaluation of tumors was performed at each institution. Data recruitment was conducted between April 1, 2016 and March 31, 2018. The data center was located at Gifu University, Innovative and Clinical Research Promotion Center. The questionnaire concerning the sequence of diagnosis and treatment, patients’ background information at diagnosis, treatment status of systemic chemotherapy, radiotherapy as initial treatment and its clinical response, surgical and pathological information, postoperative complications, postoperative treatment, and confirmation of outcome were registered via an online entering system (Electric Data Capture System: EDC system). Clinical response to chemotherapy was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST).23 Postoperative complications were graded according to Clavien–Dindo grade.24 Pathological response was evaluated according to the Japanese classification4 or Mandard Tumor Regression Grade (TRG)25 classification.
2.3 Statistical analysis
Data analyses were entrusted to the Department of Biomedical Statistics and Bioinformatics of Kyoto University. Comparisons of continuous variables were made using the t-test. Fisher's exact test was used to compare categorical variables. P < .05 was considered to indicate statistical significance. The OS analysis was calculated using the Kaplan–Meier method, and survival was compared using the log-rank test. The OS was defined as time to death, irrespective of cause, from the date of diagnosis. P < .05 (two-sided) was defined as statistically significant. All analyses were conducted using SAS 9.4 statistical software (SAS Institute, Cary, NC).
2.4 Ethical management
All procedures were conducted in accordance with the ethical standards of the respective committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. The study was approved by the Institutional Review Board of Gifu University and each institution, and was registered with the University Hospital Medical Information Network (UMIN) Clinical Trial Registry (UMIN000022321).
3 RESULTS
3.1 Patients
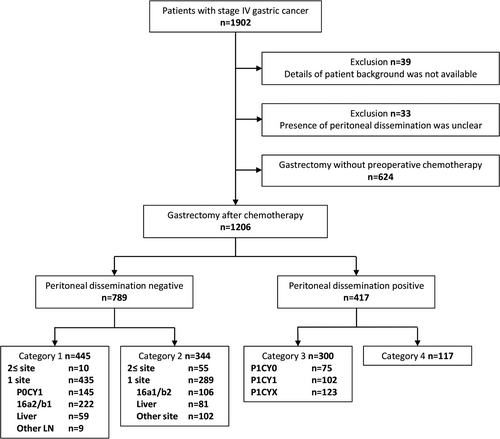
In total, 1902 patients with stage IV gastric cancer from 55 institutions (1329, 362, and 211 patients from Japan, Korea, and China, respectively) were registered in the Electric Data Capture system. Seventy-two patients were excluded because the patient background or details of metastatic status were not available. In addition, 624 patients who underwent surgery without preoperative chemotherapy were excluded, and 1206 patients were analyzed for short- and long-term outcomes (Figure 1). The number of patients in categories 1, 2, 3, and 4 were 445, 344, 300, and 117, respectively. Details of patient background at initial diagnosis are shown in Table 1. The final number of patients accrued by countries was 776 from Japan, 323 from Korea, and 107 from China.
| Characteristics | Category 1 | Category 2 | Category 3 | Category 4 | All categories |
|---|---|---|---|---|---|
| n = 445 | n = 344 | n = 300 | n = 117 | n = 1206 | |
| Age | 61.7 (10.6) | 58.8 (11.8) | 56.8 (12.4) | 54.1 (14.1) | 58.9 (12.0) |
| Sex | |||||
| Male | 305 (68.5) | 247 (71.8) | 174 (58.0) | 69 (59.0) | 795 (65.9) |
| Female | 140 (31.5) | 97 (28.2) | 126 (42.0) | 48 (41.0) | 411 (34.1) |
| Country | |||||
| China | 33 (7.4) | 55 (16.0) | 15 (5.0) | 4 (3.4) | 107 (8.9) |
| Korea | 67 (15.1) | 131 (38.1) | 73 (24.3) | 52 (44.4) | 323 (26.8) |
| Japan | 345 (77.5) | 158 (45.9) | 212 (70.7) | 61 (52.1) | 776 (64.3) |
| Histology | |||||
| Differentiated | 173 (38.9) | 140 (40.7) | 61 (20.3) | 37 (31.6) | 411 (34.1) |
| Undifferentiated | 215 (48.3) | 124 (36.0) | 199 (66.4) | 47 (40.2) | 585 (48.5) |
| Unknown | 6 (1.3) | 13 (3.8) | 2 (0.7) | 2 (1.7) | 23 (1.9) |
| Other | 50 (11.2) | 64 (18.6) | 34 (11.3) | 31 (26.5) | 179 (14.8) |
| Unevaluated | 1 (0.2) | 3 (0.9) | 4 (1.3) | 0 (0) | 8 (0.7) |
| cT grade | |||||
| T1a(M) | 1 (0.2) | 3 (0.9) | 1 (0.3) | 0 (0) | 5 (0.4) |
| T1b(SM) | 2 (0.4) | 6 (1.7) | 1 (0.3) | 0 (0) | 9 (0.7) |
| T2(MP) | 14 (3.1) | 25 (7.3) | 5 (1.7) | 2 (1.7) | 46 (3.8) |
| T3(SS) | 98 (22.0) | 88 (25.6) | 45 (15.0) | 19 (16.2) | 250 (20.7) |
| T4a(SE) | 273 (61.3) | 152 (44.2) | 205 (68.3) | 67 (57.3) | 697 (57.8) |
| T4b(SI) | 44 (9.9) | 31 (9.0) | 33 (11.0) | 18 (15.4) | 126 (10.4) |
| TX | 13 (2.9) | 39 (11.3) | 10 (3.3) | 11 (9.4) | 73 (6.1) |
| cN grade | |||||
| N0 | 55 (12.4) | 31 (9.0) | 84 (28.0) | 5 (4.3) | 175 (14.5) |
| N1 | 79 (17.8) | 43 (12.5) | 65 (21.7) | 19 (16.2) | 206 (17.1) |
| N2 | 149 (33.5) | 106 (30.8) | 90 (30.0) | 27 (23.1) | 372 (30.8) |
| N3a | 124 (27.9) | 90 (26.2) | 34 (11.3) | 36 (30.8) | 284 (23.5) |
| N3b | 18 (4.0) | 33 (9.6) | 10 (3.3) | 15 (12.8) | 76 (6.3) |
| NX | 20 (4.5) | 41 (11.9) | 17 (5.7) | 15 (12.8) | 93 (7.7) |
Note
- Continuous values are expressed as mean (SD).
- Categorical values are expressed as number (%).
3.2 Preoperative chemotherapy
Details of the first-line chemotherapy regimens are summarized in Table 2. The predominant regimen was the combination of S-1 and cisplatin (SP)7 in all categories. The combination of docetaxel, cisplatin, and S-1 (DCS)26-28 and docetaxel and S-1 (DS)11, 29, 30 were common regimens in categories 1, 2, and 3; however, the combination of capecitabine and oxaliplatin (capeOX),31 capecitabine and cisplatin (XP),8 and 5-fluorouracil with l-leucovorin and oxaliplatin (FOLFOX)32 were more frequently administered than DCS in category 4. The median duration of preoperative chemotherapy, which represented the period between the disease diagnosis and gastrectomy, was 92 d in category 1, 135.5 d in category 2, 158 d in category 3, and 174 d in category 4 (Table 3). Concerning the response to preoperative chemotherapy, the overall response rate (ORR), which was the ratio of patients showing complete response (CR) and partial response (PR), for all patients in each category was 54.1% in category 1, 72.6% in category 2, 39.0% in category 3, and 61.6% in category 4 (Table 3). Of note, the other regimens, which shared 24.7% in Table 2, included a variety of combinations such as new agents in the clinical trials, intraperitoneal chemotherapy and original regimen of each institution (data not shown).
| Regimens | Category 1 | Category 2 | Category 3 | Category 4 | All categories |
|---|---|---|---|---|---|
| n = 445 | n = 344 | n = 300 | n = 117 | n = 1206 | |
| DCS | 56 (12.6) | 33 (9.6) | 28 (9.3) | 5 (4.3) | 122 (10.1) |
| SP | 222 (49.9) | 124 (36.0) | 94 (31.3) | 33 (28.2) | 473 (39.2) |
| DS | 23 (5.2) | 11 (3.2) | 25 (8.3) | 5 (4.3) | 64 (5.3) |
| XP | 14 (3.1) | 23 (6.7) | 8 (2.7) | 9 (7.7) | 54 (4.5) |
| SOX | 16 (3.6) | 22 (6.4) | 11 (3.7) | 2 (1.7) | 51 (4.2) |
| CapeOX | 9 (2.0) | 16 (4.7) | 13 (4.3) | 10 (8.5) | 48 (4.0) |
| FOLFOX | 9 (2.0) | 19 (5.5) | 8 (2.7) | 7 (6.0) | 43 (3.6) |
| 5-FU+CDDP | 5 (1.1) | 4 (1.2) | 1 (0.3) | 2 (1.7) | 12 (1.0) |
| CPT11+CDDP | 4 (0.9) | 5 (1.5) | 1 (0.3) | 0 (0) | 10 (0.8) |
| S-1 | 4 (0.9) | 7 (2.0) | 11 (3.7) | 5 (4.3) | 27 (2.2) |
| Paclitaxel | 1 (0.2) | 0 (0) | 2 (0.7) | 0 (0) | 3 (0.2) |
| Capecitabine | 0 (0) | 0 (0) | 0 (0) | 1 (0.9) | 1 (0.1) |
| Other | 82 (18.4) | 80 (23.3) | 98 (32.7) | 38 (32.5) | 298 (24.7) |
| Trastuzumab | |||||
| Administered | 22 (4.9) | 28 (8.1) | 11 (3.7) | 14 (12.0) | 75 (6.2) |
Abbreviations
- CapeOX, oxaliplatin plus capecitabine; CDDP, cisplatin; CPT-11, irinotecan; D, docetaxel plus S-1; DCS, docetaxel, cisplatin, and S-1; SP, cisplatin plus S-1; FOLFOX, 5-fluorouracil with l-leucovorin and oxaliplatin; SOX, oxaliplatin plus S-1; XP, capecitabine plus cisplatin.
- Categorical values are expressed as number (%).
- Continuous values are expressed as mean (SD).
| Factors | Category 1 | Category 2 | Category 3 | Category 4 | All categories |
|---|---|---|---|---|---|
| n = 445 | n = 344 | n = 300 | n = 117 | n = 1206 | |
| Duration (d) | 92 (75–148.5) | 135.5 (84.25–240) | 158 (92–236) | 174 (117–272) | 124 (81–209) |
| Response | |||||
| CR | 30 (6.7) | 28 (8.1) | 16 (5.3) | 7 (6.0) | 81 (6.7) |
| PR | 211 (47.4) | 222 (64.5) | 101 (33.7) | 65 (55.6) | 599 (49.7) |
| SD | 92 (20.7) | 55 (16.0) | 43 (14.3) | 22 (18.8) | 212 (17.6) |
| Non-CR/PD | 48 (10.8) | 6 (1.7) | 100 (33.3) | 10 (8.5) | 164 (13.6) |
| PD | 14 (3.1) | 20 (5.8) | 11 (3.7) | 10 (8.5) | 55 (4.6) |
| NE | 50 (11.2) | 13 (3.8) | 29 (9.7) | 3 (2.6) | 95 (7.9) |
Abbreviations
- CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluated.
- Continuous values are expressed as median (interquartile range).
- Categorical values are expressed as number (%).
- Clinical response to chemotherapy was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST guideline, v. 1.1.
3.3 Surgical treatment and pathological response
Surgical procedures and pathological evaluation of resected specimens are detailed in Table 4A and Table 4B. Total gastrectomy was performed in 67.5% of all patients. D3 lymphadenectomy33, 34 was applied to 25.2% of patients in category 1; in contrast, it was applied to 12.2%, 2.0%, and 6.0% of patients in categories 2, 3, and 4, respectively. In categories 1 and 2, ~75% of patients achieved R0 resection; however, R0 resection was achieved in <60% of patients in categories 3 and 4. Pathological response in the primary tumor was evaluated using the Japanese classification of gastric carcinoma4 in 773 patients, TRG25 classification in 69 patients, and was not available in 364 patients. The predominant pathological grade was 1a and was identified in 39.3% of patients (304/773) in whom the response was evaluated using the Japanese classification of gastric carcinoma,4 and grade 3 was identified in 7.8% of those patients (60/773). Concerning the pathological diagnosis of lymph node metastasis, ypN0 was identified in 26.5% of all patients. On the other hand, in ~40% of patients lymph node metastasis with ypN3a or ypN3b was identified, although chemotherapy was administered before surgery.
| Characteristics | Category 1 (n = 445) | Category 2 (n = 344) | Category 3 (n = 300) | Category 4 (n = 117) | All categories (n = 1206) |
|---|---|---|---|---|---|
| Surgical procedure | |||||
| TG | 300 (67.4) | 206 (59.9) | 226 (75.3) | 82 (70.1) | 814 (67.5) |
| DG | 143 (32.1) | 123 (35.8) | 70 (23.3) | 34 (29.1) | 370 (30.7) |
| PG | 2 (0.4) | 12 (3.5) | 3 (1.0) | 1 (0.9) | 18 (1.5) |
| Unresected | 0 (0) | 1 (0.3) | 0 (0) | 0 (0) | 1 (0.1) |
| Other | 0 (0) | 2 (0.6) | 1 (0.3) | 0 (0) | 3 (0.2) |
| Lymphadenectomy | |||||
| D0 | 6 (1.3) | 2 (0.6) | 3 (1.0) | 1 (0.9) | 12 (1.0) |
| D1 | 52 (11.7) | 54 (15.7) | 88 (29.3) | 27 (23.1) | 221 (18.3) |
| D2 | 271 (60.9) | 239 (69.5) | 200 (66.7) | 80 (68.4) | 790 (65.5) |
| D3 | 112 (25.2) | 42 (12.2) | 6 (2.0) | 7 (6.0) | 167 (13.8) |
| N/A | 4 (0.9) | 7 (2.0) | 3 (1.0) | 2 (1.7) | 16 (1.3) |
| Residual tumor | |||||
| R0 | 339 (76.2) | 257 (74.7) | 177 (59.0) | 66 (56.4) | 839 (69.6) |
| R1 | 55 (12.4) | 33 (9.6) | 50 (16.7) | 18 (15.4) | 156 (12.9) |
| R2 | 51 (11.5) | 53 (15.4) | 71 (23.7) | 31 (26.5) | 206 (17.1) |
| RX | 0 (0) | 1 (0.3) | 2 (0.7) | 2 (1.7) | 5 (0.4) |
Abbreviations
- DG, distal gastrectomy; PG, proximal gastrectomy; TG, total gastrectomy.
- Continuous values are expressed as mean (SD).
- Categorical values are expressed as number (%).
| Characteristics |
Category 1 (n = 445) |
Category 2 (n = 344) |
Category 3 (n = 300) |
Category 4 (n = 117) |
All categories (n = 1206) |
|---|---|---|---|---|---|
| ypT grade | |||||
| T1a(M) | 11 (2.5) | 15 (4.4) | 3 (1.0) | 1 (0.9) | 30 (2.5) |
| T1b(SM) | 31 (7.0) | 24 (7.0) | 9 (3.0) | 8 (6.8) | 72 (5.8) |
| T2(MP) | 51 (11.5) | 55 (16.0) | 16 (5.3) | 9 (7.7) | 131 (10.9) |
| T3(SS) | 133 (29.9) | 107 (31.1) | 82 (27.3) | 41 (35.0) | 363 (30.1) |
| T4a(SE) | 154 (34.6) | 87 (25.3) | 152 (50.7) | 44 (37.6) | 437 (36.2) |
| T4b(SI) | 34 (7.6) | 24 (7.0) | 26 (8.7) | 9 (7.7) | 93 (7.7) |
| TX | 31 (7.0) | 32 (9.3) | 12 (4.0) | 5 (4.3) | 80 (6.6) |
| Pathological response | |||||
| Grade 0 | 16 (3.6) | 10 (2.9) | 10 (3.3) | 1 (0.9) | 37 (3.1) |
| Grade1a | 125 (28.1) | 68 (19.8) | 86 (28.7) | 25 (21.4) | 304 (25.2) |
| Grade1b | 68 (15.3) | 45 (13.1) | 37 (12.3) | 11 (9.4) | 161 (13.3) |
| Grade 2 | 90 (20.2) | 38 (11.0) | 67 (22.3) | 16 (13.7) | 211 (17.5) |
| Grade 3 | 29 (6.5) | 19 (5.5) | 8 (2.7) | 4 (3.4) | 60 (5.0) |
| TRG1 | 3 (0.7) | 3 (0.9) | 1 (0.3) | 0 (0) | 7 (0.6) |
| TRG2 | 2 (0.4) | 9 (2.6) | 1 (0.3) | 4 (3.4) | 16 (1.3) |
| TRG3 | 8 (1.8) | 9 (2.6) | 2 (0.7) | 1 (0.9) | 20 (1.7) |
| TRG4 | 6 (1.3) | 5 (1.5) | 0 (0) | 3 (2.6) | 14 (1.2) |
| TRG5 | 2 (0.4) | 4 (1.2) | 3 (1.0) | 3 (2.6) | 12 (1.0) |
| NE | 96 (21.6) | 134 (39.0) | 85 (28.3) | 49 (41.9) | 364 (30.2) |
3.4 Postoperative complications
The number of patients with postoperative complications after conversion therapy is detailed in Table 5A. In total, 290 patients (24.0%) had some postoperative complications in any Clavien–Dindo grade. Three patients presented with Clavien–Dindo grade V postoperative complications, and the overall fatal-postoperative complication rate was 0.2%. Of these, one patient presented with anastomotic leakage in category 1, one presented with acute myocardial infarction in category 2, and one presented with cardiopulmonary arrest in category 2. The number of patients who presented with Clavien–Dindo grade II and more severe was 248 (20.6%). Details of postoperative complications are shown in Table 5B. The most frequently identified complication was pancreatic fistula in cases in which Clavien–Dindo grade was either limited to grade II and more severe or grade IIIa and more severe.
| Category | |||||
|---|---|---|---|---|---|
| 1 (n = 445) | 2 (n = 344) | 3 (n = 300) | 4 (n = 117) | Total (n = 1206) | |
| Clavien–Dindo grade | |||||
| I | 13 (2.9) | 13 (3.8) | 9 (3.0) | 7 (6.0) | 42 (3.5) |
| II | 47 (10.6) | 41 (11.9) | 27 (9.0) | 9 (7.7) | 124 (10.3) |
| IIIa | 52 (11.7) | 19 (5.5) | 13 (4.3) | 6 (5.1) | 90 (7.5) |
| IIIb | 6 (1.3) | 5 (1.5) | 4 (1.3) | 1 (0.9) | 16 (1.3) |
| Iva | 5 (1.1) | 2 (0.6) | 1 (0.3) | 1 (0.9) | 9 (0.7) |
| IVb | 3 (0.7) | 1 (0.3) | 1 (0.3) | 1 (0.9) | 6 (0.5) |
| V | 1 (0.2) | 2 (0.6) | 0 (0) | 0 (0) | 3 (0.2) |
| Total | 127 (28.5) | 83 (24.1) | 55 (18.3) | 25 (21.4) | 290 (24.0) |
Note
- Patients were allocated to each Clavien–Dindo grade according to the worst grade identified in each patient.
- Values are expressed as number (%).
| Wound infection | Thrombosis | Anastomotic leakage | Abdominal abscess | Pancreatic fistula | Pneumonia | Others | |
|---|---|---|---|---|---|---|---|
| Category | |||||||
| 1 | 23 | 6 | 13 | 26 | 30 | 11 | 56 |
| 2 | 15 | 1 | 7 | 12 | 10 | 3 | 54 |
| 3 | 7 | 2 | 10 | 12 | 13 | 3 | 21 |
| 4 | 4 | 0 | 1 | 1 | 2 | 2 | 18 |
| Clavien–Dindo grade | |||||||
| I | 15 | 1 | 1 | 0 | 3 | 1 | 36 |
| II | 23 | 6 | 11 | 18 | 16 | 13 | 63 |
| IIIa | 10 | 1 | 11 | 28 | 35 | 4 | 32 |
| IIIb | 0 | 1 | 2 | 4 | 1 | 0 | 9 |
| IVa | 1 | 0 | 1 | 1 | 0 | 0 | 6 |
| IVb | 0 | 0 | 4 | 0 | 0 | 1 | 1 |
| V | 0 | 0 | 1 | 0 | 0 | 0 | 2 |
| Total | 49 | 9 | 31 | 51 | 55 | 19 | 149 |
Note
- Values are expressed as number of patients who presented each complication.
3.5 Survival
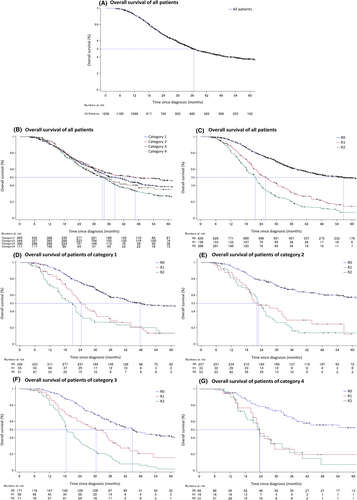
The OS is shown in Figure 2. The median survival time (MST) of all 1206 patients who underwent conversion therapy and NAC was 36.7 mo (95% confidence interval [CI]: 34.4–40.0) (Figure 2A). The MST in each category was 38.4 mo (33.5–44.1) in category 1, 46.6 mo (35.3–82.2) in category 2, 33.4 mo (29.4–37.0) in category 3, and 34.1 mo (26.9–47.5) in category 4 (Figure 2B). The MST was significantly longer in patients who underwent R0 resection (56.6 mo [46.4–74.5]) than in those who underwent R1 resection (25.8 mo (22.4–30.2]) and R2 resection (21.7 mo [18.6–22.8]) (P < .001) (Figure 2C). Similarly, the MST was significantly longer in patients who underwent R0 resection than in those who underwent R1 or R2 resection in each category. The MST of patients who underwent R0, R1, and R2 resection in each category were 47.8 mo (40.7–95.2), 24.4 mo (20.7–30.2), and 20.9 mo (15.2–24.1) (P < .001) in category 1 (Figure 2D); 116.7 mo (61.2–not reached), 22.1 mo (18.5–32.5), and 22.8 mo (19.2–25.8) (P < .001) in category 2 (Figure 2E); 44.8 mo (37.9–60.4), 30.4 mo (20.6–37.0), and 18.5 mo (17.4–22.6) (P < .001) in category 3 (Figure 2F); and not reached (37.2–not reached), 23.4 mo (15.1–29.4), and 23.5 mo (17.7–32.3) (P < .001) in category 4 (Figure 2G).
3.6 Pathological response to chemotherapy and survival of the patients
To evaluate the OS of patients who underwent conversion therapy according to their pathological response in the primary lesion, patients who achieved R0 resection were selected (n = 839). Among these, the pathological response was evaluated using the Japanese classification4 in 591 patients, TRG classification25 in 36 patients, and was not evaluated in 212 patients. Because of incompatibility between the Japanese classification and TRG classification and the small number of patients in the TRG classification group, the analysis was conducted only in patients in whom the pathological response was evaluated using the Japanese classification. The number of patients in pathological grades 0, 1a, 1b, 2, and 3 were 24, 210, 119, 179, and 59, respectively. Patients were divided into two groups: the responder group included patients with pathological grades 2 or 3, and the nonresponder group included patients with pathological grades 0, 1a, or 1b. Patient background, surgical outcomes, and details of pathological evaluation are summarized in Table S1A,B. Concerning the clinical response of these two groups, the ORR was 66.0% in patients with grades 2 or 3 and 53.0% in patients with grades 0, 1a, or 1b (P = .0021). The median OS of patients in those two groups were 95.2 mo (62.1–not reached) and 36.2 mo (31.6–42.4) (P < .0001), respectively.
3.7 Long-term outcomes of patients who underwent conversion therapy with peritoneal metastasis, para-aortic LN, and liver metastasis
As the category classification is still a mixture of various types of metastasis, extent of disease spread, and degree of tumor burden, the survival of patients was analyzed separately for each of the three major metastatic pathways: peritoneal, lymphatic, and hepatic.
3.8 Peritoneal metastasis
Category 3 included patients with macroscopic peritoneal dissemination, in which the diagnosis was confirmed by a direct method (P-direct) with diagnostic staging laparoscopy or exploratory laparotomy, and patients with peritoneal dissemination diagnosed by an indirect method (P-indirect), such as radiological examinations. To clarify the long-term outcomes of patients with P0CY1 (category 1) disease and patients with peritoneal dissemination detected by direct methods, subanalysis of the OS of patients with these diseases treated with surgery after systemic chemotherapy was also conducted. Evaluation of curability of the peritoneal disease was performed according to the Japanese classification of gastric carcinoma 3rd edition4 in each institution and investigators evaluated the R0/R1/R2 by the biopsy of the macroscopic nodules, peritoneal cytology at the time of operation. Total peritonectomy was not performed in this cohort in any country.
Patient background, preoperative chemotherapy, surgical results, and pathological diagnoses are summarized in Table S2A,B. The number of patients with P0CY1 disease and P (+)-direct were 145 and 156, respectively. Macroscopic type 4 tumors were identified in approximately half of these patients in both groups. The extent of peritoneal dissemination in patients with P (+)-direct was P1 in 52 patients, P2 in 39 patients, and P3 in 65 patients. The median duration of preoperative chemotherapy was 84 d for P0CY1 disease and 167 d for P (+)-direct. R0 resection was achieved in 73.8% of patients with P0CY1 disease and 63.5% with P-direct disease.
The OS of patients with P0CY1 disease and P (+)-direct is shown in Figure S1. The MST of patients with P0CY1 disease who underwent R0, R1, and R2 resection were 42.4 mo (34.7–not reached), 26.8 mo (23.3–38.4), and 20.9 mo (8.9–28.3) (P = .0002), respectively. The MST of patients with P (+)-direct who underwent R0, R1, and R2 resection were 41.8 mo (33.8–49.7), 27.3 mo (18.3–35.1), and 21.3 mo (17.7–29.7) (P < .0001), respectively.
3.9 Para-aortic lymph node metastasis in station LN No 16a2/b1 or 16a1/b2
This study included patients who underwent surgery after systemic chemotherapy for para-aortic lymph node metastasis in station LN No 16a2/b1 in category 1 and for metastasis in station LN No. 16a1/b2 in category 2. To clarify the long-term outcomes of these patients, we performed a subanalysis comparing the OS of patients between the two groups where patients with any other distant metastasis were excluded.
Patient background, duration, and clinical response to preoperative chemotherapy, surgical results, and pathological evaluation in patients with LN No 16a2/b1 and No 16a1/b2 are shown in Table S3A,B. The ORR of patients with LN No 16 a2/b1 was 65.3% and that of patients with LN No 16 a1/b2 was 76.4%. The number of patients who underwent D3 lymph node dissection was 95 (42.8%) in patients with LN No 16a2/b1 and 26 (24.5%) in patients with LN No 16a1/b2.
The OS of all patients with LN No 16a2/b1 and LN No 16a1/b2 are shown in Figure S2. The OS of patients with LN No 16a1/b2 was longer than that of patients with LN No 16a2/b1. The MST was 54.3 mo (32.6–not reached) in patients with LN No 16a1/b2 and 33.5 mo (29.2–43.3) in patients with LN No 16a2/b1 (P = .044) (Figure S2A). The MST of patients who underwent R0 resection was 116.7 mo (45.3–not reached) in patients with LN No 16a1/b2 and 44.7 mo (33.5–96.7) in patients with LN No 16 a2/b1 (P = .026) (Figure S2B).
3.10 Liver metastasis
In the CONVO-GC-1 study, patients with solitary liver metastasis, up to 5 cm in diameter, were included in category 1, those with liver metastasis >5 cm or with multiple liver metastases in category 2, and those with peritoneal dissemination in addition to liver metastasis in category 4. The number of patients in each category was 64 in category 1, 102 in category 2, and 11 in category 4. The MST of patients in each category was 95.2 mo (42.6–not reached) in category 1, 46.6 mo (30.0–not reached) in category 2, and not reached (15.1–not reached) in category 4. There was no statistical difference in the OS among the three categories (P = .18) (Figure S3A). Thus, to analyze the OS of patients with liver metastasis who were treated with conversion therapy according to the number of metastatic lesions, subanalysis was conducted among patients with liver metastasis, but without any other distant metastasis, in categories 1 and 2 combined. These patients were divided into three groups according to the number of liver metastasis at diagnosis: 1) patients with solitary liver metastasis regardless of the metastatic lesion size; 2) patients with two lesions of liver metastasis; and 3) patients with three or more lesions of liver metastasis. Patients in category 4 were excluded because the impact on survival of peritoneal metastasis was unclear due to the small number of patients. Patient background, preoperative chemotherapy, surgical results, and pathological diagnoses are summarized in Table S4A,B. There were 63 patients with one lesion, 24 with two lesions, and 53 with three or more lesions. R0 resection was achieved in 85.7%, 87.5%, and 81.1% of patients with one, two, and ≥3 lesions, respectively. The OS of these patients is shown in Figure S3B. The MST of patients with one lesion was 95.2 mo (42.6–not reached), two lesions was 46.6 mo (27.2–not reached), and three or more lesions was 56.6 mo (28.5–not reached) (P = .26).
4 DISCUSSION
This is the first retrospective study to reveal the short- and long-term outcomes of conversion therapy for stage IV gastric cancer on a scale of a thousand patients. The initial analysis was performed on postoperative complications because it is a crucial issue in conversion therapy for patients with stage IV gastric cancer, by which the timing of postoperative chemotherapy may be deferred. In the present analysis, postoperative complications were identified in 24.0% of patients with any Clavien–Dindo grade and 20.6% of patients with grade II or higher. Those data can be underestimated because of the retrospective nature of the study. However, the present result is acceptable compared with those demonstrated by the JCOG0405 phase II NAC trial with the SP regimen34 and the JCOG 1002 NAC phase II trial with the DCS regimen,28 whose postoperative complication rates were 55.1% and 30.6%, respectively, as evaluated by the National Cancer Institute Common Toxicity Criteria for Adverse Events.35, 36
Moreover, the current study demonstrated a remarkably long MST of patients who underwent conversion surgery, especially R0 resection, in each category of classification. Of these, the pathological response of the primary lesion was revealed to be a good prognostic marker, as demonstrated elsewhere.37 Interestingly, the MST of patients in category 1 was not necessarily superior to that of other categories, in spite of the impression that the prognosis of patients with resectable metastasis is better than that of patients with initially unresectable metastases. These results prompted us to further investigate the OS of patients with peritoneal disease, para-aortic lymph node, and liver metastasis.
Important results were detected by the subanalysis of patients with P0CY1 and P (+). Notably, the MST was favorable and similar between patients with P0CY1 and P (+) if R0 resection was achieved. Surgeries for curative intent on P0CY1 and P (+) after chemotherapy are acceptable, and it is important to confirm the CY0 and P0 situation by staging laparoscopy or a second-look operation in order to perform R0 resection. In contrast, a fatal event was continuously identified over 4 years after surgery with P (+) disease, and it was not often observed in patients with P0CY1 afterward. This may suggest a difference in potential curability between P0CY1 and P (+) disease. However, further follow-up would be required to confirm the difference.
Another interesting result was obtained by the subanalysis of the OS of patients with LN No 16a2/b1 metastasis and patients with LN No 16a1/b2, which was paradoxical. The MST of patients with LN No 16a1/b2 metastasis was longer than that of patients with LN No 16a2/b1 metastasis. One reason for this may be a selection bias of patients for surgery. As LN No 16a2/b1 metastasis is generally regarded as technically resectable,38 the operation with D3 LN dissection may have been performed irrespective of the NAC response. In contrast, for patients with LN No 16a1/b2 metastasis, curative surgery with LN dissection ranging from <D3 to D3 plus additional removal of a wider range of metastasis was performed only if the tumor had responded favorably to induction chemotherapy. Surgery aimed at R0 resection for para-aortic lymph node metastasis can be a good treatment strategy after NAC for LN No 16a2/b1 metastasis or induction chemotherapy for LN No 16a1/b2 metastasis as conversion therapy.
Concerning liver metastasis in patients with gastric cancer, controversy remains as to whether synchronous removal at the time of primary resection is recommended.39 Solitary40 or two lesions41 of liver metastasis were associated with better survival; however, the OS of patients with multiple liver metastases was not satisfactory. In those studies, preoperative chemotherapy was administered in only 17.6%-22.3% of all patients, and many patients were treated with surgery plus postoperative chemotherapy or surgery alone. In contrast, MST of patients with two lesions or three or more lesions in the current study was more acceptable and compared well with that of patients with solitary liver metastasis. Even in cases with multiple liver metastases, long survival can be expected if the R0 operations are successfully performed as conversion therapy.
As for the survival of the patients with postoperative complications, there was no significant survival difference between the patients with or without postoperative complications after R0 resection in each category (data not shown).
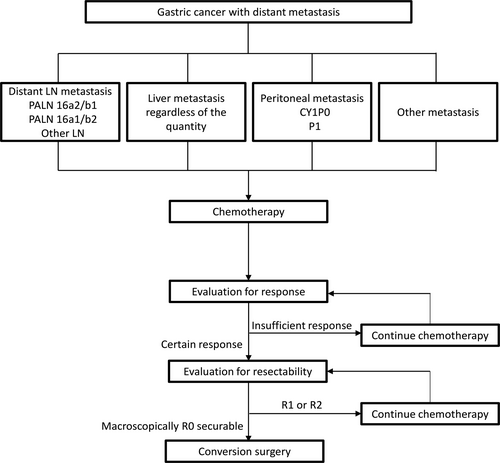
We suggest an inclusive treatment strategy for patients with stage IV gastric cancer (Figure 3); namely, systemic chemotherapy, which is NAC for potentially resectable disease or palliative chemotherapy for marginally resectable and unresectable disease, administered as the initial treatment. In cases where a certain response to chemotherapy is confirmed, resectability is subsequently evaluated. Patients in whom R0 resection can be achieved may be favorable candidates for conversion surgery. Macroscopically and/or microscopically, if residual tumors are apprehended in resectability-evaluation or a certain response is not confirmed in response-evaluation, further chemotherapy should be administered, and reevaluation performed to avoid missing an opportunity for conversion surgery. Patients with a certain response to chemotherapy and who underwent R0 resection as conversion surgery could expect promising survival regardless of the site of metastasis, even though these metastases were identified in multiple organs or locations. In other words, the operation could be considered for the patients with category 1 (NAC indication) and patients with liver metastasis of category 2, provided the metastatic lesions were evaluated as technically resectable. However, basically, the patients with stable disease after short-term chemotherapy in the categories 2, 3, and 4 (conversion surgery) is not recommended. Taking into consideration the malignant potential of gastric cancer in general, a decision to proceed to surgery should be made with caution, especially among patients who had been regarded as technically/oncologically noncurable at the initial diagnosis.
This study has several limitations. First, this was an international multicenter retrospective study, and the background of the treatment may have differed among countries. In addition, information on the proportion of patients who became candidates for surgery among each category is unavailable. Second, the period in which the patients were treated extended over a decade and the surgical procedure and chemotherapy may have changed. Further subanalysis of this project is ongoing, including the timing of operation, postoperative chemotherapy, and the differences among countries.
In conclusion, surgery aiming at R0 operation after induction chemotherapy could now be considered as an established treatment strategy for stage IV gastric cancer, not only for technically resectable metastasis but also for marginally resectable and initially unresectable metastasis.
ACKNOWLEDGMENTS
The authors thank Ms. Kikue Sato for assistance in statistical analysis, Mami Matsumaru for data collection, and Mami Sakurai for institutional interaction and research coordination. This work was supported by grants from FACO and grants for the research promotion committee of the JGCA.
STUDY GROUP AFFILIATIONS
Gifu University, Tochigi Cancer Center, Kanagawa Cancer Center, Gunma Prefectural Cancer Center, Kyoto Prefectural University of Medicine, Hakodate Goryoukaku Hospital, Shizuoka Cancer Center, Dokkyo Medical University, Ishikawa Prefectural Central Hospital, Osaka Prefectural General Medical Center, Kitano Hospital, Osaka National Hospital, Nagoya University Graduate School of Medicine, Hiroshima City Hiroshima Citizens Hospital, Kochi Medical School, Hamamatsu University Hospital, Osaka City University Graduate School of Medicine, Kobe University Graduate School of Medicine, Hyogo College of Medicine, Kanazawa Medical University, Hiroshima University, Tokyo Medical and Dental University, The University of Tokyo, Kitasato University School of Medicine, Gifu Municipal Hospital Department of Surgery, Iwate Medical University, Kyushu University, Chiba Cancer Center, Okayama University, Cancer Institute Hospital, Kumamoto University, Mie University, Shizuoka General Hospital, Kagoshima University, National Cancer Center Hospital East, National Cancer Center Hospital, The Jikei University, Sapporo medical university, Keio university, Kyoto University, Saitama Medical University International Medical Center, Osaka University, Fukushima Medical University, Samsung Medical Center, Yonsei Cancer Center, The Catholic University of Korea, Seoul National University Hospital, Graduate School of Cancer Science and Policy, Research Institute and Hospital, National Cancer Center, Peking University Cancer Hospital, Shan Xi Tumor hospital, Yantai Yuhuangding Hospital affiliated to Qingdao University, The First Affiliated Hospital of Zhejiang Chinese Medicine University, Southern Medical University, Chinese People’s Liberation Army General Hospital, The Fourth Affiliated Hospital, Hebei Medical University.
DISCLOSURE
Conflict of interest: Dr. Yoshida reports grants and personal fees from Taiho Pharm, Asahi Kasei Pharma, Chugai Pharm., Covidien Japan, Daiichi Sankyo, Eisai, Eli Lilly Japan, Johnson & Johnson, MerkSerono, MSD, Nippon Kayaku, Novartis, Ono Pharm., Otsuka Pharm., Sanofi, Takeda Pharm., Tsumura, Yakult Honsha, grants from Abbott, Abbvie, Astellas, Biogen Japan, Celgene, GlaxoSmithKline, KCI, Koninklijke Philips, Kyowa Kirin, Meiji Seika Pharma, Toray Medical, personal fees from AstraZeneca, Bristol-Myers Squibb, Denka, EA Pharma, Olympus, Pfizer, Sanwa Kagaku Kenkyusho, SBI Pharma, Teijin Pharma, TERUMO, outside the submitted work.
Human rights statement and informed consent: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and in compliance with the Helsinki Declaration of 1964 and later versions. All hospitals disclosed information to patients. Participating patients were excluded only when they specified that they were unwilling to participate.




